During their life cycle, mammals acquire immunity towards gastrointestinal nematode (GIN) parasites, as they are continuously exposed to their infective forms. However, the effective expression of this acquired immunity can break down during certain points in time, e.g. during the periparturient period(Reference Houdijk, Jessop and Kyriazakis1). Such a periparturient relaxation of immunity (PPRI) plays an important role in the epidemiology of GIN infections. PPRI is associated with a higher GIN burden and nematode egg excretion(Reference Barger2, Reference Beasley, Kahn and Windon3), and as such the periparturient host is a major source of infection for its parasite-naïve offspring.
A nutrient partitioning framework has identified the hypothesis that PPRI has a nutritional basis: scarce nutrients may be preferentially used to meet the elevated nutritional demands of the reproductive effort at the expense of the immune response during the periparturient period(Reference Coop and Kyriazakis4). Consequently, at times of nutrient scarcity, increased nutrient supply would be expected to reduce the degree of PPRI to parasites and lead to reduced worm burden and worm egg excretion. There is indeed a significant body of evidence to support the view that protein supplementation in mammals, such as small ruminants, reduces the degree of PPRI(Reference Houdijk, Athanasiadou, t' Mannetje, Ramirez-Aviles, Sandoval-Castro and Ku-Vera5, Reference Kyriazakis and Houdijk6). Similarly, feeding foods with higher crude protein (CP) content to lactating rodents exposed to the GIN Nippostrongylus brasiliensis has also resulted consistently in reduced worm burden(Reference Houdijk, Jessop and Knox7–Reference Normanton, Houdijk and Jessop9). However, in the above studies, the animals were fed ad libitum and the increased dietary protein contents resulted in increased feed intake per se, which confounded the effects of protein supply with other dietary factors such as energy supply. It has been suggested that dietary protein and energy deficiency may have different effects on host defence mechanisms(Reference Koski and Scott10), while evidence from ruminant studies suggests that moderate protein, but not energy deficiency, may increase the degree of periparturient parasitism(Reference Donaldson, van Houtert and Sykes11). In ruminants, however, the effects of energy and protein nutrition may be difficult to assess separately due to the modifying role of the rumen(Reference Houdijk, Jessop and Kyriazakis1). Since such confounding effects can be readily avoided in non-ruminants, our objective was to dissect, for the first time in a monogastric model, the effects of moderate energy and protein restriction on PPRI to GIN parasites. We hypothesised that, at times of dietary protein and energy scarcity, increasing protein supply would be more effective in reducing the degree of PPRI than increasing energy supply.
Experimental methods
Animals, housing and feeding strategy during gestation
The experiment described below was approved by the Scottish Agricultural College Ethics Review Committee (ED AE 24/2007) and carried out under Home Office authorisation (PPL 60/3626). One hundred and sixteen second-parity female rats were housed in a room, where ambient temperature was maintained at 21°C, relative humidity ranged from 45 to 65 % and artificial lighting was provided between 08·00 and 18·00 hours. The rats were individually housed in solid-bottomed cages, with fresh sawdust provided weekly. Shredded plastic bubble wrapping for nesting material was provided 3 d before the expected parturition date. Wire-bottomed cages were used during mating and for faeces collection during the primary infection, as described previously(Reference Houdijk, Jessop and Knox12). For mating, female rats were placed with a proven male breeder and mating was confirmed through the presence of a vaginal plug.
Until mating was confirmed, the rats were given ad libitum access to standard rat chow (Rat and Mouse no. 3; Special Diet Services, Witham, Essex, UK). After mating was confirmed, the rats were given ad libitum access to a high-protein food, with 210 g digestible CP and 16·4 MJ metabolisable energy (ME)/kg DM until 10 d into gestation to allow for establishment of pregnancy and placental development. The rats were then fed with a low-protein food, containing 60 g CP and 17·3 MJ ME/kg DM, which was continued until parturition. This feeding protocol was used to reduce body protein reserves during the second half of gestation in order to maximise the degree of protein scarcity during lactation when the rats would be fed low-protein foods(Reference Houdijk, Jessop and Knox7, Reference Pine, Jessop and Oldham13).
Feeding treatments
Six feeding treatments(Reference Houdijk, Jessop and Kyriazakis1–Reference Kyriazakis and Houdijk6) were designed, consisting of restrictedly feeding one of two levels of predetermined ME supply at one of three levels of predetermined CP supply (Fig. 1). Feeding treatments 1–3 and 4–6 were calculated to supply 1·05 and 1·40 MJ ME/kg parturition body weight (PBW) per d, respectively. These planes of ME nutrition were 90 % of the previously observed mean ME intakes on low- and high-protein foods, respectively(Reference Jones, Houdijk and Knox8). In addition, CP supply was calculated to increase incrementally from scarce to more than adequate at each level of ME supply. This was 6·6, 13·3 and 19·9 g/kg PBW per d for the low ME level, and 13·3, 19·9 and 26·5 g/kg PBW per d for the high ME level treatments. These CP allowances were chosen to reflect the range of achieved mean daily CP intake of similar foods in previous experiments under ad libitum feeding(Reference Houdijk, Jessop and Knox7–Reference Normanton, Houdijk and Jessop9) using dam PBW as a scaling factor. Variation in dietary CP content was achieved through the isoenergetic exchange of casein with digestible carbohydrates (starch/sucrose). The resulting composition of the experimental foods is presented in Table 1.
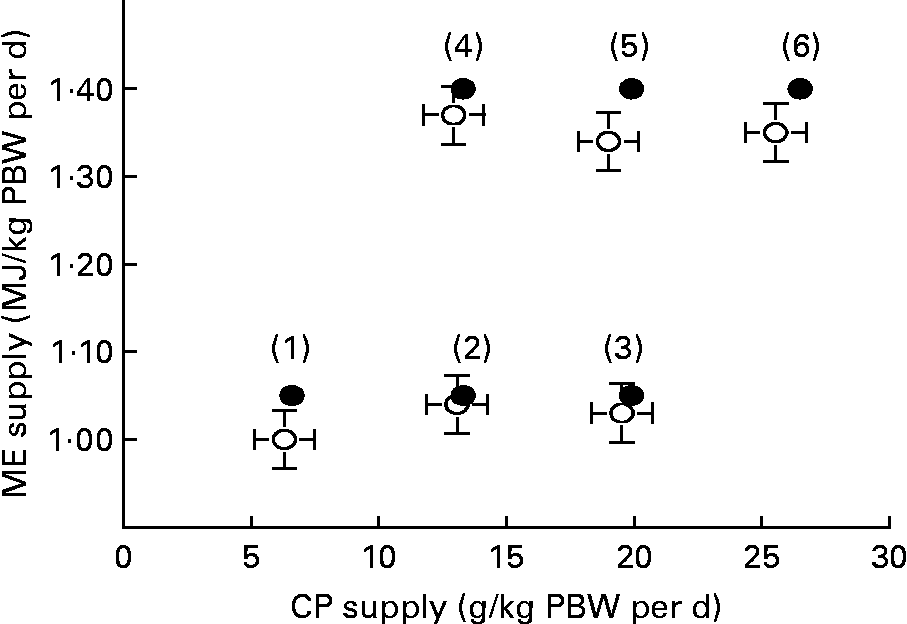
Fig. 1 Planned (●) and achieved (○, with se) dietary supply of metabolisable energy (ME, MJ/kg parturition body weight (PBW) per d) and crude protein (CP, g/kg PBW per d) from six experimental feeding treatments over the first 11 d of lactation in rats, re-infected with Nippostrongylus brasiliensis.
Table 1 Composition and analysis of the experimental food used during lactation

ME, metabolisable energy; CP, crude protein.
* Food ME contents was calculated by multiplying its content of protein (casein), digestible carbohydrates (starch, sucrose and maize starch) and fat (maize oil) with the ME contents of protein (17 MJ/kg), carbohydrates (17 MJ/kg) and fat (38 MJ/kg)(Reference Astrup, Tremblay, Gibney, Lanham-New and Cassidy Vorster58).
Foods were offered on a daily basis in increasing amounts during lactation, reflecting the natural increase in food intake observed in previous experiments with this animal–parasite model system(Reference Houdijk, Jessop and Knox7–Reference Normanton, Houdijk and Jessop9). We omitted a priori feeding treatments with very low or high CP:ME ratios that would jeopardise food intake, as earlier work has shown that voluntary intake on such foods was unacceptably low in lactating animals(Reference Friggens, Hay and Oldham14, Reference Oldham and Friggens15). Thus, our experimental design can be described as having an incomplete 4 × 2 factorial arrangement (Fig. 1).
Infection protocol
All rats received a primary infection of 1600 third-stage infective larvae of N. brasiliensis on day − 37 (with day 0 as mean achieved parturition date), which were suspended in 0·5 ml sterile PBS that was subcutaneously injected in the hind leg. A secondary infection of 1600 third-stage infective larvae N. brasiliensis was administered on day 2 to a subgroup of rats. At the same time, control rats (primary infected only) were sham-infected through subcutaneous injection of 0·5 ml sterile PBS only.
Experimental design
The effects of the six feeding treatments used were assessed on lactational performance and parasitological variables on days 8 and 11 of lactation (corresponding to days 6 and 9 post secondary infection, respectively). The two sampling time points post-secondary infection were included, as previous data(Reference Jones, Houdijk and Knox8) suggested that the nutritional sensitivity of host resistance may differ over time. All rats in these twelve feeding treatment–endpoint combinations (six feeding treatments × two endpoints) received secondary infection. In addition, control rats that did not receive secondary infection were included until day 8 of lactation for each feeding treatment to assess the effect of re-infection on lactational performance (dam and litter weight gain), i.e. six additional control treatments. This resulted in a total of eighteen treatments. Rats blocked for PBW were randomly allocated to these eighteen treatments on the morning of the parturition date. Total sample size aimed for was seven for infected rats and six for control rats. However, the minimum realised sample size for each of the eighteen resulting treatments was five.
Measurements
Body weight and food intake
The rats were weighed daily throughout the experiment, and daily weights taken post-parturition was used to calculate lactational dam weight gain. The pups were counted and the whole litter was weighed daily from day 0 of lactation. Litter size was standardised at twelve pups on day 1 and this was further reduced to ten pups on day 2 of lactation to ensure pup survival and to have equal initial nutrient demands. Litter weight from day 2 onwards was used to calculate litter weight gain during lactation. Because of the restricted feeding regimen, refusals were not expected during lactation. However, food intake was measured daily and any refusals observed during the lactation period were weighed in order to calculate mean achieved CP and ME intake. Foods offered during lactation were sampled during their preparation for the analysis of DM, CP (Kjeldahl-N × 6·38), diethyl ether extract, ash and acid-detergent fibre (Table 1).
Faecal egg counts
A faecal egg count (FEC, in eggs/g of fresh faeces) was performed 7 d after the primary infection (day − 30) to confirm the presence of infection. A second FEC was performed on day − 23 to confirm that the expected parasite expulsion had taken place. To this effect, faeces were collected through overnight housing on bottom-wired cages(Reference Houdijk, Jessop and Knox12) and FEC were performed using a modified saturated salt flotation method(Reference Christie and Jackson16).
Worm burden and nematode eggs in the colon
The rats were killed through gradually increasing ambient CO2 concentration, followed by CO2 asphyxiation for parasitological assessment (see later) and immune responses(Reference Jones, Houdijk and Sakkas17). The small intestine was removed and stored in formaldehyde for subsequent worm burden assessment (number, sex and stage of maturity according to worm morphology). The contents of the large intestine were weighed and assessed for worm eggs, as described for FEC above. This was then multiplied by the weight of the large intestinal contents to obtain the total number of nematode eggs in the colon (EIC). The latter measure is preferred over the FEC equivalent of the colon contents, as feeding treatments used could have biased colon contents volume, and thus the concentration of EIC contents.
Calculations and statistical analysis
Data were analysed using restricted maximum likelihood, to account for the incomplete 4 × 2 factorial design of the CP–ME combinations used. The effects of CP supply, ME supply, endpoint and their two-and three-way interactions were assessed on dam body weight gain, litter weight gain, worm burden, percentage of female worms and EIC. In order to investigate for the effect of infection per se, a separate restricted maximum likelihood analysis was used to assess the effects of CP supply, ME supply, infection status and their two and three-way interactions on dam body weight gain and litter growth until day 8 of lactation. For both statistical assessments, interactions that did not approach significance at P < 0·05 were omitted from the final models used.
Because of their skewed nature, the EIC and worm burden were transformed according to log(n+1) to normalise data before statistical analysis. These data are reported as back-transformed means with their 95 % CI, as described previously(Reference Houdijk, Jessop and Knox7). All statistical analyses were performed using Genstat 11 for Windows release 11.1, 2008 (VSN International, Hemel Hempstead, Hertfordshire, UK).
Results
Faecal egg counts during the primary infection and performance until parturition
FEC taken on day − 37 and − 30 averaged 3563 (95 % CI 351, 319) eggs/g and 0 (95 % CI 0, 0) eggs/g, respectively. Initial mean body weight was 345 (se 4·0) g. During the first 10 d of gestation, the rats grew from 355 (se 3·6) to 383 (se 4·3) g and had an average DM intake of 20·6 (se 0·40) g/d. From then onwards and until parturition, the pregnant rats continued to gain weight to a mean of 451 (se 5·1) g and had an average DM intake of 18·7 (se 0·51) g/d, which dropped to an average of 7·1 (se 0·62) g/d just before parturition. Dam mean PBW averaged 360 (se 4·6) g, mean litter size averaged 14·2 pups (se 0·27) g and mean litter weight averaged 74·1 (se 0·99) g.
Achieved metabolisable energy and crude protein intake
Fig. 1 shows the expected and achieved least squares mean intake of ME and CP over the 11 d lactating period/kg PBW per d. Small numbers of feed refusal were observed for each feeding treatment. Consequently, target CP and ME supply were not exactly met. However, as intended, feeding treatment did not significantly affect achieved ME intake, within the low (P = 0·130) and the high level of ME supply (P = 0·897). Infection did not affect mean achieved ME intake (P = 0·290) and CP intake (P = 0·738).
Dam weight gain
The effects of feeding treatments (Fig. 2(a)) and infection for each feeding treatment (Fig. 2(b)) on daily dam weight gain were shown. Dam weight gain was significantly affected by dietary CP supply (P < 0·001) and ME supply (P < 0·001), but not by the endpoint (P = 0·401; data not shown). Dams at a low level of ME supply lost more weight than dams at a high level of ME supply. An increasing level of CP supply resulted in increased dam weight gain at both levels of ME supply (Fig. 2(a)). Infection did not affect dam weight gain (Fig. 2(b); P = 0·494), while no significant interactions among any of the factors involved were observed (P>0·10).

Fig. 2 Dam weight gain across four levels of dietary supply of crude protein (CP) and two levels of metabolisable energy (ME, 1·05 and 1·40 MJ/kg parturition body weight (PBW)/d) over the first 11 d of lactation in rats (a) re-infected with Nippostrongylus brasiliensis (□, low energy; ![]() , high energy) and (b) across these feeding treatments for infected and non-infected control lactating rats (□, infected;
, high energy) and (b) across these feeding treatments for infected and non-infected control lactating rats (□, infected; ![]() , control). Values are means with their standard errors represented by vertical bars.
, control). Values are means with their standard errors represented by vertical bars.
Litter weight gain
The effects of feeding treatments (Fig. 3(a)) and infection for each feeding treatment (Fig. 3(b)) on daily litter weight gain are shown. Litter weight gain was affected by CP supply (P < 0·001) and ME supply (P = 0·011), but not by the endpoint (P = 0·247; data not shown). Litters from low ME dams gained less weight than litters from high ME dams. An increasing level of CP supply resulted in higher litter weight gain at both levels of ME supply (Fig. 3(a)). Infection status (Fig. 3(b)) significantly affected litter weight gain (P = 0·015). Although the interaction between CP supply, ME supply and infection status was not formally significant (P = 0·113), litters of infected dams allocated to feeding treatments 1 and 2 showed lower weight gain than their non-infected counterparts.

Fig. 3 Litter weight gain across four levels of dietary supply of crude protein (CP) and two levels of metabolisable energy (ME, 1·05 and 1·40 MJ/kg parturition body weight (PBW per d) over the first 11 d of lactation in rats re-infected with (a) Nippostrongylus brasiliensis (□, low energy; ![]() , high energy) and (b) across these feeding treatments for infected and non-infected control lactating rats (□, infected;
, high energy) and (b) across these feeding treatments for infected and non-infected control lactating rats (□, infected; ![]() , control). Values are means with their standard errors represented by vertical bars.
, control). Values are means with their standard errors represented by vertical bars.
Total worm burden, eggs in colon and worm burden composition
The effects of feeding treatments on total worm burden for day 8 (Fig. 4(a)) and day 11 of lactation (Fig. 4(b)) were shown, while Fig. 5 shows such effects for EIC on the same days. Total worm burden was significantly affected by CP supply (P = 0·023) and endpoint (P = 0·013), but were not influenced by ME supply (P = 0·155). Worm burden decreased with increasing level of CP supply at both levels of ME supply and was lower on day 11 than on day 8 of lactation (Fig. 4(a) and (b)). Although Fig. 4 suggests that these effects of CP supply were more pronounced on day 8 than on day 11, this interaction was not formally significant (P = 0·248). EIC were not affected by dietary CP (P = 0·204) and ME (P = 0·720) supply, which were significantly higher on day 11 than on day 8 (Fig. 5(a) and (b); P = 0·004). There were no significant interactions between any of the above factors for EIC (P>0·10). Fig. 6 shows the percentage of female worms in the worm burden. ME supply (P = 0·470) and endpoint (P = 0·161) did not affect worm burden composition. However, increasing CP supply reduced the percentage of female worms (P = 0·049), while the magnitude of this effect was greater at the lower level of ME supply (P = 0·022; Fig. 6).

Fig. 4 Backtransformed mean worm burden, with backtransformed lower and upper limits of transformed error bars as 95 % CI, taken on (a) day 8 (□, low energy; ![]() , high energy) and (b) day 11 (□, low energy;
, high energy) and (b) day 11 (□, low energy; ![]() , high energy), following re-infection with Nippostrongylus brasiliensis on day 2 of lactation in rats fed four levels of dietary crude protein (CP) and two levels of metabolisable energy supply (1·05 and 1·40 MJ/kg parturition body weight (PBW) per d).
, high energy), following re-infection with Nippostrongylus brasiliensis on day 2 of lactation in rats fed four levels of dietary crude protein (CP) and two levels of metabolisable energy supply (1·05 and 1·40 MJ/kg parturition body weight (PBW) per d).

Fig. 5 Backtransformed mean total eggs in colon, with backtransformed lower and upper limits of transformed error bars as 95 % CI, taken on (a) day 8 (□, low energy; ![]() , high energy) and (b) day 11 (□, low energy;
, high energy) and (b) day 11 (□, low energy; ![]() , high energy), following re-infection with Nippostrongylus brasiliensis on day 2 of lactation in rats fed four levels of dietary crude protein (CP) and two levels of metabolisable energy supply (1·05 and 1·40 MJ/kg parturition body weight (PBW) per d).
, high energy), following re-infection with Nippostrongylus brasiliensis on day 2 of lactation in rats fed four levels of dietary crude protein (CP) and two levels of metabolisable energy supply (1·05 and 1·40 MJ/kg parturition body weight (PBW) per d).

Fig. 6 Percentage of female worms in worm burden arising from re-infection with Nippostrongylus brasiliensis in lactating rats at four levels of dietary crude protein (CP) and two levels of metabolisable energy supply (1·05 and 1·40 MJ/kg parturition body weight (PBW per d); □, low energy; ![]() , high energy. Values are means with their standard errors represented by vertical bars.
, high energy. Values are means with their standard errors represented by vertical bars.
Discussion
Previous studies carried out with this rodent–nematode parasite model have consistently shown that reducing nutrient scarcity through increasing dietary CP content during lactation results in a reduced degree of parasitism(Reference Houdijk, Jessop and Knox7–Reference Normanton, Houdijk and Jessop9). In these studies, ad libitum feeding of high-CP foods resulted in reduced worm burden, but because food intake of high-CP foods increased, improved resistance could have arisen from the increased intake of any nutrient and/or energy. The present experiment aimed to account for this uncertainty by testing the hypothesis that parasitism during lactation is sensitive to CP supply per se by increasing levels of dietary CP supply at constant levels of ME supply. At the same time, the design allowed us to assess whether a moderate dietary ME restriction compromises lactating host resistance to parasites. We discuss our result that CP supply, but not ME supply, affects lactational resistance to N. brasiliensis. It is unlikely that the incomplete 4 × 2 factorial design used (Fig. 1) biased our conclusions; re-analysis of the data omitting those arising from feeding treatments 1 and 6, thus addressing our hypothesis with a balanced 2 × 2 factorial design, confirmed that worm burden was affected by increased CP supply (P = 0·035) and not by ME supply (P = 0·174), and although the effects of CP supply on worm burden were again more pronounced on day 8 than on day 11, as in the incomplete factorial design, there was no formally significant interaction between CP supply and time (P = 0·063).
The experimental design created the required conditions in order to test our hypotheses. In the present experiment, restricted feeding at 90 % of the achieved feed intake observed in previous experiments avoided increased feed intake, which has been observed when animals are offered foods with higher CP content. The success of the experimental design is further evidenced by the small numbers of food refusal that occurred across feeding treatments and the consequently similar level of ME intake at increasing levels of CP intake, within each of the two different planes of ME supply (Fig. 1). With regard to performance data, the lower level of ME supply resulted in higher dam weight loss and lower litter gain. This is consistent with previous findings on the effects of energy nutrition on lactational performance of rats(Reference Kliewer and Rasmussen18, Reference Passos, Ramos and Moura19). At the same time, increased CP supply resulted in smaller dam weight loss and larger litter weight gain. Since the last increment of CP supply did not further increase lactational performance, the range of CP supply achieved ranged from scarce to more than adequate. However, it cannot be discounted that the ME supply was still limiting at the highest level of ME supply used, as only two levels of ME were used.
Lactation imposes significant protein demands on mammals(Reference Friggens, Hay and Oldham14). At times of dietary protein scarcity, additional protein may be derived from labile body protein. However, the amount of labile body protein that can be mobilised is limited(Reference Pine, Jessop and Oldham13), and this limitation was further exacerbated by feeding low-protein food during late gestation. At the same time, parasitised hosts would be expected to have additional protein requirements to activate or maintain an effective protective response to parasites(Reference Kyriazakis and Houdijk6). This is because the effector mechanisms of the immune response are highly proteinaceous in nature and inflammation and immune system activation are characterised by the synthesis of specific proteins that play crucial roles in the defence of the host against pathogens and the modulation of the immune response(Reference Coop and Kyriazakis20, Reference Le Floc'h, Melchior and Obled21). Thus, feeding foods with a sufficiently low CP content to lactating parasitised hosts, as in the present experiment, would be expected to result in protein scarcity, with penalties on both lactational performance and expression of immunity to parasites. We considered changes in the number of adult nematodes in the small intestine and EIC to be the result of changes in the degree of expression of immunity to N. brasiliensis, as they provide the ultimate measure on how the host copes with the challenge(Reference Normanton, Houdijk and Jessop22). Increasing dietary CP supply resulted in a significant reduction in worm burden. This agrees with results from studies with periparturient mammalian hosts, including rats(Reference Houdijk, Jessop and Knox7, Reference Jones, Houdijk and Knox8), ewes(Reference Donaldson, van Houtert and Sykes23–Reference Kahn, Knox and Gray26) and dairy goats(Reference Chartier, Etter and Hoste27, Reference Etter, Chartier and Hoste28). However, to our knowledge, the present experiment is the first to have assessed independently the effects of CP and ME supply on resistance to parasites in a non-ruminant periparturient model.
Increased CP supply did not affect EIC, although there was a substantial decrease at the higher levels of CP supply. The absence of a significant nutritional sensitivity of EIC in the presence of such effects on total worm burden concurred with substantially larger variation (see Fig. 5). While it cannot be excluded that this may have arisen to some extent from the lower than expected number of replicates, and variation in fecundity in response to dietary treatments, we have discussed previously that this may arise from the relatively large numbers of steps involved in the nematode egg counting technique used(Reference Houdijk, Jessop and Knox12). Perhaps, to some extent, this variation could have been reduced through repeated measures of FECs and assessment of daily nematode egg excretion during secondary infection. However, earlier attempts suggest that the highly digestible nature of the semi-synthetic foods used would not have allowed consistent production of the relatively large volumes of faeces required for such purpose(Reference Normanton, Houdijk and Jessop22).
In addition to reducing worm burden, the increasing level of CP supply resulted in a reduced percentage of female worms in the worm burdens, especially at the lower level of ME supply. Increased CP supply reduced the percentage of female worms not only in earlier studies using the same host–parasite system(Reference Houdijk, Jessop and Knox7), but also following protein supplementation of sheep infected with Haemonchus contortus (Reference Wallace, Bairden and Duncan29). It has been shown that female adult nematodes are usually expelled at a higher rate than male adult nematodes(Reference Africa30, Reference Jarrett, Jarrett and Urquhart31) and that rats infected solely with female adult N. brasiliensis develop a stronger immunity than rats infected with male worms(Reference Ogilvie32). Taken together, these observations suggest that the expression of immunity may be more effective towards female than towards male N. brasiliensis (Reference Houdijk, Jessop and Knox12). Since female worms are longer and heavier than male worms(Reference Kassai33), it would be reasonable to assume that the mean worm length and the weight of the worm burden in the dams receiving lower protein was larger than in the dams fed higher levels of protein. For abomasal infections in sheep, it has been suggested that hosts control worm mass rather than worm number, since longer and heavier worms may cause more damage to the host(Reference Stear, Strain and Bishop34). If such arguments hold for small-intestinal infections as well, then having a worm burden with shorter and lighter worms would have been beneficial to the rats fed higher levels of protein.
In the present experiment, moderate ME restriction imposed during lactation did not affect worm burdens, the worm sex ratio or the number of nematode EIC. Studies in ruminants have likewise shown that gastrointestinal parasitism is not sensitive to moderate changes in ME supply(Reference Donaldson, van Houtert and Sykes23, Reference Bown, Poppi and Sykes35). Activation and maintenance of an immune response are expected to be energetically demanding processes(Reference Colditz36) and immune cells require high levels of glucose(Reference Fox, Hammerman and Thompson37). This has been evidenced by the lower amount of fat deposits in infected mice compared with their uninfected counterparts(Reference Coltherd, Bünger and Kyriazakis38, Reference Kristan and Hammond39). It should be noted that the high energetic demand for milk synthesis in lactating mammals can, at least to some degree, be met through mobilisation of maternal adipose tissue(Reference Oftedal40). An energy restriction during lactation results in increased body fat mobilisation in many species of mammals, including rats(Reference del Rosario Ayala, Racotta and Hernández-Montes41–Reference Parmley, Machado and McNamara45). It cannot be excluded that the effects of dietary ME supply on worm burden were not observed because the likely higher rate of body fat mobilisation in the low-ME dams may have provided sufficient ME to overcome ME scarcity for the expression of immunity. The effects of energy (caloric) restriction on resistance to parasites have also been assessed in growing rodents(Reference Koski, Su and Scott46–Reference Lunn, Northrop and Wainwright48). In contrast to our findings, energy restriction was consistently associated in these studies with higher worm burden. Careful consideration of the extent and the duration of the energy restriction imposed in these experiments suggests that these were substantially greater and longer than those used in the present study. For example, Koski et al. (Reference Koski, Su and Scott46) applied an energy restriction of up to 25 % for 6 weeks; Kristan(Reference Kristan47) imposed an energy restriction of 40 % for almost 7 months, and Lunn et al. (Reference Lunn, Northrop and Wainwright48) used an energy restriction of 40 % for 3 weeks. In contrast, we imposed a moderate ME restriction of 25 % for no longer than 11 d. Growing animals, when nutritionally restricted, mobilise fat while growing protein or retaining their protein reserves(Reference Kyriazakis and Emmans49, Reference Kyriazakis and Emmans50), while severe and prolonged feed restriction leads to combined fat and protein losses(Reference Hornick, Van Eenaeme and Gerard51). Thus, we cannot exclude the possibility that a more severe ME restriction could also have affected host resistance to parasites in our model system.
Re-infection with N. brasiliensis did not affect achieved DM intake and maternal body weight gain but reduced litter body weight gain (a proxy for milk production) during lactation with the consequence of infection on litter body weight being more pronounced for feeding treatments 1 and 2 (Fig. 3(b)). This suggests that infection can reduce lactational performance provided that the level of protein–energy malnutrition is sufficiently low. Indeed, at higher levels of ME intake, re-infection with N. brasiliensis did not affect litter weight gain(Reference Houdijk, Jessop and Knox7, Reference Jones, Houdijk and Knox8). Anorexia has not been observed in the aforementioned studies using the same host–parasite model, which is consistent with the view that it mainly characterises primary infections(Reference Kyriazakis, Tolkamp and Hutchings52, Reference Kyriazakis53). The absence of a systematic effect of infection on litter weight in the rats during lactation supports the view that scarce nutrient allocation to milk production takes priority over expression of immunity in parasite-immunised periparturient mammals(Reference Coop and Kyriazakis20). This is because in the converse situation, infection would have been expected to penalise litter growth in any nutritional environment, and not only when both CP and ME supply are sufficiently low (treatments 1 and 2) to reduce resilience(Reference Houdijk, Jessop and Knox7). Variable effects of nematode infection on food intake and/or dam and pup performance have been observed in other host–parasite systems(Reference Kristan54–Reference Willis and Poulin56). However, these experiments are not considered comparable to the present experiment, as they involved parasite-naïve animals. Such hosts are expected to prioritise resource allocation to cover the nutritional requirements of expression of immunity towards parasites over those of maintenance and growth(Reference Kyriazakis and Houdijk6).
In conclusion, the present results support the view that the degree of PPRI to N. brasiliensis is sensitive to changes in protein but not moderate energy supply. This is shown by a decrease in the worm burden, which was achieved in the present experiment with increasing levels of protein intake. Future work may focus on the involvement of specific, essential amino acids in these responses, as there is an increasing body of evidence for amino acid-specific effects on host immune responses(Reference Li, Yin and Li57). Increasing CP intake resulted in a reduction of the percentage of female worms in the remaining worm burden, which could suggest a more effective expression of immunity towards female worms. This could be beneficial to hosts, as female worms are larger and heavier than male worms and may cause more damage to parasitised hosts. The performance data suggest that secondary parasitic infections may have significant effects on host lactational performance, especially at times of protein–energy malnutrition.
Acknowledgements
The authors thank the staff of the March Building at the University of Edinburgh for animal husbandry, and Ian Nevison (Biomathematics and Statistics Scotland) for statistical advice. The present work was supported by the Biotechnology and Biology Sciences Research Council (BBSRC). The Scottish Agricultural College and the Moredun Research Institute received support from the Scottish Government, Rural and Environmental Research and Analysis Directorate. P. S. is grateful to the Hellenic State Scholarship Foundation for the provision of a postgraduate scholarship. None of the authors has a conflict of interest in relation to the present study. The present study has resulted from the postgraduate studies of P. S. and is part of his PhD thesis. All other authors have contributed equally to the study.









