There is an epidemiological association between peripheral arterial disease (PAD) and periodontal disease (PD)(Reference Seinost, Wimmer and Skerget1–Reference Dietrich, Webb and Stenhouse3), both of which are aggravated by inflammatory processes(Reference Van Dyke4, Reference Brevetti, Giugliano and Brevetti5). Nutritional factors influence this significant inflammatory burden in affected individuals(Reference Calder, Albers and Antoine6). PAD patients experience a high risk of ischaemic events (Reference Brevetti, Giugliano and Brevetti5) and so need adequate secondary prevention strategies. These include modification of dietary habits(Reference Gerhard-Herman, Gornik and Barrett7), since a growing body of evidence emphasises the role of nutrition in the pathophysiology and management of both PAD(Reference Gardner, Bright and Ort8–Reference Ruiz-Canela and Martínez-González18) and PD(Reference Najeeb, Zafar and Khurshid19–Reference Hujoel and Lingstrom21). PAD patients in particular are poorly nourished, with an insufficient intake of fibre(Reference Gardner, Bright and Ort8, Reference Nosova, Bartel and Chong15), vitamin E(Reference Gardner, Bright and Ort8–Reference Lane, Magno and Lane10), folic acid(Reference Gardner, Bright and Ort8, Reference Lane, Magno and Lane10) and n-3 PUFA(Reference Lane, Magno and Lane10, Reference Nosova, Bartel and Chong15) and high intakes of Na(Reference Gardner, Bright and Ort8, Reference Nosova, Bartel and Chong15), cholesterol(Reference Gardner, Bright and Ort8, Reference Nosova, Bartel and Chong15) and saturated fat(Reference Gardner, Bright and Ort8, Reference Nosova, Bartel and Chong15).
Poor oral health is a common condition, especially among the elderly, and is associated with an increased risk for atherosclerotic CVD(Reference Dietrich, Webb and Stenhouse3). PD causes chewing discomfort, impairs the functional ability to eat and negatively affects diet quality and the intake of essential nutrients(Reference Sheiham and Steele22, Reference Beaudette, Fritz and Sullivan23). Such patients show a reduced intake of vitamin C, folic acid, Mg and fibre as compared with healthy controls(Reference Staudte, Kranz and Volpel24). Individuals with impaired oral health avoid foods that are difficult or painful to chew(Reference Beaudette, Fritz and Sullivan23, Reference Marcenes, Steele and Sheiham25). These foods include fresh vegetables and fruit, nuts, seeds and whole grain products, among others, which are sources of essential nutrients and dietary fibre(Reference Beaudette, Fritz and Sullivan23).
Since the impact of PD severity on dietary intake (DI) and quality in patients with CVD has not been elucidated, the present study aimed (1) to evaluate the effect of PD severity on diet quality and DI of nutrients relevant for CVD in patients with PAD and (2) to compare DI of this patient population with the current dietary recommendations for CVD. We hypothesised that the severity of PD in PAD patients influences DI and that the consumption of nutrients relevant to CVD will differ from current dietary guidelines.
Methods
Study population and design
The present study evaluated data from a single-centre, prospective, randomised, open trial conducted at the Division of Angiology, Medical University of Graz, Austria, investigating the influence of periodontal therapy on vascular inflammation and function in patients with PAD (PeriPAD trial). PeriPAD was registered as a randomised controlled trial at the Deutsches Register Klinischer Studien (https://drks-neu.uniklinik-freiburg.de/drks_web/) ID: 00004554. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethics Committee of the Medical University of Graz, Austria (EK-Nr. 24-456 ex 11/12). Written informed consent was obtained from all subjects/patients. Recruitment took place between March 2013 and January 2015.
Patients who fulfilled the following key inclusion criteria were invited to enrol in the study: written informed consent; current or previously diagnosed symptomatic PAD (Rutherford classification 2–4 (intermittent claudication or rest pain)) and documented luminal stenosis >70 % on ultrasound or angiography or a history of endovascular or surgical revascularisation; periodontal disease determined by the Periodontal Screening and Recording (PSR) Index; signed informed consent form.
The exclusion criteria were defined as PAD Rutherford category 5 and 6 (tissue damage/loss); no natural teeth present; life expectancy <6 months; unstable cerebrovascular disease and CVD; clinically apparent infectious disease (e.g. pneumonia and symptomatic urinary tract infection); systemic inflammatory disease (e.g. chronic inflammatory bowel disease, rheumatoid arthritis, vasculitis by clinical assessment); periodontal treatment within 6 months prior to the study; mouth infection other than periodontitis; uncontrolled diabetes; pregnancy; age <18 years.
Clinical examination/testing
A cohort of 421 consecutive patients was screened for inclusion and exclusion criteria. The study visit was performed in the morning after an overnight fast at the Outpatient Clinic for Preventive Vascular Medicine at the Division of Angiology.
A general medical history was taken. Demographic data, anthropometric data, data on CVD risk factors, medication and pertinent vascular examination records were collected. Blood was sampled to determine circulating biomarkers and blood pressure was measured. The ankle brachial index was measured by trained clinical staff according to current standards and guidelines(Reference Rooke, Hirsch and Misra26). Symptomatic PAD was defined as ankle brachial index <0·9 and intermittent claudication or a history of endovascular or surgical revascularisation.
If PAD was diagnosed and at least one original tooth was present, patients were referred to the Division of Prosthodontics and Periodontology, Dental School, Medical University of Graz, to determine dental inclusion criteria. Patients who met the inclusion criteria were enrolled in the study after providing informed consent.
Blood sampling and biochemical analysis
A venous blood sample was drawn from an antecubital vein in the morning after an overnight fast. Analysis was performed on campus by the university’s certified laboratory to guarantee rapid processing. Laboratory methods or values were not changed during the study. Automated analysers (Cobas® 8000, Roche; ARCHITECT®, Abbott GmbH & Co. KG; XE 5000®, Sysmex; BNA II nephelometer analyser, Siemens,; IDS-iSYS immunoassay system, IDS) were used to measure serum concentrations of TAG, HDL-cholesterol, total cholesterol, glucose, C-reactive protein, cholesterol/HDL ratio and IL-6. The Friedewald formula was used to determine LDL-cholesterol concentrations. The laboratories were certified according to ISO 9001:2008.
Periodontal examination
The periodontal screening examination included the assessment of the general oral and dental history, determination of the PSR Index and an orthopantomogram. All dental parameters were assessed by experienced periodontists. PSR is an internationally accepted method to examine the periodontium and to detect periodontal diseases(27, Reference Landry and Jean28). Patients were classified according to their PSR stage: ‘healthy’ (PSR stage 0), ‘gingivitis’ (PSR stage 1 + 2), ‘moderate periodontitis’ (PSR stage 3) and ‘severe periodontitis’ (PSR stage 4).
Dietary assessment
DI was assessed by 24-h food recall (24-h recall) and a thirty-six-item self-administered qualitative FFQ. Participants were asked to report their usual consumption frequency of the thirty-six listed food items and categories during the previous 3 months with frequency options ranging from ‘almost never or <1/month’ to ‘>3 times/d’. This recently validated and published, self-administered FFQ has proved to be a good measure of dietary quality in a population of people aged 55 years and older(Reference Freisling, Elmadfa and Schuh29). The information derived from our FFQ was used to calculate the food frequency index (FFI). The development of this score has been described elsewhere(Reference Freisling, Elmadfa and Schuh29).
Since elderly patients sometimes have difficulty filling out questionnaires by themselves, a trained dietitian was available for support, checked the FFQ for completeness and undertook the structured 24-h recall together with the patients.
Nutrient intake from 24-h recall was subsequently calculated using nut.s software (nutritional.software, dato Denkwerkzeuge). Our results were compared with the D-A-CH reference values for nutrient supply (German Nutrition Society (DGE), the Austrian Nutrition Society and the Swiss Society for Nutritional Research)(30) and recommendations for CVD prevention of the European Society of Cardiology (ESC)(Reference Piepoli, Hoes and Agewall31) and American Heart Association/American College of Cardiology (AHA/ACC)(Reference Eckel, Jakicic and Ard32–Reference Smith, Benjamin and Bonow34).
Statistical analysis
Statistical analyses were as follows: For descriptive statistics, means and standard deviations were calculated for normally distributed data; otherwise, medians and interquartile ranges (25th–75th percentiles) were calculated (Table 1). Normal distribution of data was tested by the Kolmogorov–Smirnov test. The Kruskal–Wallis test was used to determine differences in nutrient intake (a P value of <0·007 was considered statistically significant after Bonferroni correction(Reference Bland and Altman35) to account for multiple testing) (Table 2) and in macronutrient consumption (Table 3) between the three periodontal disease groups. Spearman’s correlation coefficient was used in correlation analysis. All analyses were completed with SPSS for Windows version 23 (SSCP Inc.).
Table 1. Patient characteristics (n 412)
(Mean values and standard deviations; numbers and percentages; median and interquartile range (IQR))
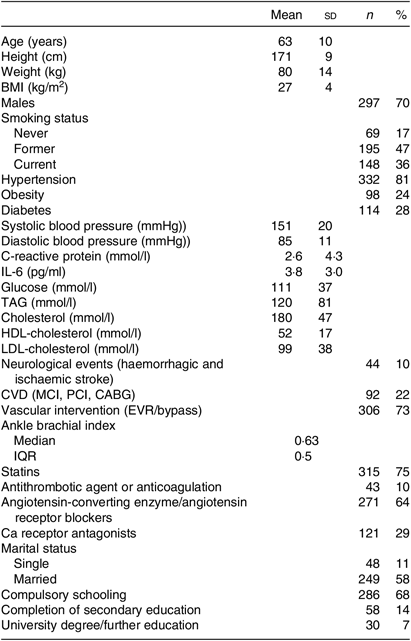
MCI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; EVR, endovascular revascularisation.
Table 2. Dietary intake (24-h recall) of nutrients relevant for CVD and diet quality in peripheral arterial disease patients with gingivitis, moderate periodontitis and severe periodontitis
(Medians and 25th–75th percentiles)
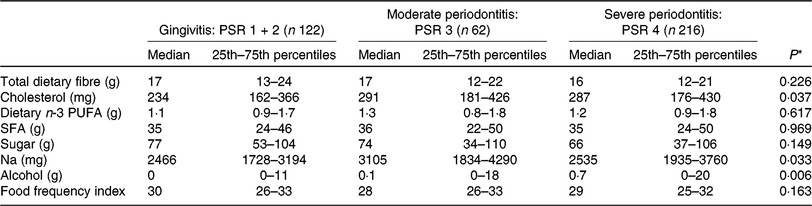
PSR, Periodontal Screening and Recording Index.
* P value was calculated from the Kruskal–Wallis test for continuous variables.
Table 3. Comparison of macronutrient intake among peripheral arterial disease patients with gingivitis, moderate periodontitis and severe periodontitis
(Medians and 25th–75th percentiles)
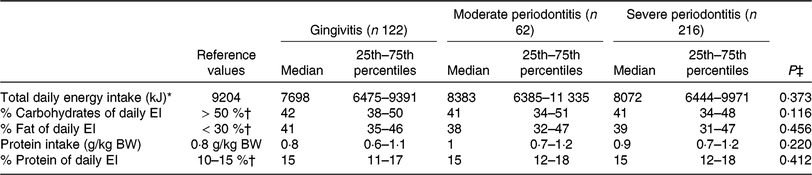
EI, energy intake; BW, body weight.
* D-A-CH reference values for male subjects, 51–65 years, physical activity level = 1·4 was used(30).
† Percentage of daily energy intake.
‡ P values were calculated from the non-parametric Kruskal–Wallis test.
Recommendations for the intake of specific nutrients were obtained from the guidelines for CVD prevention by the ESC(Reference Piepoli, Hoes and Agewall31) and AHA/ACC(Reference Eckel, Jakicic and Ard32).
Results
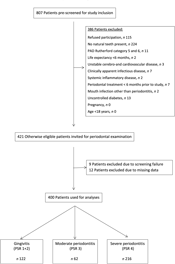
A total of 807 patients were screened for study inclusion, and 386 patients were not eligible due to various reasons (see Fig. 1). Finally, 421 patients were included for analysis. Nine patients had to be excluded for analysis due to screening failure. Twelve patients had to be excluded due to missing dental data, leaving 400 participants for analysis.
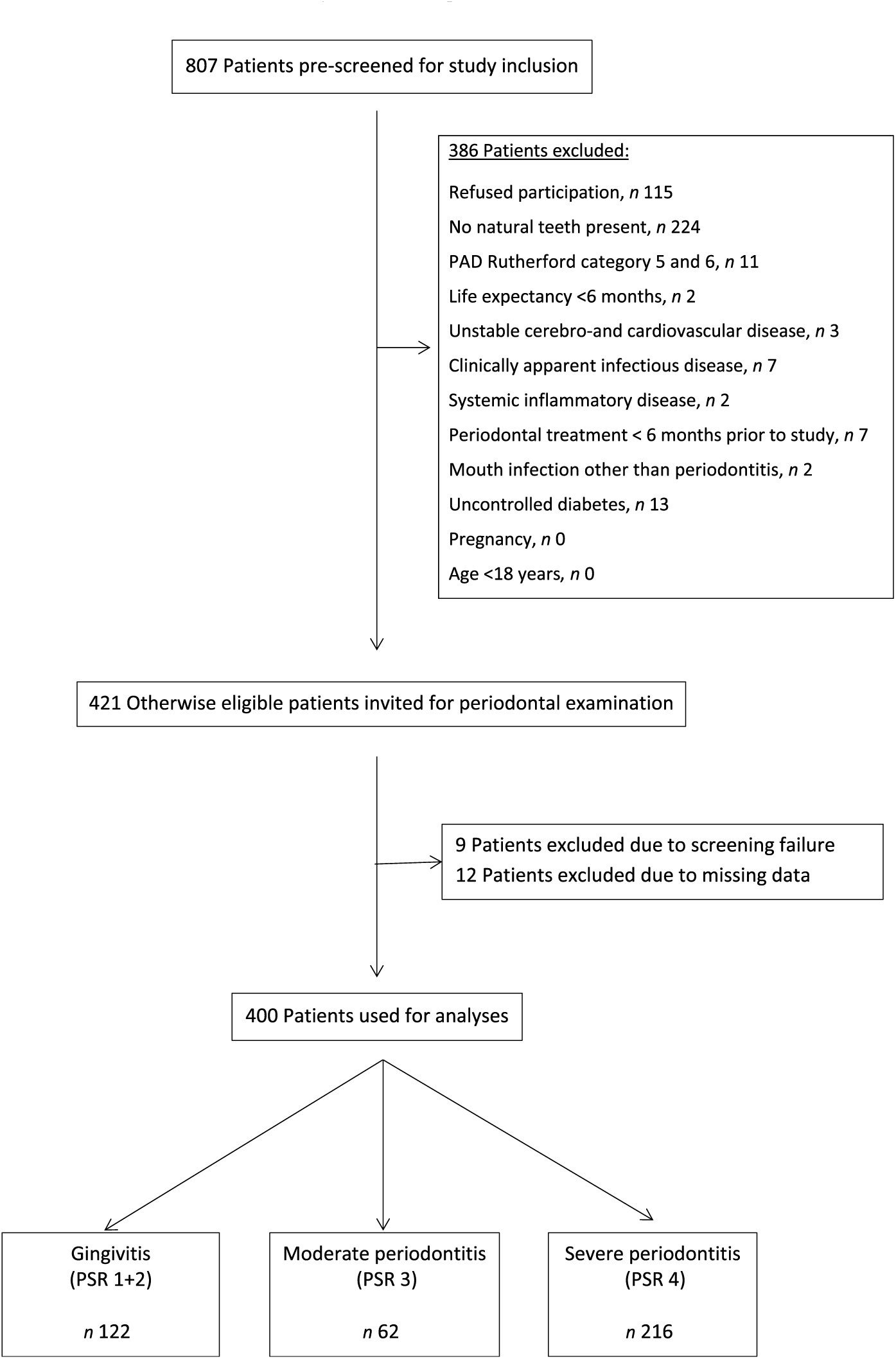
Fig. 1. Flow diagram of the screening process and enrolment of patients. PAD, peripheral arterial disease; PSR, Periodontal Screening and Recording Index.
Patients’ characteristics are summarised in Table 1. A flow chart of the screening process is shown in Fig. 1.
Impact of periodontal disease severity on dietary intake and quality
We found a very high prevalence of PD among our PAD patients (99·8 %), of whom 53·9 % (n 216) were diagnosed with severe periodontitis. Only one of the patients was classified as dentally healthy.
Nutritional intake was stratified according to the severity of PD. No difference in DI of total fibre, cholesterol, n-3 PUFA, SFA, sugar and Na was seen among PAD patients with gingivitis, moderate periodontitis and severe periodontitis. Alcohol consumption differed between PD stages and showed a positive correlation with PD severity (P = 0·001; r 0·159) (Table 2). Post hoc testing revealed a significant difference in median alcohol intake between patients with gingivitis and severe periodontitis (P = 0·001).
PD severity and patients’ median number of teeth(Reference Ruiz-Canela and Martínez-González18, Reference Lane, Magno and Lane10–Reference Staudte, Kranz and Volpel24) did not correlate with other investigated nutritional parameters and FFI. The FFI indicated no difference in diet quality among the PD stages.
Impact of periodontal disease severity and number of teeth on macronutrient composition
The non-parametric Kruskal–Wallis test showed no difference in macronutrient intake between the three PD stages. However, daily macronutrient intake (in %) differed from D-A-CH reference values for nutrient supply(30). On average, the subjects consumed fewer than the daily reference value of 9204 kJ/d for men 51 years of age and older at a physical activity level of 1·4(30). Dietary fat intake was well above the recommended 30 % of total daily energy intake (TEI)(30) (Table 3), which 80 % of the participants exceeded. Median daily carbohydrate (CHO) consumption of all subjects was below the recommended intake value (D-A-CH: 50–60 % of TEI(30)) with 41·5 % of TEI. Protein intake of this patient population was found to be adequate (Table 3).
Dietary intake relative to recommendations
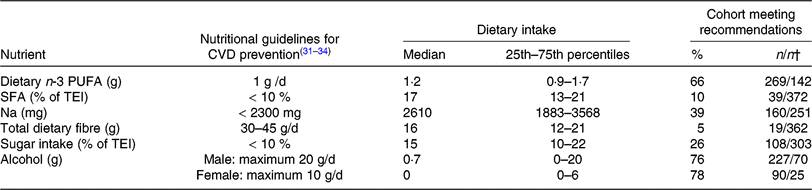
Table 4 displays the nutritional intake of patients in comparison with established guidelines for CVD prevention.
Table 4. Nutrient intake in peripheral arterial disease patients compared with guidelines for CVD prevention*
(Medians and 25th–75th percentiles; percentages and numbers of patients meeting recommendations)
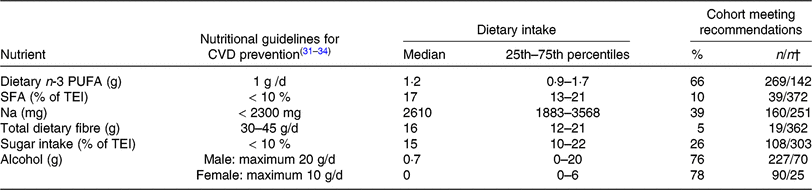
TEI, total daily energy intake; ESC, European Society of Cardiology; AHA, American Heart Association; ACC, American College of Cardiology.
* The Table summarises the subjects’ nutritional intake, represented as proportions (%) relative to recommendations for nutrient intake. The values used for comparison were obtained from guidelines for CVD prevention by the ESC(Reference Piepoli, Hoes and Agewall31) and for Na intake by the AHA/ACC(Reference Eckel, Jakicic and Ard32). Recommendations for daily dietary n-3 PUFA (1 g/d) from the AHA(Reference Kris-Etherton33, Reference Smith, Benjamin and Bonow34) were used, as the ESC guidelines do not include specific recommendations for dietary n-3 PUFA intake. Dietary n-3 PUFA refers to total dietary n-3 PUFA (i.e. α-linolenic acid, EPA, docosapentaenoic acid and DHA).
† Number of patients meeting the recommendations/number of patients not meeting recommendations.
The recommended daily amount of n-3 PUFA(Reference Kris-Etherton33, Reference Smith, Benjamin and Bonow34) was consumed by two-thirds of our patients with both PAD and PD, with 1·7 % of the patients reporting the daily use of n-3 PUFA supplements. Our subjects showed elevated intake levels of SFA (90 % exceeded the cut-off point of 10 % of TEI, as recommended by the ESC(Reference Piepoli, Hoes and Agewall31)), Na and sugar. An inadequate intake of total dietary fibre was reported (95 % of subjects did not meet the ESC cut-off(Reference Piepoli, Hoes and Agewall31)). Recommendations for alcohol consumption were met by >70 % of this patient population (Table 4).
Discussion
The main finding of this cross-sectional study was that overall DI of nutrients relevant for CVD and diet quality did not differ depending on the severity of PD in patients with PAD. Nevertheless, macronutrient consumption and intake of nutrients relevant for CVD greatly deviated from current dietary guidelines.
Impact of periodontal disease severity on dietary intake and quality
Previous studies have reported an impairment of the functional ability to eat in patients affected with PD and poor oral health. This contributed to poor composition and quality of the patient’s diet(Reference Beaudette, Fritz and Sullivan23). In a study by Staudte et al. (Reference Staudte, Kranz and Volpel24), approximately 50 % of the patients with chronic periodontitis experienced oral discomfort while eating. An individual’s dental condition and tooth loss, which may be related to PD, can also influence DI of essential nutrients and the selection of foods consumed(Reference Beaudette, Fritz and Sullivan23–Reference Marcenes, Steele and Sheiham25). It could therefore be suspected that patients with a more severe degree of PD would have more trouble chewing and so would not consume enough essential nutrients. Foods difficult to masticate include many with a healthful profile such as fruits, raw vegetables, nuts and whole grain bread, among others(Reference Beaudette, Fritz and Sullivan23, Reference Marcenes, Steele and Sheiham25).
In our study, the prevalence of moderate and severe periodontitis in screened patients was 69 %, which is similar to what has been reported in the literature(Reference Ahn, Shin and Han36). Despite this high prevalence of PD among our PAD patients, the degree of PD did not affect diet quality and intake of nutrients relevant for CVD. Nevertheless, we observed a significant difference in alcohol intake between patients with severe periodontitis and those with gingivitis. Alcohol consumption was the only parameter presenting a significant positive correlation with PD severity and has previously been shown to negatively affect PD(Reference Lages, Costa and Cortelli37) and to increase the risk for PAD(Reference Ogilvie, Lutsey and Heiss17, Reference Bell, Daskalopoulou and Rapsomaniki38).
Number and distribution of natural teeth have also been shown to influence DI, food choice and amount of nutrients consumed, probably due to the reduced ability to chew(Reference Sheiham and Steele22, Reference Marcenes, Steele and Sheiham25), but we did not find any correlation between the number of teeth and PD severity with the nutritional parameters investigated. These contradictory findings might be explained by the fact that the median age of our participants was lower than in Sheiham et al. (Reference Marcenes, Steele and Sheiham25), and the median number of eighteen natural teeth in our patients was still fairly high. Marcenes et al. (Reference Marcenes, Steele and Sheiham25) defined acceptable oral health by the presence of >20 natural teeth and stated that subjects with >21 teeth consumed more essential nutrients.
Impact of periodontal disease severity and number of teeth on macronutrient composition
We hypothesised that in PAD patients, concomitant presence of PD alters the composition of the patients’ diet. Contrary to expectations, we observed no difference in macronutrient composition among the three PD stages. However, CHO and fat intake of our patients differed from current reference values. D-A-CH reference values for daily intake of CHO, fat and protein are 50–60 %, 25–30 % and 10–15 % of TEI, respectively(30).
Protein intake was adequate in our patients, but CHO intake was far too low in all PD groups. In contrast, 80 % of our patients exceeded the recommended fat intake of 30 % of TEI. This is in agreement with Brostow et al. (Reference Brostow, Hirsch and Pereira12), who showed in sixty-four PAD patients that the percentage of energy consumed as fat also exceeded the recommendations.
Our results stand in contrast to Gardner et al. (Reference Gardner, Bright and Ort8), who examined the DI of forty-six subjects with PAD and reported a mean macronutrient composition very close to recommendations (17 % protein, 51 % CHO and 30 % fat). Our findings also disagree with Hamasaki et al. (Reference Hamasaki, Kitamura and Kawashita39), demonstrating a low intake of dietary fat in patients with advanced PD (23·2 ± 7·1 %). In this specific study the ‘weighting capacity technique’ was used to determine DI (with this method, food intake was distributed in proportion to family size by dividing by the number of people in the family)(Reference Hamasaki, Kitamura and Kawashita39). The greater sample size of our investigation and the different methods of dietary assessment might explain the contradictory results. Our representative cross-sectional results might reflect the general DI of the Austrian population(Reference Elmadfa40), with a lower than recommended intake of CHO, but increased intake of dietary fat and adequate intake of protein.
Dietary intake relative to European Society of Cardiology and American Heart Association/American College of Cardiology recommendations
Strategies for secondary prevention of CVD include the modification of dietary intake and habits. Accordingly, lifestyle and nutritional guidelines for the management of CVD have been developed by various institutions including the ESC(Reference Piepoli, Hoes and Agewall31), AHA and ACC(Reference Eckel, Jakicic and Ard32–Reference Smith, Benjamin and Bonow34). Specific dietary parameters relevant for CVD, and therefore for patients affected with PAD, include n-3 fatty acids, SFA, Na, dietary fibre, sugar intake and alcohol consumption(Reference Piepoli, Hoes and Agewall31–Reference Smith, Benjamin and Bonow34). The results of the present study demonstrate that the consumption of these nutrients diverged greatly from guidelines.
Several classes of fatty acids have been shown to influence cardiovascular risk factors and outcomes(Reference Naqvi, Davis and Mukamal11). The prospective Prevención con Dieta Mediterránea (PREDIMED) trial showed that the intake of n-3 PUFA may be associated with a reduced risk for developing PAD(Reference Ruiz-Canela, Estruch and Corella41). Generally, PAD patients appear to have an inadequate intake of vegetable lipids and hence essential fatty acids(Reference Antonelli-Incalzi, Pedone and McDermott9). n-3 Fatty acids have also been suggested to play a role in PD. Using National Health and Nutrition Examination Survey (NHANES) III data, Naqvi et al. (Reference Naqvi, Buettner and Phillips42) found an inverse association of n-3 fatty acid intake with lower prevalence of periodontitis. In our large cohort of PAD patients suffering from concomitant PD, 66 % reached the AHA/ACC recommendation of at least 1 g/d of n-3 PUFA. A similar result was obtained in a recent prospective study by Nosova et al. (Reference Nosova, Bartel and Chong15), who showed that 59 % of their veteran subjects suffering from PAD reached this recommended cut-off. Another previous study by Lane et al. (Reference Lane, Magno and Lane10) demonstrated the reduced consumption of PUFA among PAD patients and suggested a protective effect of n-3 fatty acids against CAD and PAD.
A low intake of SFA, another class of fatty acids, is also of major importance in CVD prevention. Dietary SFA have been associated with an increased risk for PAD(Reference Naqvi, Davis and Mukamal11). Gardner et al. (Reference Gardner, Bright and Ort8) demonstrated that 80 % of their PAD patients exceeded the recommended DI of SFA. These results are confirmed by our study, where more than 90 % exceeded the recommended upper value.
Adequate fibre intake has previously been shown to have an inverse association with PAD(Reference Ruiz-Canela and Martínez-González18). Our data confirm the findings of Nosova et al. (Reference Nosova, Bartel and Chong15), who showed a low intake of fibre and high intake of Na among their PAD patients. In our cohort poor nutrition was reflected in high intake of Na and sugar, whereas more than 90 % of our subjects did not reach the recommended intake of dietary fibre. This is in accordance with Gardner et al. (Reference Gardner, Bright and Ort8), who showed that only 26 % of their PAD patients reached the recommended intake for dietary fibre. It reflects a low intake of high-fibre foods, such as whole grain products, fruits and vegetables, all of which are difficult to chew. Since our patients were also suffering from PD, this might have further influenced fibre intake. Periodontal inflammation and loose teeth make it difficult to eat high-fibre foods. Staudte et al. (Reference Staudte, Kranz and Volpel24) also demonstrated a lower intake of fibre in patients with periodontitis than in healthy controls.
The findings of our study highlight important implications for secondary prevention of CVD and public health, although we could not demonstrate that PD influences DI in PAD patients. Nevertheless, these patients should be encouraged to follow the ESC, AHA/ACC and D-A-CH dietary guidelines. Dietary modification and enhancement of nutritional strategies (including management of alcohol intake) should be considered as key components in secondary prevention to reduce CV risk in this population.
Limitations
There are limitations to this cross-sectional, observational study. The patient population is not representative of a general population since predominately Caucasian PAD patients were studied. Furthermore, we did not include PAD patients with Fontaine Stage IV (defined by skin necrosis or ulcers), and our patients were not compared with a healthy control group. Another limitation is that DI was recorded via 1-d 24h-recall and a FFQ. A 7-d dietary recall would have been of advantage to evaluate the patients’ dietary intake and habits in more detail, also capturing foods that are not eaten daily (e.g. fish), but was not feasible in our patient population due to age, cognitive impairment and other co-morbidities. Nevertheless, the methods we used are widely accepted tools for dietary assessment and can be a reliable indicator of trends in dietary habits within a population. In addition, to avoid potential errors in DI reporting, a trained dietitian was always available to help patients with the questionnaires.
Conclusion
In our sample of patients with PAD and concomitant PD, we did not observe differences in DI of nutrients known to be relevant for CVD and diet quality in relation to the severity of the PD, though macronutrient intake did differ from D-A-CH recommendations. Our most relevant finding is that our patients showed poor nutrition with regard to DI of Na, fibre, sugar, n-3 fatty acids and SFA, which differed greatly from current recommendations of the ESC, AHA and ACC. Future studies should focus on nutritional modification and intervention in order to study the effect on the progression of PD and CV risk in PAD patients.
Acknowledgements
We are grateful to the Austrian Science Fund (FWF) for funding and to the patients who were willing to provide their data.
This work was supported by the FWF (grant number KLI 256 B00). The FWF had no role in the design, analysis or writing of this article.
The authors take full responsibility for the content to which they have contributed. A. H., B. A., G. W., B. M., H. M., R. G., S. H., F. Q. and G. S. have no conflict of interest to disclose related to the manuscript. A. H., G. S., G. W., B. A., R. G. were responsible for study design; A. H., G. S., G. W., B. A., B. M. conducted the research; F. Q. and A. H. analysed the data and performed statistical analysis; and S. H. and H. M. were involved in manuscript preparation.