Hypertension (HTN) affects >one billion people. Despite advances, blood pressure (BP) remains uncontrolled in approximately 50 % of individuals treated with medication( Reference Cutler, Sorlie and Wolz 1 , Reference Egan, Zhao and Axon 2 ). There is a need for novel, effective therapies for primary and secondary prevention of HTN.
Nitric oxide (NO) is a major vasodilating molecule and plays a critical role in vascular homoeostasis and BP regulation( Reference Moncada and Higgs 3 ). Reduced NO bioavailability, either through decreased production or increased consumption, has been associated with endothelial dysfunction and implicated in the development of prehypertension( Reference Plavnik, Ajzen and Christofalo 4 ) and HTN( Reference Giansante and Fiotti 5 ). Multiple studies have demonstrated that plasma and/or urinary NO metabolites are significantly lower in hypertensives compared with matched controls( Reference Linder, Kiowski and Bühler 6 – Reference Heiss, Lauer and Dejam 11 ). In addition, NO levels correlate positively with vascular function( Reference Heiss, Lauer and Dejam 11 ) and peak brachial artery dilation( Reference Casey, Beck and Braith 12 ), but negatively with BP( Reference Forte, Copland and Smith 7 , Reference Ghasemi, Zahediasl and Syedmoradi 9 ). Thus, the restoration of NO signalling provides an attractive mechanism to control HTN.
Dietary nitrate has been suggested to be an important contributor to NO signalling in humans, with the first human report of an anti-hypertensive effect in 2006. A 3-d double-blind, cross-over study of 0·1 mmol/kg per d sodium nitrate resulted in a significant 3·7-mmHg reduction in diastolic BP (DBP) in young, healthy volunteers( Reference Larsen, Ekblom and Sahlin 13 ). A 2008 study demonstrated marked hypotensive effect of NO3 − (−10·4/8 mmHg), which correlated with increased plasma nitrite( Reference Webb, Patel and Loukogeorgakis 14 ).
Since then, research regarding dietary nitrate and CVD has increased. Indeed, several reviews have detailed the existing pre-clinical/clinical evidence, as well as possible mechanisms, for dietary nitrate and its potential role in BP regulation( Reference Gilchrist, Shore and Benjamin 15 – Reference Gee and Ahluwalia 19 ). A 2013 systematic review and meta-analysis concluded that dietary nitrate can reduce systolic BP (SBP) by 4·4 mmHg (P<0·001) and DBP by 1·1 mmHg (P=0·06)( Reference Siervo, Lara and Ogbonmwan 16 ). This and another review( Reference Hobbs, George and Lovegrove 17 ) noted inverse associations between nitrate dose of SBP reduction (r 2 0·45; P=0·033). However, it is important to note that these reviews focused mostly on normotensive, healthy, normal-weight males in acute interventions and there is a lack of data regarding hypertensives.
Nonetheless, high habitual intake of dietary nitrate has been inversely associated with HTN risk( Reference Bahadoran, Mirmiran and Ghasemi 20 , Reference Golzarand, Bahadoran and Mirmiran 21 ). Further, a proof-of-principal study in fifteen untreated, grade 1 hypertensives demonstrated that a single nitrate dose (3·5 mmol as beetroot juice) increased plasma nitrite by 150 % and decreased 24-h BP (−11·2/−9·6 mmHg; P<0·001), as well as arterial stiffness, compared with control (P<0·05)( Reference Ghosh, Kapil and Fuentes-Calvo 22 ). In a subsequent unblinded and uncontrolled, pilot study, we demonstrated that 14-d dietary nitrate could increase serum NO metabolites in controlled and uncontrolled hypertensives but selectively lowered BP and arterial stiffness in uncontrolled hypertensives only( Reference Ghosh, Kapil and Fuentes-Calvo 22 ). However, recent randomised controlled trials (RCT) of dietary nitrate in hypertensives provided inconsistent results. One of these RCT demonstrated that 4 weeks of 6·45 mmol dietary nitrate significantly reduced BP( Reference Kerley, Dolan and Cormican 23 ). However, a subsequent cross-over RCT reported no effect of 1 week of the same nitrate dose( Reference Kapil, Khambata and Robertson 24 ).
Considering these inconsistencies, we wanted to follow on from our pilot study and assess, in a more rigorous manner, the effect of 7-d daily dietary nitrate on ambulatory BP, plasma nitrate/nitrite, lipids and renal indices among treated but uncontrolled hypertensives.
Methods
Trial design
This was a 7-d, double-blind, randomised, placebo (PL)-controlled, cross-over trial to assess the effect of dietary nitrate. Subjects were tested on three separate occasions – baseline (day 1), midpoint (day 8) and endpoint (day 15) – before and after each intervention period (Fig. 1).
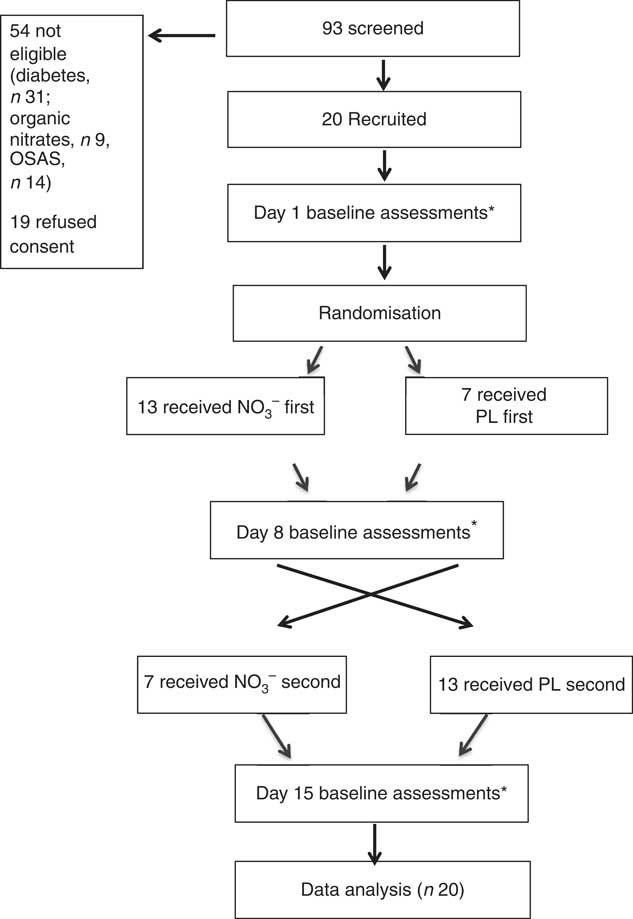
Fig. 1 Trial design. NO3 −, nitrate-rich beetroot juice; PL, placebo. * Assessments included 24-h ambulatory blood pressure, blood draw and assessments of dietary nitrate intake.
Study participants
Subjects with known or suspected uncontrolled HTN, established on diverse anti-hypertensive regimens, were recruited from specialist clinics and invited to wear an ambulatory BP monitor (ABPM) for 24 h. We excluded subjects with controlled HTN (<130/80 mmHg), as well as those with kidney disease, diabetes, cognitive impairment or sleep apnoea, and those taking organic nitrates. The study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee of Connolly Hospital, Dublin, Ireland.
Intervention
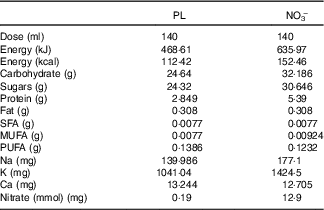
This trial used NO3 − and nitrate-depleted beetroot juice (PL) as previously described( Reference Gilchrist, Winyard and Fulford 25 ). Independent testing and analysis of the NO3 − and PL were conducted previously( Reference Gilchrist, Winyard and Fulford 25 ) and the specific compositions are displayed in Table 1.
Table 1 Composition of nitrate-depleted (placebo; PL) and nitrate-rich beetroot juice (NO3 −)
A physician not involved in data gathering (L. C.) generated the allocation sequence, whereas a nutrition researcher enrolled subjects (C. P. K.). After baseline assessments, subjects were randomised to consume 140 ml of NO3 (12·9 mmol nitrate) at approximately 09.00 hours each morning for 7 d followed by PL (0·5 mmol nitrate) for 7 d or the cross-over condition. We selected this dose as the nitrate content is attainable with a diet rich in vegetables( Reference Sobko, Marcus and Govoni 26 ).
During the trial, all subjects were provided with written and verbal instructions not to alter behaviours that are known to influence NO kinetics, including dietary, tobacco, alcohol, exercise or medication habits and not to use mouthwash or antibiotics, which are known inhibitors of dietary nitrate reduction to nitrite.
Subjects took their seventh and final dose of each beverage 2–3 h before their midpoint and endpoint assessments. In this manner, the juice was most active during waking hours and testing coincided with peak physiological and biochemical effect( Reference Kerley, Dolan and Cormican 23 , Reference Kerley, Cahill and Bolger 27 ). Compliance with the juice was assessed with a daily diary.
Outcome measures
On all 3 testing days (days 1, 8 and 15) in an identical manner and at the same time of day, non-fasting blood was drawn and subjects were fitted with an ABPM for 24 h as previously described( Reference Ghosh, Kapil and Fuentes-Calvo 22 ).
Lifestyle assessment
Weekly dietary nitrate intake was estimated at each time point using the ‘Nitrate Veg Table’( Reference Lidder and Webb 28 ). This table asks subjects to report how many times in the previous week they consumed foods rich in nitrate and provides a composite score for weekly nitrate intake. At each time point, we also asked detailed questions regarding the subjects exercise, alcohol, medication, tobacco and alcohol habits.
Biochemical analysis
On testing days, venous blood samples were drawn into serum and plasma tubes that have a low nitrate/nitrite content before ABPM setup. Serum was analysed locally for routine lipid (total cholesterol, LDL, HDL) and renal (sodium, potassium, creatinine) parameters.
The half-life of NO is <1 s( Reference Moshage, Kok and Huizenga 29 ). Therefore, direct determination of NO in vivo is difficult. However, measurement of NO metabolites (nitrate/nitrite) in biological fluids reflects NO bioavailability( Reference Kleinbongard, Dejam and Lauer 30 ). Therefore, the plasma tube was centrifuged at 4000 rpm and 4°C for 10 min immediately after phlebotomy. Plasma was subsequently immediately extracted into Eppendorf cryovials, frozen at −80°C and later analysed for nitrate/nitrite with the current ‘gold-standard’, ozone-based chemiluminescence analysis (NO analyser, NOA280i; Sievers), which is the most accurate and sensitive NO metabolite detection method, as previously described( Reference Maher, Arif and Madhani 31 ).
Statistical methods
The primary outcome was 24-h SBP. Our sample size was based on the primary outcome of mean 24-h ambulatory BP. At α=0·05, we estimated that nineteen participants would provide 80 % power, assuming an sd of 3·8 mmHg, to detect a 2·6-mmHg difference in the mean 24-h ambulatory BP based on our pilot study( Reference Kerley, Dolan and Cormican 23 ).
The difference between baseline and post-interventions was calculated for all variables, denoted as ΔNO3 − and ΔPL. Ambulatory BP were analysed by using mixed models in SAS 9·0 software by using the PROC MIXED command (SAS Institute Inc.). The subject was included as a random factor in each model. Fixed effects included the treatment (active or PL) and the order of treatments to account for a possible ‘carry over’ effect. The statistical difference between ΔNO3 − and ΔPL for other outcome variables was calculated using paired t tests. Results were expressed as means and standard deviations. All statistical tests were conducted at the two-sided 0·05 significance level.
Results
Study population
Of ninety-three subjects screened, twenty were recruited. There were no dropouts. Baseline characteristics are displayed in Table 2. The cohort comprised mainly male (65 %), Caucasian (90 %) and obese (mean BMI=30 kg/m2) individuals, and most had a history of CVD. All subjects were prescribed ≥1 anti-hypertensive medication. There were no reported adverse events. The juices were well tolerated, and as reported previously( Reference Webb, Patel and Loukogeorgakis 14 , Reference Kerley, Dolan and Cormican 23 , Reference Kerley, Cahill and Bolger 27 ) fourteen subjects (70 %) reported transient, red/pink urine (beetruria).
Table 2 Baseline characteristics (Mean values and standard deviations; numbers and percentages)
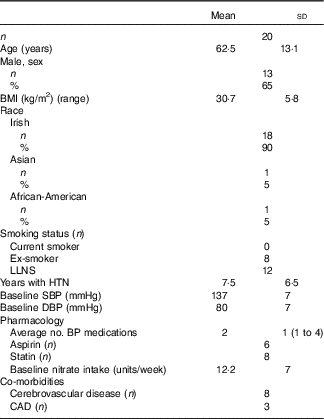
LLNS, lifelong non-smoker; HTN, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; CAD, coronary artery disease.
Lifestyle results
Throughout the trial, there was no reported change in prescribed medication, or habitual tobacco/alcohol/exercise habits between visits. Dietary nitrate intakes were constant on both group and individual levels at all three time points (12·2 nitrate ‘units’ weekly).
Biochemical results
Plasma nitrite levels increased significantly after NO3 − (P=0·001) but not after PL (P=0·3) (Table 3). There was no difference at any stage between lipid or renal parameters (Table 3).
Table 3 Biochemical results (Mean values and standard deviations)
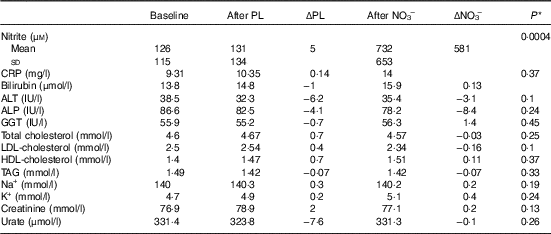
PL, placebo; ΔPL, change following PL compared with baseline; NO3 −, nitrate-rich beetroot juice; ΔNO3 −, change following NO3 − compared with baseline; CRP, C-reactive protein; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase.
* P values derived from paired t tests of ΔNO3 − v. ΔPL.
Ambulatory blood pressure results
Average ABPM wear time was similar at all three time points with an average of >30 successful readings taken. Mean group values for 24-h, day and night SBP and DBP decreased after NO3 − (Table 4). There was no observed treatment effect regarding either SBP or DBP (24 h, day or night).
Table 4 Ambulatory blood pressure (ABP) results (Mean values and standard deviations; differences and 95 % confidence intervals)
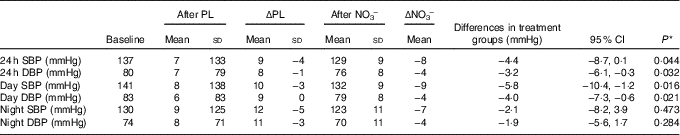
PL, placebo; ΔPL, change in ABPM following PL compared with baseline; NO3 −, nitrate-rich beetroot juice; ΔNO3 −, change in ABPM following NO3 − compared with baseline; SBP, systolic blood pressure; DBP, diastolic blood pressure; ABPM, ambulatory blood pressure monitor.
* P values derived from mixed model analysis as described.
Discussion
Daily dietary nitrate for 7 d was well tolerated and led to increased NO metabolites and reduced 24-h and day BP. Our trial extends the growing literature regarding the hypotensive effect of dietary nitrate as we recruited highly selected individuals with uncontrolled HTN but already established on anti-hypertensive medication. Further, we controlled for factors influencing NO, including diet, alcohol, smoking, exercise and medication.
We observed significant increases in plasma nitrite, which were greater here (126 to 732 μM) than in our pilot study (100 to 175 μM)( Reference Kerley, Dolan and Cormican 23 ). This can be explained by the timing of phlebotomy. Here, non-fasting blood samples were taken 2–3 h after subjects consumed juice, coinciding with peak NO metabolite bioavailability( Reference Webb, Patel and Loukogeorgakis 14 , Reference Kerley, Dolan and Cormican 23 , Reference Kerley, Cahill and Bolger 27 ).
We observed significant decreases in 24-h (−8/−4 mmHg) and day BP (−9/−4 mmHg) profiles but no significant effect at night. Considering that all subjects consumed juice 2–3 h before their morning clinic visit and the peak biochemical and physiological response to exogenous nitrate is reported at 2–3 h( Reference Webb, Patel and Loukogeorgakis 14 , Reference Kerley, Dolan and Cormican 23 , Reference Kerley, Cahill and Bolger 27 ), the lack of effect at night is not surprising.
It is noteworthy that BP values decreased after PL, as well as after NO3 −. It is possible that other bioactive components may affect BP. For example, antioxidants, flavonoids, anthyocianates, polyphenols and betaine may up-regulate nitric oxide synthase expression, decrease oxidative stress and increase NO metabolite bioavailability( Reference Rodriguez-Mateos, Hezel and Aydin 32 ). These components and nitrate may have individual and synergistic additive effects, even at low doses( Reference Rodriguez-Mateos, Hezel and Aydin 32 ). Interestingly and importantly, if BP values had not decreased after PL, the statistical significance of the anti-hypertensive effect of NO3 − would have been even greater.
Despite the growing interest in dietary nitrate, there remains a lack of data regarding hypertensives. An acute unblinded, uncontrolled pilot study demonstrated that a single dose of dietary nitrate (3·5 mmol) could increase plasma nitrite by 150 % and decrease 24-h BP (−11·2/−9·6 mmHg; P<0·001)( Reference Ghosh, Kapil and Fuentes-Calvo 22 ). In a subsequent unblinded and uncontrolled, pilot study we demonstrated that 14-d dietary nitrate increased serum NO metabolites in controlled and uncontrolled hypertensives but selectively lowered BP and arterial stiffness in uncontrolled hypertensives only( Reference Kerley, Dolan and Cormican 23 ). However, two recent, well-conducted trials provided conflicting evidence. A double-blind, randomised, controlled trial of drug-treated (n 34) and drug-naïve (n 34) hypertensives demonstrated that 28 d of 6·45 mmol dietary nitrate significantly reduced clinic BP, home BP and 24-h ABPM( Reference Kapil, Khambata and Robertson 24 ). A subsequent randomised, PL-controlled, double-blind cross-over trial assessed twenty-seven individuals with treated HTN after 7 d of high-nitrate beetroot juice (6·45 mmol) and 7 d of nitrate-depleted beetroot juice (0·5 mmol). Despite significant increases in nitrate/nitrite, there was no difference in home BP or 24-h ABPM( Reference Bondonno, Liu and Croft 33 ).
It is noteworthy that we used double the dose of nitrate in our trial compared with these trials (12·9 v. 6·45 mmol)( Reference Kapil, Khambata and Robertson 24 , Reference Bondonno, Liu and Croft 33 ). We selected this dose as the nitrate content is attainable with a diet rich in vegetables( Reference Gilchrist, Winyard and Fulford 25 ). For example, the Dietary Approaches to Stop Hypertension (DASH), traditional Japanese, Mediterranean and vegetarian diets are all high in nitrate (approximately 10–20 mmol)( Reference Sobko, Marcus and Govoni 26 , Reference Nadtochiy and Redman 34 , Reference Lin, Allen and Li 35 ). Further, the nitrate content of these diets has been suggested to mediate the major cardioprotective effects( Reference Sobko, Marcus and Govoni 26 , Reference Nadtochiy and Redman 34 , Reference Lin, Allen and Li 35 ). National dietary data surveys show average daily nitrate intakes in the USA and Europe to be 0·5–3·0 mmol/d( Reference Mensinga, Speijers and Meulenbelt 36 , Reference Gangolli, van den Brandt and Feron 37 ), reflecting a processed diet in low in vegetables.
The inconsistencies may be due to differing dosing regimens, intervention periods and patient demographics. The authors suggest that use of anti-hypertensives may diminish the effect of exogenous nitrate. However, half of the subjects in the Kapil et al. trial( Reference Kerley, Dolan and Cormican 23 ) were on anti-hypertensives as were our subjects here and in our pilot study( Reference Ghosh, Kapil and Fuentes-Calvo 22 ). A plausible explanation, which the authors mention( Reference Kapil, Khambata and Robertson 24 ), is that the baseline BP was quite well controlled and therefore an additional decrease is unlikely. Indeed, we observed a similar effect in our pilot study where values in controlled hypertensives did not decrease( Reference Ghosh, Kapil and Fuentes-Calvo 22 ). According to a recent review, dietary nitrate appears to be most hypotensive in those with higher baseline BP( Reference Lara, Ashor and Oggioni 38 ). We speculate that this may be owing to cross-talk between endothelial NO synthase and the nitrate–nitrite–NO pathways, whereby beneficial effects of nitrate are likely to be more pronounced when NO synthase is compromised (e.g. HTN). In addition, it has been demonstrated that the abundance and activity of a nitrite reductase enzyme is higher in those with higher BP( Reference Ghosh, Kapil and Fuentes-Calvo 22 ). Other factors likely to influence the effect of dietary nitrate include habitual dietary intake, baseline NO bioavailability, as well as response to exogenous nitrate. Response to exogenous nitrate is complex and determined by oral microbiota, gastric pH and O2 tension among others( Reference Kerley 39 ).
Trial strengths
This was a double-blind, randomised, PL-controlled cross-over trial. The use of a PL juice means that we could fully blind and randomise this trial. The use of ABPM provides a robust measure of BP. There were no dropouts and there was no change in multiple measured factors known to influence NO kinetics throughout the trial, and no participant used antibiotics or mouthwash (both known inhibitors of nitrate reduction to nitrite) during the trial. Compliance with the beetroot juice was 100 %.
Trial limitations
We included a small sample. However, considering the cross-over design of our trial and the samples studied in previous nitrate studies combined with our sample size calculation, we feel that twenty well-characterised subjects can provide useful information in this context. This trial was of short duration (7 d), designed to assess the efficacy and tolerability of daily dietary nitrate in uncontrolled hypertensives. We did not include a washout period as the pharmacokinetics of dietary nitrate reveal that biochemical and physiological effects of nitrate ingestion are absent after approximately 24 h. Therefore, subjects completed the first arm of the study on day 8 and commenced the second arm of the study on day 9.
Conclusion
Our results suggest that dietary nitrate has anti-hypertensive effects among uncontrolled hypertensives in conjunction with increased NO metabolites. This effect is consistent with the majority of animal model, pre-clinical and clinical data. Future studies are required to ascertain the long-term effect of dietary nitrate on BP in humans, particularly in light of evidence that dietary nitrate appears more effective in those with higher baseline BP and/or those who are treatment naïve. This intriguing concept has potential implications for multiple vulnerable populations, for example those with resistant HTN and hypertensives who refuse/cannot tolerate medication.
Acknowledgements
We wish to acknowledge the patients who agreed to participate in this research. We also wish to acknowledge the Irish Heart Foundation who supported this work. However, the Irish Heart Foundation had no role in the design, analysis or writing of this article.
C. P. K. made substantial contributions to study conception and design, data acquisition analysis and interpretation of data and has drafted the submitted article. E. D. made substantial contributions to study conception and design and analysis/interpretation of data. P. E. J. made substantial contributions to biochemical analysis and analysis/interpretation of data. L. C. made substantial contributions to study conception and design and analysis/interpretation of data. C. P. K., E. D., P. E. J. and L. C. have provided final approval of the version to be published. E. D., P. E. J. and L. C. have revised the submitted article critically for important intellectual content. C. P. K. and L. C. have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that there are no conflicts of interest.








