It is accepted that marine fish and crustacean are generally unable to synthesise n-3 and n-6 long-chain PUFA (LC-PUFA) from their respective precursors, linolenic acid (18 : 3n-3) and linoleic acid (18 : 2n-6)(Reference Tocher1). Therefore, LC-PUFA such as EPA (20 : 5n-3), DHA (22 : 6n-3) and arachidonic acid (20 : 4n-6) are considered as essential fatty acids (EFA) for marine fish and crustacean. Numerous studies have demonstrated that DHA, EPA and arachidonic acid play important roles in survival and growth of marine fish and crustacean larvae(Reference Copeman, Parrish and Brown2), and dietary deficiencies result in reduced survival, poor growth and prolonged intermolt periods of crustaceans(Reference Suprayudi, Takeuchi and Hamasaki3). However, it was also reported that excessive dietary LC-PUFA levels could result in growth depression(Reference Yang, Zhang and Tan4). The EFA must be included in the diet at adequate levels to fulfil requirements for growth, survival and development(5). Moreover, fish oil remains the main but limited source of EFA, especially n-3 LC-PUFA, in aquafeeds(Reference Nasopoulou and Zabetakis6). Hence, it is important to determine the optimal dietary n-3 LC-PUFA requirements of marine fish and crustaceans.
The n-3 LC-PUFA requirements have been studied for several crustacean species. In swimming crab (Portunus trituberculatus) (initial weight 200–300 g) at the stage of ovarian development, optimal dietary n-3 LC-PUFA level was 6·0–8·0 mg/g of dry diet with a DHA:EPA ratio of 2·0(Reference Feng7,Reference Wang8) and a suitable supplement of arachidonic acid was 0·6–2·4 mg/g of diet(Reference Yang9). Other studies showed that the optimum dietary n-3 LC-PUFA requirements for P. trituberculatus (initial weight 2·17 and 24·00 g) were 23·5 and 23·3 mg/g of diet when DHA:EPA ratios were 0·9 and 1·1, respectively(Reference Zhang10,Reference Hu11) . The optimal n-3 LC-PUFA requirement was 5·0 and 8·9 mg/g of diet for juvenile Pacific white shrimp (Litopenaeus vannamei) (initial weight 1·43 and 0·50 g)(Reference Yang, Zhang and Tan4,Reference González-Félix, Gatlin Iii and Lawrence12) , respectively. It was also demonstrated that the optimum n-3 LC-PUFA requirements were higher in subadult than adult L. vannamei (Reference Zhang, Wang and Tan13). Therefore, previous studies have revealed that quantitative EFA requirements may vary with culture species, stage of development, dietary ingredients, and with dietary LC-PUFA (DHA:EPA ratio)(5). Many investigations of n-3 LC-PUFA requirements for marine fish or crustacean used diets based on fishmeal and/or fish oil where the natural occurrence of n-3 LC-PUFA in these marine ingredients strongly influences the proportions of dietary fatty acids. Thus, purified or semi-purified artificial diets are required to properly evaluate n-3 LC-PUFA requirements. Previous studies also demonstrated that n-3 LC-PUFA requirements may also be affected by dietary lipid level(Reference Izquierdo14). For example, n-3 LC-PUFA requirement increased from 12·0–22·0 to 27·0–32·0 mg/g of dry weight when dietary lipid level increased from 10 to 15 % in red sea bream (Pagrosomus major)(Reference Takeuchi, Toyota and Watanabe15). The n-3 LC-PUFA requirement of red drum (Sciaenops ocellatus) did not exceed 3·8 mg/g of diet when dietary lipid levels were 7·4 %, while the optimal n-3 LC-PUFA level ranged from 28·7 to 51·2 mg/g of diet when dietary lipid level was 18·3 %(Reference Williams and Robinson16,Reference Brinkmeyer and Holt17) . Hence, in order to determine the n-3 LC-PUFA requirement of marine crustaceans, purified diets must be used and dietary lipid level and the ratio of dietary LC-PUFA must also be considered. However, studies on the relationship between these factors and dietary LC-PUFA requirement are few. Whether there was a relationship in crustaceans between dietary lipid level and n-3 LC-PUFA requirement existed was unknown, and how dietary lipid level affects n-3 LC-PUFA requirement of crustaceans was not clear.
The mud crab is distributed widely throughout the Indian ocean and Indo-Pacific regions and is a commercially important marine crab species due to its short growth cycle, high adaptability and nutritional value(Reference Li, Ai and Liu18). In China, S. paramamosain has become major marine aquaculture crustaceans in recent years and the culture technology and techniques have constantly improved(Reference Li, Ai and Liu18). In 2018, the yield of farmed mud crabs (mainly S. paramamosain) reached 157 712 tons(19). However, the development of commercial feed for S. paramamosain has lagged in the crab farming industry, and there are few reports on the nutritional requirements of mud crab(Reference Zhao, Wen and Li20–Reference Dong, Tong and Zhang23), although dietary lipid levels of 8·52–11·63 % (optimum 9·50 %) could maintain growth performance(Reference Zhao, Wen and Li20). The requirement for dietary LC-PUFA in swimming crab was supposed to range from 6·0 to 23·6 mg/g of diets(Reference Feng7,Reference Wang8,Reference Zhang10,Reference Hu11) , and the optimum dietary DHA:EPA ratio for juvenile crab was about 1·0. However, no information was available concerning n-3 LC-PUFA requirements of S. paramamosain. Hence, the objective of the present study was to determine the optimal n-3 LC-PUFA requirement at two dietary lipid levels and to evaluate n-3 LC-PUFA supplementation on growth performance, tissue fatty acid profiles and expression levels of genes related to fatty acid biosynthesis and lipid metabolism in S. paramamosain.
Methods
Ethics statement
The present study was performed in strict accordance with the Standard Operation Procedures of the Guide for Use of Experimental Animals of Ningbo University. The experimental protocol and procedures were approved by the Institutional Animal Care and Use Committee of Ningbo University.
Diet preparation
Ten isonitrogenous purified diets (approximately 45 % crude protein) were formulated with 0 (control), 0·75, 1·50, 2·25 and 3·00 % n-3 LC-PUFA (DHA:EPA ratio approximately 1:1) at two dietary lipid levels of 7 and 12 %. The analysed n-3 LC-PUFA values were 0·15, 7·4, 14·8, 19·8 and 24·9 mg/g at 7 % lipid, and 0·10, 6·8, 13·2, 19·0 and 25·6 mg/g at 12 % lipid, respectively. Casein and soya protein concentrate were used as protein sources, and semi-pure DHA, EPA, arachidonic acid, palmitic acid and soya lecithin were used as lipid sources (Table 1), with palmitic acid used to alter and balance dietary 7 and 12 % lipid levels without impacting LC-PUFA levels or metabolism. Arachidonic acid and cholesterol were supplemented to maintain normal growth and molting according to data from P. trituberculatus and Scylla serrata (Reference Yang9,Reference Sheen24) . The fatty acid profiles of the experimental diets were presented as mg/g in Table 2. All the ingredients were purchased from Ningbo Tech-Bank Feed Co. Ltd and ground into fine powder with particle size <177 μm. The micro-components, such as vitamin and mineral premixes, were then mixed using the progressive enlargement method. EPA, DHA, palmitic acid, soyabean lecithin and distilled water (400 g/kg) were then added to the premixed dry ingredients and mixed until homogenous in a Hobart-type mixer. Cold-extruded pellets were produced (F-26, machine factory of South China University of Technology), and the pellet strands cut into uniform pellet sizes (two pellet sizes: 2·0 mm diameter, 4·0 mm length; 4 mm diameter, 6·0 mm length) using a granulating machine (G-250, machine factory of South China University of Technology), steamed for 30 min at 90°C and then air-dried to approximately 10 % moisture. The dried diets were sealed in vacuum-packed bags and stored at −20°C until used.
Table 1. Formulation and proximate composition of the experimental diets (DM, %)
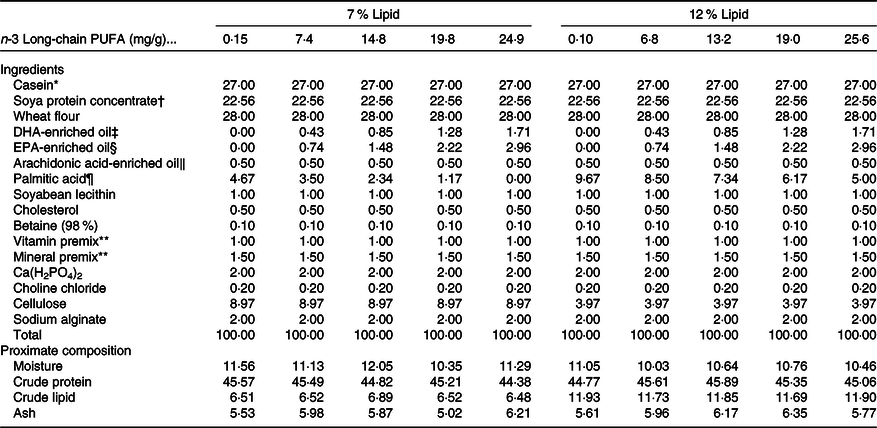
* Casein, 89·55 % crude protein and 0·2 % crude lipid.
† Soya protein concentrate, 69·88 % crude protein and 0·51 % crude lipid.
‡ DHA-enriched oil, DHA content, 406·5 mg/g oil.
§ EPA-enriched oil, EPA content, 462·5 mg/g oil; DHA content, 235·6 mg/g oil.
‖ Arachidonic acid-enriched oil, arachidonic acid content, 468·0 mg/g oil.
¶ Palmitic acid, palmitic acid content, 97 % of total fatty acids, in the form of methyl esters; Shanghai Yiji Chemical Co. Ltd, China.
** Vitamin premix and mineral premix were based on Jin et al. (Reference Jin, Wang and Huo61).
Table 2. Fatty acid compositions of the experimental diets (mg/g, DM)
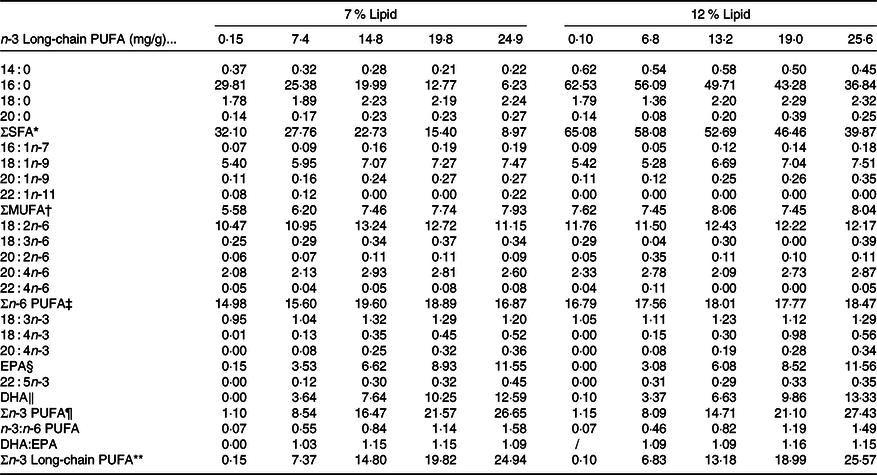
* SFA: 14 : 0, 16 : 0, 18 : 0, 20 : 0.
† MUFA: 16 : 1n-7, 18 : 1n-9, C0 : 1n-9.
‡ n-6 PUFA: 18 : 2n-6, 18 : 3n-6, 20 : 2n-6, 20 : 4n-6, 22 : 4n-6.
§ EPA, 20 : 5n-3.
‖ DHA, 22 : 6n-3.
¶ n-3 PUFA: 18 : 3n-3, 18 : 4n-3, 20 : 4n-3, EPA, 22 : 5n-3, DHA.
** n-3 Long-chain PUFA: 20 : 4n-3, EPA, 22 : 5n-3, DHA.
Experimental crabs and feeding trial
Juvenile mud crabs were obtained from Jia-Shun Aquatic Cooperatives. Before the experiment, the crabs were acclimated in a cement pool and fed a commercial feed (45 % crude protein, 8 % crude lipid; Ningbo Tech-Bank Corp.) for 2 weeks. There were three replicates (fifteen crabs per replicate) for each diet treatment. At the beginning of feeding trial, a total of 450 juvenile crabs (30·55 ± 0·75 g/crab) were then randomly allocated to individual cellular systems (each cell, 0·33 m × 0·23 m × 0·15 m, length × width × height; online Supplementary Fig. S1)(Reference Li, Ai and Liu18,Reference Zhao, Wen and Li20) . Each cell was half filled with a continuous flow of seawater (300 ml/min), and crabs were fed once daily at 18.00 hours to apparent satiation with 6–8 % of wet body weight during the feeding duration(Reference Unnikrishnan and Paulraj25). Faeces and uneaten feed from each cell were removed daily. Any dead crabs were taken out and weighed as soon as being observed, and the number of molts were calculated and recorded daily.
During the experimental period, the temperature of flowing water in the crab cells was 26–32°C, salinity was approximately 26–28 g/l, pH was 7·7–8·0, the ammonia nitrogen was lower than 0·05 mg/l and dissolved oxygen was 6·5–7·0 mg/l. Salinity, pH, ammonia nitrogen and dissolved oxygen in the cellular systems were measured by the YSI Pro plus. The feeding trial lasted for 8 weeks.
Sample collection
At the beginning of the feeding trial, ten juvenile mud crabs were randomly culled and stored at −20°C as the initial samples. At the end of trial, crabs were starved for 24 h and then counted and weighed to determine weight gain (WG), specific growth rate (SGR) and molting frequency, which were calculated per replicate. In each replicate, haemolymph samples from three to five crabs were taken from the pericardial cavity using a 2 ml syringe, placed into 1·5 ml microfuge tubes and centrifuged at 956 g for 10 min at 4°C (Eppendorf centrifuge 5810 R). The supernatant was collected and stored at −80°C until further analysis. Hepatopancreas and muscle samples were dissected from the same crabs that had blood drawn. The hepatopancreas samples were divided into two portions, one was stored at −20°C for proximate composition and fatty acid profile analysis (three crabs per replicate), and the other was frozen immediately in liquid N2 and stored at −80°C for gene expression analysis (three crabs per replicate). Muscle samples were stored at −20°C for analysing proximate composition and fatty acid profile (three crabs per replicate). Samples collected from the same replicate were pooled prior to analysis.
Biochemical analysis
Proximate composition and fatty acids
The crude protein, crude lipid, moisture and ash content of diets, muscle and hepatopancreas of the crabs were determined according to the method of the Association of Official Analytical Chemists(26). The moisture content was determined by drying the samples to a constant weight at 105°C. The crude protein contents (N × 6·25) were assayed by the Dumas combustion method with a protein analyzer (FP-528, LECO). Crude lipid was measured via the petroleum ether extraction method using a Soxtec System HT (SX360, OPSIS), and the ash content was determined after incineration in a muffle furnace at 550°C for 8 h.
Fatty acid compositions of diets, hepatopancreas and muscle were analysed as described in detail previously(Reference Gao, Koshio and Ishikawa27). In brief, total lipid was extracted with chloroform–methanol (2:1, v/v) and fatty acid methyl esters were then produced from total lipid by methanolic sulphuric acid with butylated hydroxytoluene as an antioxidant. Methyl tricosanoate (23 : 0; Sigma Aldridge Trading Co. Ltd) was used as an internal standard at 1·0 mg/ml hexane. GC (Agilent Technologies GC-MS 7890B-5977A) was used to analyse fatty acid methyl esters with fatty acids identified by reference to known standards and presented as percentages of area.
Real-time quantitative PCR analysis of fatty acid biosynthesis and lipid metabolism genes in hepatopancreas
Total RNA was extracted from hepatopancreas samples using Trizol reagent (Invitrogen); the quantity and quality of total RNA were assessed using a Nano DropND-1000 spectrophotometer (NanoDrop Technologies) and 1·2 % denaturing agarose gel electrophoresis. The 260/280 nm absorbance ratios of all samples ranged from 1·86 to 2·00, indicating a satisfactory purity of the RNA samples. The RNA was dissolved in 30 μl Recombinant DNase I (RNase-free) (Takara) and stored at −80°C until use. The cDNA was synthesised for quantitative reverse-transcriptase PCR (qPCR) using the PrimeScript™ RT Reagent Kit (Takara) according to the manufacturer’s instructions.
Elongation factor-1α was used as a housekeeping gene after the stability of elongation factor-1α expression was confirmed. Specific primers for elongase of very long-chain fatty acids 4 (ELOVL4), -6 fatty acyl desaturase (Δ6 FAD), sterol regulatory element binding protein-1 (SREBP-1), fatty acid synthase (FAS), glucose-6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (6PGD), hormone-sensitive TAG lipase (HSL), acyl-CoA oxidase (ACO), carnitine palmitoyltransferase I and II (CPTI and CPTII) used for RT-qPCR were designed using Primer Premier 5.0 software (online Supplementary Table 1). The expression of mRNA was determined by RT-qPCR (Light Cycler 96; Roche). The RT-qPCR was performed in a 20 μl reaction volume containing 10 μl of SYBR Green premix, 0·8 μl of cDNA template, 0·4 μl of each primer (10 μm) and 8·4 μl of diethyl pyrocarbonate-treated water. The RT-qPCR conditions were as follows: 95°C for 10 min; 45 cycles of 95°C for 15 s, 58°C for 15 s and 72°C for 20 s. The data were optimised using the comparative Ct (2−ΔΔCt) value method as described by Livak & Schmittgen(Reference Livak and Schmittgen28) and then subjected to statistical analysis.
Calculations and statistical analysis
The parameters were calculated as follows:
 $${\eqalign{\rm{Molting}}\,{\rm{frequency}}} = {2 \times {N_m}/({\rm{initial}}\,{\rm{number}}\,{\rm{of}}\,{\rm{crabs}}}} \cr+ {\rm{final}}\,{\rm{number}}\,{\rm{of}}\,{\rm{crabs}})$$
$${\eqalign{\rm{Molting}}\,{\rm{frequency}}} = {2 \times {N_m}/({\rm{initial}}\,{\rm{number}}\,{\rm{of}}\,{\rm{crabs}}}} \cr+ {\rm{final}}\,{\rm{number}}\,{\rm{of}}\,{\rm{crabs}})$$
where W t is the final body weight (g), W i is the initial body weight (g), t is the experimental duration in d and N m is the number of moltings.
Data were first analysed using one-way ANOVA to detect differences among all treatments. A two-way ANOVA was used to test the effects of lipid and n-3 LC-PUFA levels on growth performance, tissue compositions and fatty acid profiles, and expression of genes related to fatty acid synthesis and lipid metabolism. Data were transformed before analysis as necessary. When there were significant differences (P < 0·05), the group means were further compared using Tukey’s multiple range tests. All the results are presented as mean values with their standard errors (n 3). The two-slope broken-line regression analysis was conducted to analyse WG in response to dietary n-3 LC-PUFA level (Fig. 1). All statistical analyses were performed using SPSS 23.0 (SPSS, IBM).

Fig. 1. Relationship between dietary n-3 long-chain PUFA (LC-PUFA) levels and weight gain of juvenile mud crab fed 7 % (a) and 12 % (b) dietary lipid. The horizontal axis represents the measured dietary n-3 LC-PUFA level (DM; mg/g). The Xpot represents the optimal dietary n-3 LC-PUFA level for maximum weight gain of mud crab.
Results
Growth performance
The growth performance of crabs fed the experimental diets is shown in Table 3. Molting frequency was not affected by dietary n-3 LC-PUFA at either lipid level, but WG and SGR were significantly influenced by dietary n-3 LC-PUFA at both lipid levels. Crabs fed the diet containing 19·8 mg/g n-3 LC-PUFA had higher WG and SGR than those fed the control (0·15 mg/g) and 24·9 mg/g n-3 LC-PUFA diets at 7 % dietary lipid level. Furthermore, crabs fed the diet containing 13·2 mg/g n-3 LC-PUFA at 12 % lipid had the highest WG and SGR among all treatments. Two-slope broken-line regression analysis showed that the optimal n-3 LC-PUFA levels were estimated to be 20·1 and 12·7 mg/g at 7 and 12 % dietary lipid level, respectively (Fig. 1).
Table 3. Growth performance and molting frequency of mud crab fed the experimental diets for 8 weeks*
(Mean values with their standard errors)
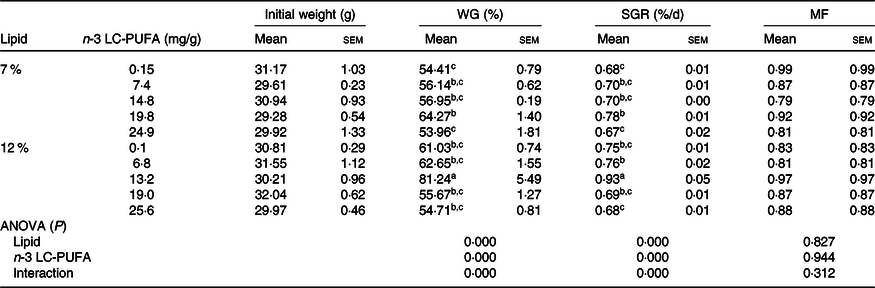
WG, weight gain; SGR, specific growth rate; MF, molting frequency; LC-PUFA, long-chain PUFA.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* A two-way ANOVA was performed to evaluate the 2 × 5 factorial design with three replicates of each treatment. Tukey’s multiple-range test was applied when significant differences (P < 0·05) were detected among dietary treatments.
Proximate composition in hepatopancreas and muscle
As shown in Table 4, moisture and lipid contents in muscle were significantly influenced by dietary n-3 LC-PUFA at both lipid levels, with lipid content in muscle significantly decreased with increased dietary n-3 LC-PUFA, although protein content in muscle was not affected by dietary n-3 LC-PUFA at either lipid level. Moisture, lipid and protein contents in hepatopancreas were significantly influenced by dietary n-3 LC-PUFA at both lipid levels, with crabs fed the control diet having the lowest lipid content in hepatopancreas among all treatments. However, protein content in hepatopancreas significantly decreased with increased dietary n-3 LC-PUFA at both lipid levels, with the highest protein content in hepatopancreas found in crabs fed the control diet.
Table 4. Proximate composition in muscle and hepatopancreas of mud crab fed the experimental diets (DM) for 8 weeks
(Mean values with their standard errors)
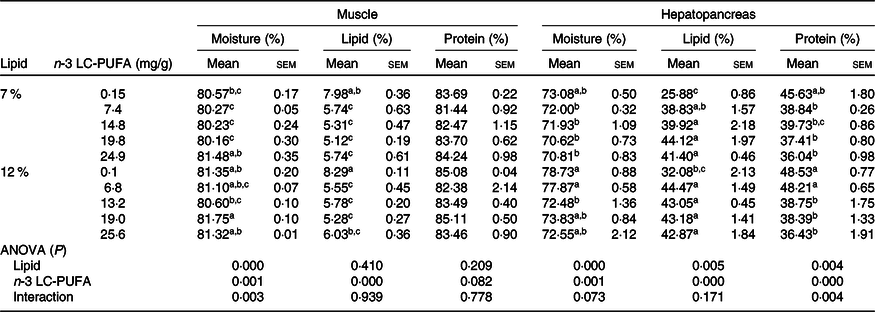
LC-PUFA, long-chain PUFA.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
Fatty acids profile of muscle and hepatopancreas
Principal component analysis was used to evaluate the overall effects of dietary n-3 LC-PUFA at two dietary lipid levels on fatty acid compositions of muscle and hepatopancreas (Fig. 2). Fatty acid compositions of crabs fed the control diet were significantly separated from those fed the n-3 LC-PUFA-supplemented diets. Full fatty acid compositions of muscle and hepatopancreas are provided in online Supplementary Tables S2 and S3.
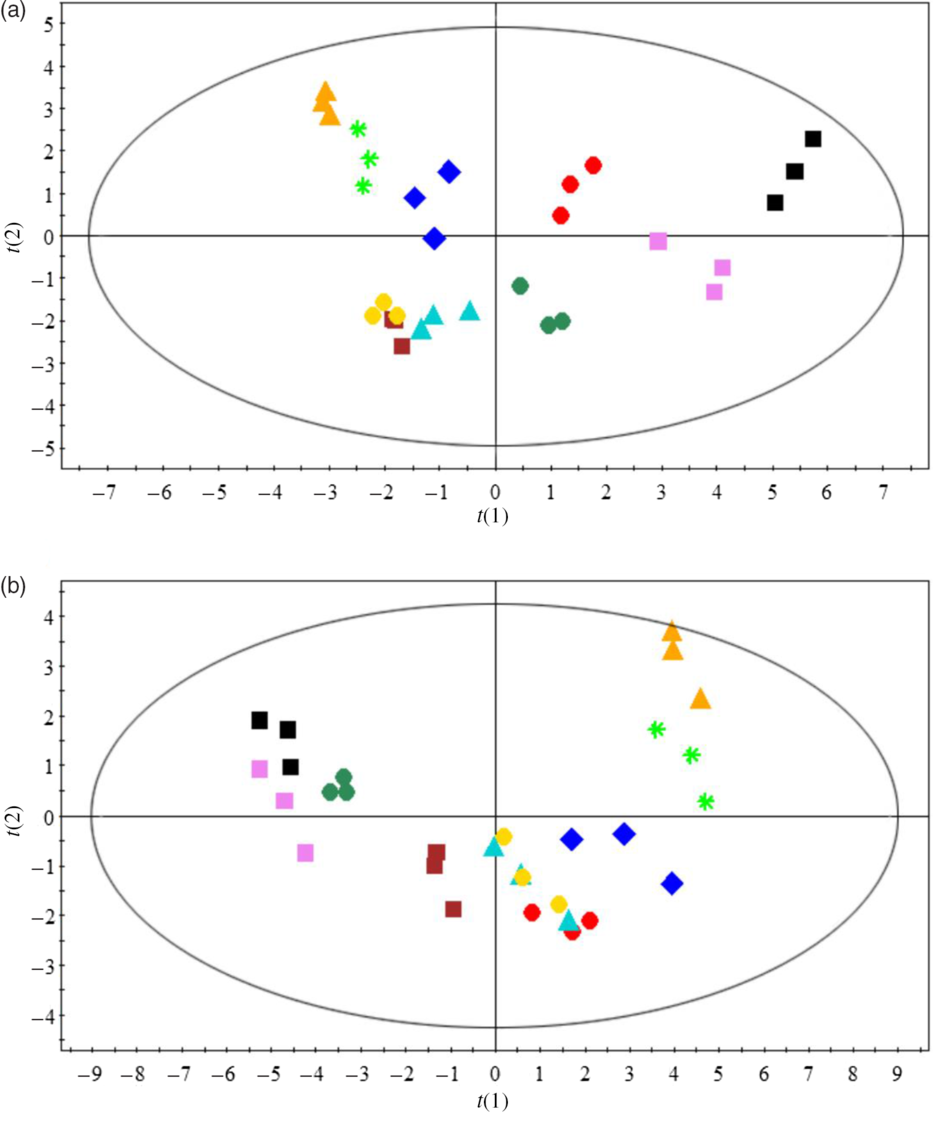
Fig. 2. Principal component analysis (PCA) score plots based on fatty acid profiles of muscle (a) and hepatopancreas (b) of crabs fed different experimental diets. For example, L7-0·15: dietary lipid and n-3 LC-PUFA levels were 7 % and 0·15 mg/g, respectively. ![]() , L7-0·15;
, L7-0·15; ![]() , L7-7·4;
, L7-7·4; ![]() , L7-14·8;
, L7-14·8; ![]() , L7-19·8;
, L7-19·8; ![]() , L7-24·9;
, L7-24·9; ![]() , L12-0·1;
, L12-0·1; ![]() , L12-6·8;
, L12-6·8; ![]() , L12-13·2;
, L12-13·2; ![]() , L12-19·0;
, L12-19·0; ![]() , L12-25·6.
, L12-25·6.
The fatty acid profiles of muscle fed the experimental diets are presented in Table 5. MUFA and n-6 PUFA contents significantly decreased with increased dietary n-3 LC-PUFA at two lipid levels. However, EPA, DHA, n-3 PUFA contents and DHA:EPA ratio significantly increased with increasing dietary n-3 LC-PUFA at both lipid levels. The EPA, DHA and n-3 PUFA contents and DHA:EPA ratio in muscle of initial crabs were higher than those fed the control diet, but lower than those fed the n-3 LC-PUFA-supplemented diets. Muscle of crabs fed all experimental diets had higher total fatty acid contents than that of initial crabs. The SFA content in muscle decreased at 7 % dietary lipid but increased at 12 % dietary lipid with dietary n-3 LC-PUFA levels increasing. The n-3:n-6 ratio in muscle increased with increased dietary n-3 LC-PUFA levels.
Table 5. Fatty acid profiles in muscle of mud crab fed the experimental diets (mg/g, DM) for 8 weeks
(Mean values with their standard errors)
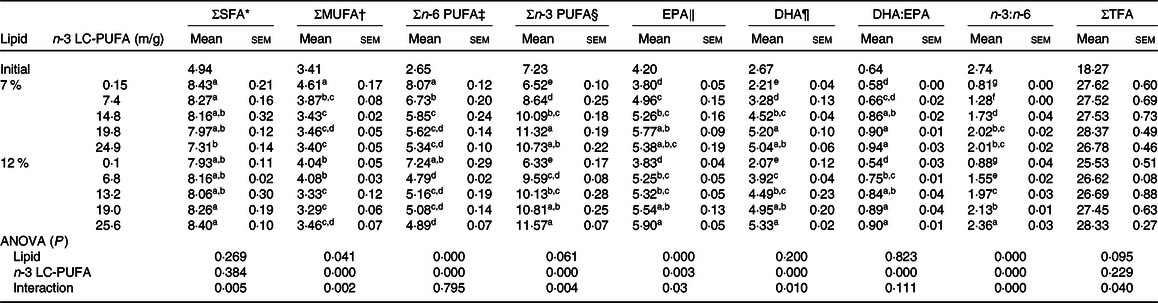
LC-PUFA, long-chain PUFA; TFA, total fatty acids.
a,b,c,d,e,f,g Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* SFA: 14 : 0, 16 : 0, 18 : 0, 20 : 0.
† MUFA: 16 : 1n-7, 18 : 1n-9, 20 : 1n-9.
‡ n-6 PUFA: 18 : 2n-6, 20 : 2n-6, 20 : 4n-6, 22 : 4n-6.
§ n-3 PUFA: 18 : 3n-3, EPA, 22 : 5n-3, DHA.
‖ EPA, 20 : 5n-3.
¶ DHA, 22 : 6n-3.
The fatty acid profiles in hepatopancreas fed the experimental diets are shown in Table 6. Crabs fed the diets supplemented with n-3 LC-PUFA had significantly higher contents of total fatty acids, MUFA, n-3 PUFA, DHA, EPA and DHA:EPA ratio in hepatopancreas than those fed the control diet at both lipid levels, and no significant differences were observed in DHA:EPA ratio of crabs fed the n-3 LC-PUFA-supplemented diets. Crabs fed the control diets at both lipid levels had lower EPA, DHA and n-3 PUFA contents in hepatopancreas than those of initial crab. The EPA, DHA and n-3 PUFA contents were higher than initial crab when crabs fed diets with higher than 7·4 and 13·2 mg/g dietary n-3 LC-PUFA at 7 and 12 % lipid levels, respectively. The DHA:EPA ratio of crabs fed diets supplemented with n-3 LC-PUFA was similar to that of initial crab and higher than those fed the control diets. The SFA content decreased at 7 % but increased at 12 % dietary lipid with increased n-3 LC-PUFA level. The n-3:n-6 PUFA ratio in hepatopancreas increased with increased dietary n-3 LC-PUFA level, but was lower than that in initial crabs. Crabs fed diets containing 12 % lipid had lower n-6 PUFA, n-3 PUFA, EPA and DHA contents in hepatopancreas than those fed the 7 % lipid diets.
Table 6. Fatty acid profiles in hepatopancreas of mud crab fed the experimental diets (mg/g, DM) for 8 weeks
(Mean values with their standard errors)
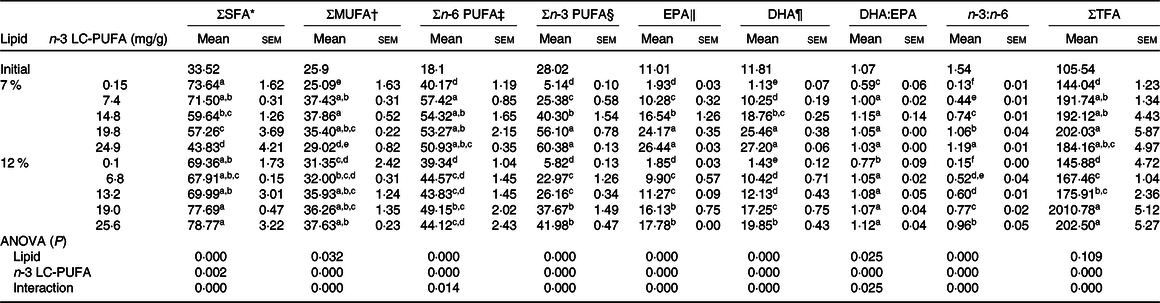
LC-PUFA, long-chain PUFA; TFA, total fatty acids.
a,b,c,d,e,f Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* SFA: 14 : 0, 16 : 0, 18 : 0, 20 : 0.
† MUFA: 16 : 1n-7, 18 : 1n-9, 20 : 1n-9, 22 : 1n-11
‡ n-6 PUFA: 18 : 2n-6, 18 : 3n-6, 20 : 2n-6, 20 : 4n-6, 22 : 4n-6.
§ n-3 PUFA: 18 : 3n-3, 18 : 4n-3, 20 : 4n-3,EPA, 22 : 5n-3, DHA.
‖ EPA, 22 : 5n-3.
¶ DHA, 22 : 6n-3.
Expression of long-chain PUFA biosynthesis in hepatopancreas
The expression of genes related to LC-PUFA biosynthesis in hepatopancreas is shown in Fig. 3. The expression levels of Δ6 FAD in hepatopancreas were up-regulated with increased dietary n-3 LC-PUFA at both lipid levels, with lowest expression level of Δ6 FAD in hepatopancreas observed in crabs fed the control diets. However, expression levels of ELOVL4 tended to decrease with increasing dietary n-3 LC-PUFA at both lipid levels. The expression levels of ELOVL4 decreased sharply when dietary n-3 LC-PUFA level was higher than 14·8 mg/g at 7 % lipid level and decreased with increasing dietary n-3 LC-PUFA levels at 12 % lipid.
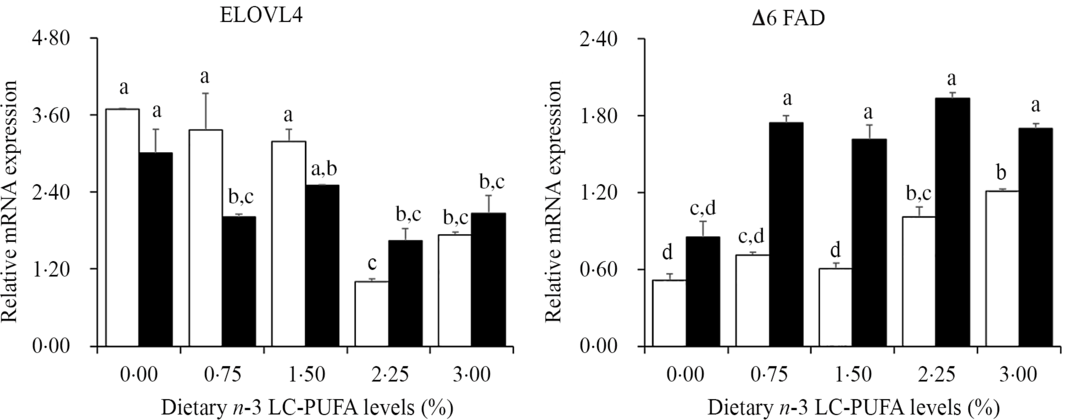
Fig. 3. Effects of different dietary lipid and n-3 long-chain PUFA (LC-PUFA) levels on relative mRNA expression levels of genes involved in LC-PUFA biosynthesis in hepatopancreas of Scylla paramamosain. ![]() , 7 % lipid level;
, 7 % lipid level; ![]() , 12 % lipid level. Values are means with their standard errors (n 3). a,b,c,d Bars bearing unlike letters are significantly different by Tukey’s test (P < 0·05). In order to include data from both dietary lipid levels, the designed n-3 LC-PUFA levels (%) were used in the X-axis. ELOVL, elongase of very long-chain fatty acids; FAD, fatty acyl desaturase.
, 12 % lipid level. Values are means with their standard errors (n 3). a,b,c,d Bars bearing unlike letters are significantly different by Tukey’s test (P < 0·05). In order to include data from both dietary lipid levels, the designed n-3 LC-PUFA levels (%) were used in the X-axis. ELOVL, elongase of very long-chain fatty acids; FAD, fatty acyl desaturase.
Expression of lipid metabolism in hepatopancreas
Fig. 4 shows the expression levels of genes involved in lipogenesis in hepatopancreas. The expression levels of SREBP-1 in hepatopancreas significantly increased as dietary n-3 LC-PUFA increased from 0·15 to 14·8 mg/g and then decreased with further increased dietary n-3 LC-PUFA at 7 % lipid level, and the highest expression level of SREBP-1 was found in crabs fed diets containing 14·8 mg/g n-3 LC-PUFA. The expression levels of G6PD showed similar trends at 7 and 12 % lipid level. Crabs fed diets containing 6·8 mg/g n-3 LC-PUFA showed the highest expression levels of 6PGD, and expression levels decreased with increased dietary n-3 LC-PUFA at 12 % lipid. The expression levels of FAS significantly decreased with increased dietary n-3 LC-PUFA at both 7 and 12 % lipid levels.
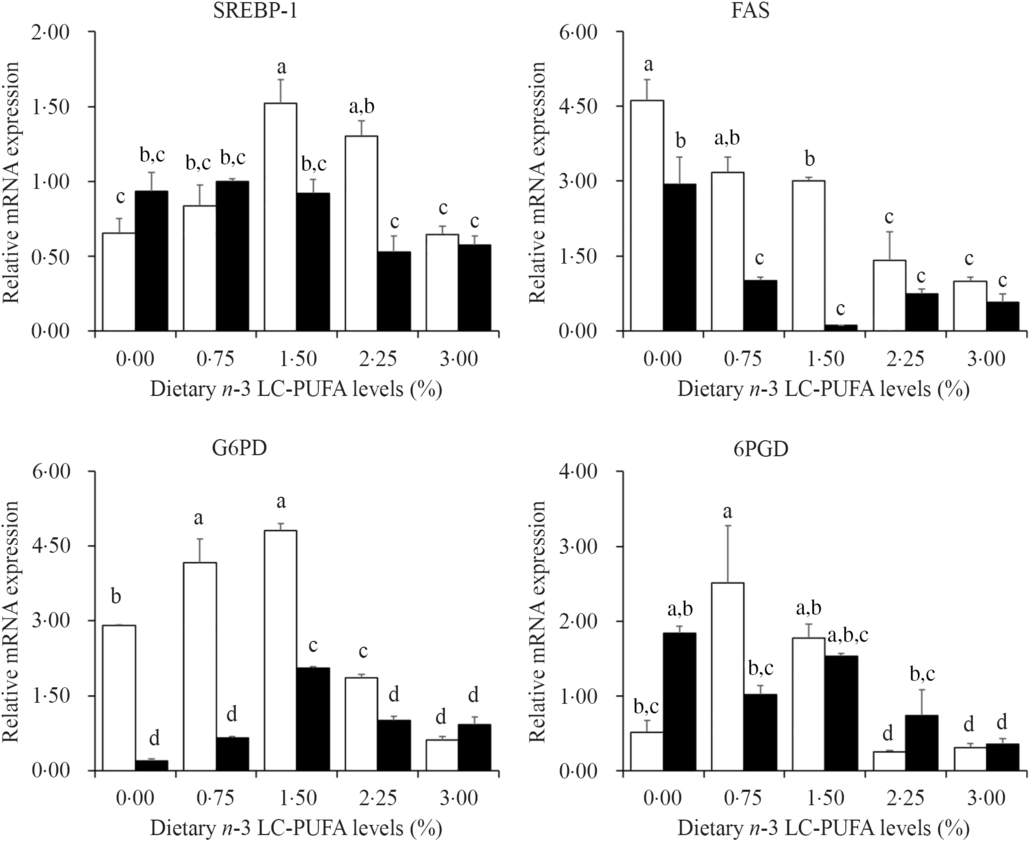
Fig. 4. Effects of different dietary lipid and n-3 long-chain PUFA (LC-PUFA) levels on relative mRNA expression of genes involved in lipogenesis in the hepatopancreas of Scylla paramamosain. ![]() , 7 % lipid level;
, 7 % lipid level; ![]() , 12 % lipid level. Values are means with their standard errors (n 3). a,b,c,d Bars bearing unlike letters are significantly different by Tukey’s test (P < 0·05). In order to include the data at both dietary lipid levels, the designed n-3 LC-PUFA levels (%) were used in the X-axis. SREBP-1, sterol regulator element-binding protein-1; FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; 6PGD, 6-phosphogluconate dehydrogenase.
, 12 % lipid level. Values are means with their standard errors (n 3). a,b,c,d Bars bearing unlike letters are significantly different by Tukey’s test (P < 0·05). In order to include the data at both dietary lipid levels, the designed n-3 LC-PUFA levels (%) were used in the X-axis. SREBP-1, sterol regulator element-binding protein-1; FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; 6PGD, 6-phosphogluconate dehydrogenase.
The relative expression levels of genes involved in lipolysis and β-oxidation are presented in Fig. 5. The expression levels of CPTI, CPTII and HSL were down-regulated with increased dietary n-3 LC-PUFA at both dietary lipid levels. However, expression levels of ACO were significantly up-regulated with increased dietary n-3 LC-PUFA.
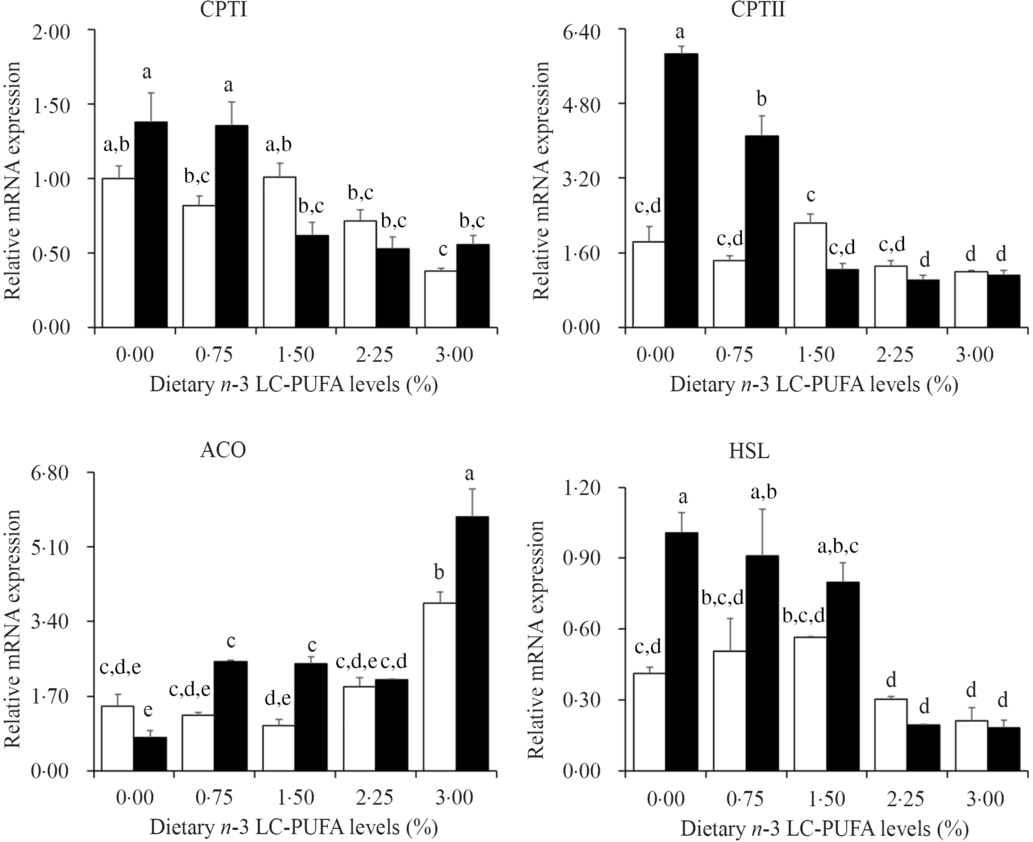
Fig. 5. Effects of different dietary lipid and n-3 long-chain PUFA (LC-PUFA) levels on relative mRNA expression of genes involved in lipolysis and β-oxidation in the hepatopancreas of Scylla paramamosain. ![]() , 7 % lipid level;
, 7 % lipid level; ![]() , 12 % lipid level. Values are means with their standard errors (n 3). a,b,c,d,e Bars bearing unlike letters are significantly different by Tukey’s test (P < 0·05). In order to include the data at both dietary lipid levels, the designed n-3 LC-PUFA levels (%) were used in the X-axis. CPT, carnitine palmitoyltransferase; HSL, hormone-sensitive TAG lipase; ACO, acyl-CoA oxidase.
, 12 % lipid level. Values are means with their standard errors (n 3). a,b,c,d,e Bars bearing unlike letters are significantly different by Tukey’s test (P < 0·05). In order to include the data at both dietary lipid levels, the designed n-3 LC-PUFA levels (%) were used in the X-axis. CPT, carnitine palmitoyltransferase; HSL, hormone-sensitive TAG lipase; ACO, acyl-CoA oxidase.
Discussion
In the present study, WG and SGR showed at first an increasing and then a decreasing trend with increased dietary n-3 LC-PUFA level regardless of dietary lipid level, and the best growth performance was observed in crabs fed diets containing 19·8 and 13·2 mg/g dietary n-3 LC-PUFA at 7 and 12 % lipid, respectively. The results indicated that excessive dietary n-3 LC-PUFA levels led to detrimental effects on growth performance of S. paramamosain, which was in agreement with previous studies in L. vannamei (Reference Yang, Zhang and Tan4) and Penaeus monodon (Reference Rees, Curé and Piyatiratitivorakul29). A hypothesis put forward to explain this negative effect was the possibility that excessive levels of dietary LC-PUFA, which have higher susceptibility to peroxidation, would result in oxidative stress(Reference Kjær, Todorcević and Torstensen30). The highest WG value was 81·24 % in the study, which was lower than 300 % in S. paramamosain (Reference Zhao, Wen and Li20) (initial weight 11·53 g) and 200 % in Eriocheir sinensis (Reference Zhao31) (initial weight 0·6 g). It was well known that the growth of crustaceans, due to an increase in WG at molt, is correlated with the molting frequency(Reference Sheen and Wu32). However, the intermolt would become longer as the individual grows larger. This also may be due to different culture species, bigger initial weight and the purified diets, which were used to reduce the effects of natural LC-PUFA in fishmeal and/or fish oil. Usually, purified diets reduce the palatability of feeds for mud crab. The farming of mud crab depends mainly on conventional simple or compound feeds including trash fish, molluscan meat and animal viscera(Reference Zhao, Wen and Li20).
Based on two-slope broken-line regression analysis of WG against dietary n-3 LC-PUFA level, the optimal dietary n-3 LC-PUFA levels (DHA:EPA = 1·1) were 20·1 and 12·7 mg/g at 7 and 12 % dietary lipid, respectively. The result observed in the present study was different to those obtained in P. trituberculatus (initial weight 2·17 and 24·00 g, respectively)(Reference Zhang10,Reference Hu11) where the optimum n-3 LC-PUFA requirement was 23·5 and 23·3 mg/g of diet when the dietary lipid was sufficient and DHA:EPA ratios were 0·9 and 1·1, respectively. Different results were also observed in other crustaceans, including Chinese mitten crab (E. sinensis), tiger shrimp (P. monodon), giant freshwater prawn (Macrobrachium rosenbergii) and L. vannamei (Reference Yang, Zhang and Tan4,Reference D’Abramo and Sheen33–Reference Wu, Wang and Cheng35) , which suggested that the optimum n-3 LC-PUFA requirement varies with species, development stages, dietary lipid level, dietary DHA:EPA ratio and feed formulation. On the other hand, the optimal n-3 LC-PUFA level decreased from 20·1 to 12·7 mg/g as dietary lipid level increased from 7 to 12 % in the present study, which showed that n-3 LC-PUFA requirements of S. paramamosain could be affected by dietary lipid level, and decreased when crabs were fed higher dietary lipid. However, the requirement of red sea bream (P. major) for dietary n-3 LC-PUFA increased from 1·2–2·2 mg/g to 2·7–3·2 mg/g of dry weight when dietary lipid level increased from 10 to 15 %(Reference Takeuchi, Toyota and Watanabe15). This difference may be due to different culture species and/or feed formulations. In the present study, 7 % dietary lipid was lower than the optimum lipid level (9·50 %) for S. paramamosain (Reference Zhao, Wen and Li20). A previous study demonstrated that L. vannamei could utilise both SFA and PUFA for energy metabolism(Reference Zhang, Wang and Tan13) and, thus, the higher n-3 LC-PUFA requirement at lower dietary lipid in S. paramamosain may be due in part to dietary n-3 LC-PUFA (e.g. EPA) being utilised to supply energy. However, information regarding the relationship between n-3 LC-PUFA requirement and dietary lipid level in crustaceans is limited and still needs to be confirmed and further studied. Additionally, WG and SGR increased significantly with increased dietary lipid level in the present study, which agreed with a previous study on mud crab investigating optimum dietary protein and lipid levels (Xin Cheng, Min Jin, Xuexi Wang, Xiaoying Hu, Mingming Zhao, Ye Yuan, Peng Sun, Lefei Jiao and Qicun Zhou, unpublished results). These results also indicated that mud crab may have a relatively high lipid requirement and/or high tolerance to increased dietary lipid level.
In the present study, crabs fed the n-3 LC-PUFA-supplemented diets had significantly higher lipid contents in hepatopancreas than those fed the control diets and no significant differences were observed in crabs fed the n-3 LC-PUFA-supplemented diets, which was similar to studies in P. trituberculatus (Reference Zhang10) and Japanese flounders (Paralichthys olivaceus)(Reference Xue, Li and Zhang36). The results indicated that the hepatopancreas was an important tissue for lipid deposition and energy storage in mud crab. Moreover, dietary n-3 LC-PUFA improved energy storage but prevented excess lipid deposition in hepatopancreas, which was supported by the data on total fatty acid content and expression of genes related to lipid metabolism in the present study. The opposite trend was observed in muscle lipid content, suggesting a differential effect of dietary n-3 LC-PUFA on lipid distribution in different tissues. It was reported that dietary n-3 LC-PUFA reduced muscle lipid content of black seabream (Acanthopagrus schlegelii)(Reference Ma, Shao and Xu37). However, dietary DHA and EPA increased the lipid content in P. olivaceus (Reference Xue, Li and Zhang36). Contradictory results about dietary LC-PUFA and lipid content were also observed in crustaceans(Reference Zhang10,Reference Hu11) . Therefore, the relationship between tissue lipid content and dietary n-3 LC-PUFA levels requires further study in crustaceans. The hepatopancreas protein content decreased with increasing dietary n-3 LC-PUFA levels at each dietary lipid, which partly agreed with the whole-body protein content in P. trituberculatus (Reference Hu11). It has been reported that SFA and MUFA are better than n-3 LC-PUFA as substrates for energy production, and high dietary levels of n-3 LC-PUFA could lead to insufficient energy supply, and thus hepatopancreas protein was also used to supply energy.
It was demonstrated in fish and crustaceans that the fatty acid compositions of liver/hepatopancreas and/or muscle reflected the fatty acid profile of diets(Reference Nasopoulou and Zabetakis6,Reference Zhang, Wang and Tan13,Reference Unnikrishnan and Paulraj25) . In the present study, the fatty acid compositions in hepatopancreas and muscle showed similar results when presented as absolute quantitative terms. Briefly, the EPA, DHA and n-3 PUFA contents in hepatopancreas increased with increased dietary n-3 LC-PUFA levels. Crabs fed diets supplemented with n-3 LC-PUFA had similar DHA:EPA ratios in hepatopancreas as initial crab and higher than those fed control diets. In muscle, the DHA:EPA ratio in crabs fed diets containing n-3 LC-PUFA was higher than that in initial crabs. On the one hand, these results indicated selective retention of DHA over EPA or other fatty acids in S. paramamosain for its greater biological value as EFA, which was reported in other species(Reference Izquierdo14). On the other hand, recent study indicated that L. vannamei had the potential ability to convert linolenic acid to EPA and DHA(Reference Chen, Li and Gan38). We speculated that S. paramamosain may be able to synthesise DHA from EPA or shorter chain PUFA, albeit the capacity may be low. Thus, when the content of DHA in hepatopancreas was insufficient, it was necessary to synthesise DHA from EPA to satisfy functional roles. The contents of total fatty acids and n-3 PUFA in hepatopancreas increased with increasing dietary n-3 LC-PUFA level, which was consistent with the lipid content data discussed above. However, the SFA content decreased at 7 % dietary lipid but increased at 12 % dietary lipid with increasing dietary n-3 LC-PUFA levels in both hepatopancreas and muscle. This may reflect S. paramamosain utilising SFA for energy metabolism when dietary energy was low as reported in L. vannamei (Reference Yang, Zhang and Tan4,Reference Zhang, Wang and Tan13) , with dietary n-3 LC-PUFA suppressing the utilisation of SFA for energy at the higher dietary lipid level. In the present study, the absolute contents of MUFA and n-6 PUFA decreased in muscle but increased in hepatopancreas with increasing dietary n-3 LC-PUFA at both dietary lipid levels, which may indicate a difference in fatty acid deposition in different tissues(Reference Yang, Zhang and Tan4). The n-3:n-6 PUFA ratio is an important index for evaluating the nutritional value of food. The FAO/WHO recommended that the lowest n-3:n-6 PUFA ratio in human food should be 0·1–0·2(39), and values above that are more beneficial. In the present study, the ratios of n-3:n-6 PUFA in hepatopancreas and muscle were much higher than 0·2, other than in hepatopancreas of crabs fed control diets, which were 0·13 and 0·15 at 7 and 12 % dietary lipid, respectively. The ratios of n-3:n-6 PUFA increased with increasing dietary n-3 LC-PUFA at both dietary lipid levels in both hepatopancreas and muscle, which indicated that dietary n-3 LC-PUFA supplementation in this experiment improved the nutritional value of mud crab. In hepatopancreas, the contents of n-6 PUFA, n-3 PUFA, EPA and DHA were lower in crabs fed the diets containing 12 % lipid than those fed 7 % lipid. This may be due to high dietary lipid improved by palmitic acid reduced the proportion of PUFA in dietary lipid, which agreed with another study on mud crab investigating the relationship between dietary lipid and optimum DHA:EPA (Xuexi Wang, Min Jin, Xin Cheng, Xiaoying Hu, Mingming Zhao, Ye Yuan, Peng Sun, Lefei Jiao, Mónica B. Betancor, Douglas R. Tocher and Qicun Zhou, unpublished results).
FAS, G6PD, 6PGD and HSL are known as important proteins involved in the mechanisms of lipogenesis and lipolysis, the gene expression levels of which could be affected by dietary fatty acid profile(Reference Jin, Monroig and Lu40,Reference Jin, Lu and Yuan41) . The function of FAS is to catalyse de novo fatty acid synthesis(Reference Chen, Luo and Liu42), and previous studies have shown that n-3 LC-PUFA down-regulate the expression of genes involved in fatty acid synthesis, particularly FAS(Reference Jin, Lu and Yuan41). 6GPD and G6PD are the key regulatory enzymes involved in NADPH production, essential for fatty acid biosynthesis(Reference Chen, Luo and Liu42,Reference Zheng, Luo and Zhu43) , while HSL is known to be involved in lipolysis(Reference Ma, Shao and Xu44). Additionally, SREBP-1 is a transcription factor regulating fatty acid, lipid and cholesterol biosynthesis pathways(Reference Zheng, Luo and Zhu43,Reference Minghetti, Leaver and Tocher45) , and a study in mouse indicated that LC-PUFA inhibit lipogenesis by down-regulating the mRNA expression of SREBP-1(Reference Kim, Takahashi and Ezaki46). There are few studies on SREBP-1 in crustaceans. SREBP-1 cDNA from the hepatopancreas of mud crab has been cloned, and it was demonstrated that the quantitative expression of SREBP-1 was highly influenced by dietary fatty acids(Reference Hao, Lin and Rong47). In the current study, the expression levels of FAS and HSL decreased with increasing dietary n-3 LC-PUFA level regardless of dietary lipid level, which agreed with results reported in black seabream(Reference Jin, Lu and Yuan41). Moreover, the SREBP-1, 6GPD and G6PD expression levels increased and then decreased when dietary n-3 LC-PUFA level increased from 0·15 to 24·9 mg/g at 7 % dietary lipid, while the expression levels of SREBP-1 and G6PD were down-regulated by increased dietary n-3 LC-PUFA at 12 % dietary lipid. These results indicated that the hepatopancreas of mud crab may require a certain level of lipid to supply energy and maintain function, and dietary n-3 LC-PUFA promotes lipogenesis when lipid intake is insufficient, while it suppresses lipogenesis to prevent the damage caused by excess lipid deposition in hepatopancreas.
The main pathway of fatty acid catabolism is β-oxidation in mitochondrial matrix and peroxisome(Reference Lu, Xu and Wang48). Both CPT and ACO are key enzymes of fatty acid β-oxidation; CPTI can participate in long-chain fatty acid oxidation, catalysing the conversion of fatty acid CoA to fatty acid carnitines for entering the mitochondrial matrix, and the fatty acyl group is transferred back to CoA by a second enzyme, CPTII(Reference Kerner and Hoppel49,Reference Li, Limbu and Ma50) . ACO is the rate-limiting enzyme for fatty acid β-oxidation in peroxisomes(Reference Lu, Xu and Wang48). In the present study, the expression levels of CPTI and CPTII showed a decreasing trend with increased dietary n-3 LC-PUFA level at both 7 and 12 % lipid levels suggesting a reduction of transport of long-chain fatty acids into the mitochondrial matrix, which led to an inhibition of fatty acid β-oxidation in agreement with the hepatopancreas lipid content data. The ACO expression levels were up-regulated by increasing dietary n-3 LC-PUFA at both 7 and 12 % lipid levels, indicating that fatty acid β-oxidation in peroxisomes was enhanced, opposite to that in mitochondria. The results highlighted the different roles of fatty acid β-oxidation in mitochondria and peroxisomes. One of the steps in DHA biosynthesis through the ‘Sprecher pathway’ is catalysed by ACO in peroxisomes(Reference Sprecher51). The up-regulation of ACO expression by dietary n-3 LC-PUFA in the present study agrees with the DHA content in hepatopancreas, which may indicate that mud crab require a high level of DHA to maintain physiological function.
Key enzymes involved in LC-PUFA biosynthesis in mud crab have been cloned, including Δ6 FAD-like(Reference Lin, Hao and Zhu52) and ELOVL4-like(Reference Lin, Hao and Huang53). It is well established that ELOVL4 effectively elongates C22 PUFA to C24 PUFA and has the potential to participate in the production of DHA in fish(Reference Li, Monroig and Navarro54,Reference Li, Monroig and Wang55) . The Δ6 FAD is regarded as the rate-limiting enzyme in the LC-PUFA biosynthetic pathway in mammals because it is the first enzyme involved in the bioconversion of C18 PUFA towards longer and more unsaturated homologues and is also involved in the synthesis of DHA from EPA(Reference Sprecher51,Reference Monroig, Li and Tocher56) . It is generally accepted that high levels of dietary LC-PUFA can suppress the expression of elongase and desaturase genes(Reference Tocher1), which may be the reason for decreased expression of ELOVL4 in the present study. A previous study in orange-spotted grouper (Epinephelus coioides) reported that ELOVL4 mRNA expression was down-regulated in response to high dietary LC-PUFA(Reference Li, Monroig and Navarro54). It was demonstrated that crabs fed soya oil diets had higher ELOVL4 and Δ6 FAD expression than those fed fish oil diets(Reference Yang, Zhang and Tan4,Reference Lin, Hao and Zhu52) , which was opposite in L. vannamei (Reference Chen, Li and Li57). In the current study, the expression of Δ6 FAD increased with increasing dietary n-3 LC-PUFA level at both dietary lipid levels, which was positively related to the expression of ACO. It was reported that both Δ6 FAD and ACO participate in DHA biosynthesis from EPA through the ‘Sprecher pathway’(Reference Sprecher51). The up-regulation of Δ6 FAD and ACO expression may therefore also indicate that mud crab need high DHA to maintain basic functions, and it is necessary to synthesise DHA from EPA. This is further evidence for the selective retention of DHA over EPA in mud crab as well as suggesting the capacity of synthesising DHA from EPA in vivo. A study in juvenile golden pompano (Trachinotus ovatus) showed a similar result, with the expression of ELOVL4, ELOVL5 and Δ6 FAD in both liver and brain increasing with increasing dietary DHA:EPA ratios(Reference Zhang, Chen and You58). Additionally, the expression of Δ6 FAD in crabs fed 12 % dietary lipid was higher than those fed 7 % dietary lipid, we speculated that higher dietary lipid contained lower proportion of n-3 LC-PUFA in the study, which may be in accordance with the result of DHA content in hepatopancreas. Additionally, the underlying regulation mechanism of ELOVL4 demonstrated that the transcription of ELOVL4 was positively mediated by LXRα directly or indirectly through the regulation of SREBP-1 transcription(Reference Li, Monroig and Wang55), and expression of Δ6 FAD was also positively regulated by SREBP-1(Reference Dong, Tan and Cai59,Reference Chen, Wang and Zhang60) , which was partly in agreement with the results in the present study. However, to the best of our knowledge, information on fatty acid and LC-PUFA biosynthesis in mud crab is lacking and studies on the above enzymes have only been at the transcriptional level. The function of these enzymes and the underlying mechanisms by which the expression of these genes is regulated needs to be studied.
Conclusion
In summary, mud crabs have a high lipid requirement and relatively high tolerance to dietary lipid levels. Based on two-slope broken-line regression analysis, the optimal n-3 LC-PUFA requirements of mud crab WG were estimated to be 20·1 and 12·7 mg/g at 7 and 12 % dietary lipid levels, respectively. Purified, high-lipid diets rich in palmitic acid reduced the n-3 LC-PUFA requirement of mud crab. Dietary n-3 LC-PUFA promoted energy storage but prevented excess lipid deposition in hepatopancreas.
Acknowledgements
The authors graciously thank Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences (NIMTE, CAS) for use of the Agilent Technologies GC-MS 7890B-5977A, USA.
This research was supported by National Key R & D Program of China (2018YFD0900400), China Agriculture Research System-48 (CARS-48), Nature Science Foundation of Zhejiang Province (LY17C190002), Key Research Program of Zhejiang Province of China (2018C02037) and Zhejiang Aquaculture Nutrition & Feed Technology Service Team (ZJANFTST2017-2). This research was also sponsored by the K. C. Wong Magna Fund in Ningbo University.
X. W. formulated the research question, designed the study, carried out the study, analysed the data and wrote the article. M. J. designed the study, assisted in the correction and developed the questions. X. C. was involved in feeding trial and laboratory assessments. J. L. assisted in feeding trial and data analysis. L. J. assisted in the correction and developed the questions. M. B. B. developed the questions, and revised the manuscript. D. R. T. assisted in developing the research questions and revising the manuscript. Q. Z. formulated the research question, designed the study and revised the manuscript. All the authors approved the final version of the manuscript.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003335














