Oesophageal cancer has the eighth highest incidence of all cancers worldwide, with 455 800 new cases and 400 200 deaths estimated to have occurred in 2012( Reference Torre, Bray and Siegel 1 ). Incidence rates of oesophageal cancer vary considerably across different geographic regions( Reference Ferlay, Soerjomataram and Dikshit 2 ), highlighting the potential role of nutritional and lifestyle factors in oesophageal cancer aetiology. Because of the lack of early clinical symptoms, oesophageal cancer is typically diagnosed at an advanced stage. Prognoses for these patients remain very poor, with a 5-year survival rate of<20 %( Reference Pakzad, Mohammadian-Hafshejani and Khosravi 3 ). Therefore, primary prevention of oesophageal cancer is a major public health priority.
There are two histological types of oesophageal cancer – squamous cell carcinoma and adenocarcinoma. Although squamous cell cancers are thought to be more strongly associated with tobacco exposure( Reference Ahsan, Neugut and Gammon 4 , Reference Kabat, Shivappa and Hebert 5 ), both types have been found to be associated with diet-related inflammation based on the dietary inflammatory index (DII®)( Reference Lu, Shivappa and Lin 6 ). On the basis of recent data collected from the Chinese National Central Cancer Registry, oesophageal squamous cell cancer (ESCC) was the most common type, being responsible for nearly 90 % of the cases of oesophageal carcinoma in China( Reference Zeng, Zheng and Zhang 7 ). It has been well established that tobacco smoking and alcohol drinking are important risk factors for ESCC( Reference Morita, Kumashiro and Kubo 8 ). A diet high in processed meat and frequent drinking of very hot beverages may also increase the risk of developing ESCC. The major aetiological factors for oesophageal adenocarcinoma (EAC) are gastro-oesophageal reflux and body fatness (as indicated by increased BMI, waist circumference and waist:hip ratio). There is some evidence that low intake of fruits and vegetables is linked to an increased risk of both ESCC and EAC( 9 ).
Chronic inflammation is a state of continuous presence of inflammatory cytokines in circulation and in the tissues, and has been shown to play a crucial role in the development of various epithelial cancers( Reference Keibel, Singh and Sharma 10 , Reference Pan, Lai and Dushenkov 11 ). In particular, it has been shown that chronic inflammation is an important risk factor in the development of oesophageal cancer, especially epithelial damage involved in ESCC( Reference O’Sullivan, Phelan and O’Hanlon 12 , Reference Corley, Kerlikowske and Verma 13 ).
Dietary habits represent modifiable factors that have been shown to influence both inflammation( Reference de Mello, Schwab and Kolehmainen 14 – Reference Shivappa, Steck and Hurley 17 ) and oesophageal cancer( Reference Lin, Yngve and Lagergren 18 – Reference Terry, Lagergren and Ye 20 ). Diet represents a complex set of exposures that often interact, and has a cumulative effect on both inflammatory responses and health outcomes. The DII was developed to assess the inflammatory potential of an individual’s diet( Reference Wirth, Burch and Shivappa 21 ). A pro-inflammatory diet is characterised by a high consumption of foods rich in SFA and carbohydrates, and a low consumption of foods rich in fibre, PUFA, flavonoids and other antioxidant dietary components. The DII has been validated in a variety of longitudinal and cross-sectional studies with various inflammatory markers, including C-reactive protein (CRP)( Reference Shivappa, Steck and Hurley 22 ), IL-6( Reference Shivappa, Hebert and Rietzschel 23 ) and TNF-α ( Reference Tabung, Steck and Zhang 24 ). The DII has been associated with the risk of colorectal cancer in case–control studies in Spain and Italy( Reference Zamora-Ros, Shivappa and Steck 25 , Reference Shivappa, Zucchetto and Montella 26 ), in three cohort studies from the USA( Reference Tabung, Steck and Ma 27 – Reference Shivappa, Prizment and Blair 29 ) and risk of various cancers in case–control studies in Italy( Reference Shivappa, Bosetti and Zucchetto 30 – Reference Shivappa, Hebert and Polesel 33 ). Specifically related to oesophageal cancer, the DII has been shown to be associated with oesophageal cancer in Italy, Iran, Sweden and Ireland( Reference Lu, Shivappa and Lin 6 , Reference Tabung, Steck and Zhang 24 , Reference Shivappa, Zucchetto and Serraino 31 , Reference Shivappa, Hebert and Anderson 34 ).
Xinjiang Uyghur Autonomous Region, located in the north-west of China, is one of the areas constituting the ‘Asian oesophageal cancer belt’( Reference Zheng, Vuitton and Sheyhidin 35 ). The objective of the present study was to investigate whether pro-inflammatory diets, as measured by the DII, are associated with increased risk of oesophageal cancer among adults residing in this remote region of China. This is the first study to examine this association in China.
Methods
Study design and participants
A hospital-based case–control study of oesophageal cancer was conducted in Urumqi and Shihezi, Xinjiang Uyghur Autonomous Region of China, between January 2008 and December 2009. Participants were recruited from the Xinjiang Tumor Hospital, Shihezi People’s Hospital, Kuitong Hospital and No. 1 Affiliated Hospital of Shihezi University. Medical records and pathology reports were reviewed to identify patients with histopathologically confirmed incident oesophageal cancer that had been diagnosed within the previous 12 months. Pathological diagnoses were based on the International Classification of Disease for Oncology (ICD-O-3 codes: C150-C155, C158, C159)( Reference Garbbert, Shimoda and Hainaut 36 ). Patients without histopathologically confirmed oesophageal cancer and those with reported memory problems were excluded. Of the total 364 incident eligible patients identified, 359 consented to participate.
During the same period, controls were recruited from inpatient wards of the Departments of Ophthalmology, Orthopaedics, Respiratory Diseases and Physiotherapy at the same hospitals. Exclusion criteria for controls were previous diagnosis of a malignant disease, on long-term medical diet and self-reported memory problems. Random numbers were generated using the software Research Randomizer (http://www.socialpsychology.org) to make a final selection whenever more controls were available than could be interviewed. Of the 400 eligible controls who were frequency-matched to cases on sex and age (±5 years), 380 eventually gave their consent to participate in the study (with a response rate of 95 %). No significant differences were found in demographic variables (age, education and marital status) between participants and non-participants in both case and control groups.
Given the sample size of 359 case patients and 380 control participants and a two-sided statistical significance level of 0·05, our study achieved more than 99 % power to detect a 2-fold increased risk of oesophageal cancer associated with pro-inflammatory diets( Reference Shivappa, Zucchetto and Serraino 31 ). For this power calculation, the prevalence of consuming a pro-inflammatory diet was estimated at 40 and 25 % in the cancer patients and control group, respectively.
The study protocol was approved by the participating hospitals and the Human Research Ethics Committee of Curtin University (approved no. HR 56/2006). Written informed consent was obtained from all participants, who were assured of confidentiality of the information provided and their right to withdraw at any time without prejudice.
Interview and exposure measurements
All participants were interviewed in-person by trained nurses, usually in the presence of their next-of-kin, to facilitate the recall of dietary habits( Reference Liang, Binns and Lee 37 ). The nurses were aware of the case–control status of the participants, but remained blind to the study hypothesis. The structured questionnaire composed sections on demographic characteristics, anthropometry, personal health and family medical histories, diet and lifestyle, including cigarette smoking and alcohol drinking. A 137-item semi-quantitative FFQ, which had been validated and included fruits, vegetables, meat, poultry, cereals and beverages commonly consumed in Northwestern China, was used to collect dietary information( Reference Zhang 38 ). Frequency and quantity of intake were recorded in detail. The reference recall period for dietary variables was set at 5 years before diagnosis for cases and 5 years before interview for controls. Participants who consumed at least 500 ml of alcoholic beverages per week were classified as ‘often’; otherwise, they were referred to as ‘never/seldom’ drinkers.
Daily intakes of carbohydrate, protein, cholesterol, fat, SFA, fibre, niacin, folic acid, thiamin, riboflavin, vitamin C, vitamin E, vitamin B6, β-carotene, Fe, Mg and Zn were estimated based on the frequency and amounts of each food or beverage item consumed using the Chinese Food Composition Tables( 39 ). The US Department of Agriculture nutrient database( 40 ) was used for the conversion to vitamin B12, MUFA, n-3, n-6, PUFA and SFA, as such values were unavailable from the Chinese Food Composition Tables.
Calculation of dietary inflammatory index scores
The development( Reference Shivappa, Steck and Hurley 17 ) and validation( Reference Shivappa, Steck and Hurley 22 ) of the DII has been explained elsewhere. Through evaluation of peer-reviewed literature published from 1950 to 2010, the score is based on 1943 articles linked to forty-five individual nutrient, food or flavonoid intake parameters. Points were assigned to each of these parameters according to whether they increased (+1), decreased (−1) or had no (0) effect on six established inflammatory biomarkers: IL-1b, IL-4, IL-6, IL-10, TNF-α and CRP. The score for each of the food parameters was weighted according to the study designs and total number of research articles. Overall parameter-specific inflammatory effect scores were then calculated based on the ratio of the total weighted number of articles:the weighted pro- and anti-inflammatory articles for each parameter followed by subtracting the anti-inflammatory fraction from the pro-inflammatory fraction. Parameters that had a robust pool of literature, that is greater than the median number of 236 weighted articles, were assigned the full value of that score. Parameters with a number of weighted articles <236 were adjusted according to the distance of their number from this median.
Actual dietary intake data from the FFQ were adjusted against a reference global daily mean and standard deviation intake for each parameter to obtain a z score. The global intake data were based on consumption data from eleven countries in different parts of the world. To reduce the effect of skewness, a common occurrence in dietary data, z scores were converted to proportions. These, in turn, were centred on zero by doubling the proportion and subtracting 1. The centred proportion for each intake parameter was multiplied by its respective parameter-specific inflammatory effect score. All of the food-parameter-specific DII scores were then summed to create the overall DII score for each participant in the study, DII=b 1×n 1+b 2×n 2+ … +b 22×n 22, where b i (i=1, …, 23) refers to the literature-derived inflammatory effect scores for each of the evaluable food parameters and n i refers to the food-parameter-specific centred percentiles, which were computed from the FFQ-derived dietary data. The steps involved in DII calculation are shown in Fig. 1. For the current study, data on twenty-three of the forty-five DII food parameters could be derived from the FFQ and were thus used for calculating the DII scores. These include energy, carbohydrate, protein, fat, SFA, iron, cholesterol, fibre, PUFA, n-3, n-6, niacin, thiamin, riboflavin, Mg, Zn, vitamin C, vitamin E, vitamin B6, B12, β-carotene, garlic and onions. Alcohol intake was not included in the DII calculation because daily consumption information was not recorded. All of these food-parameter-specific DII scores are then summed to create the overall DII score for each subject in the study.
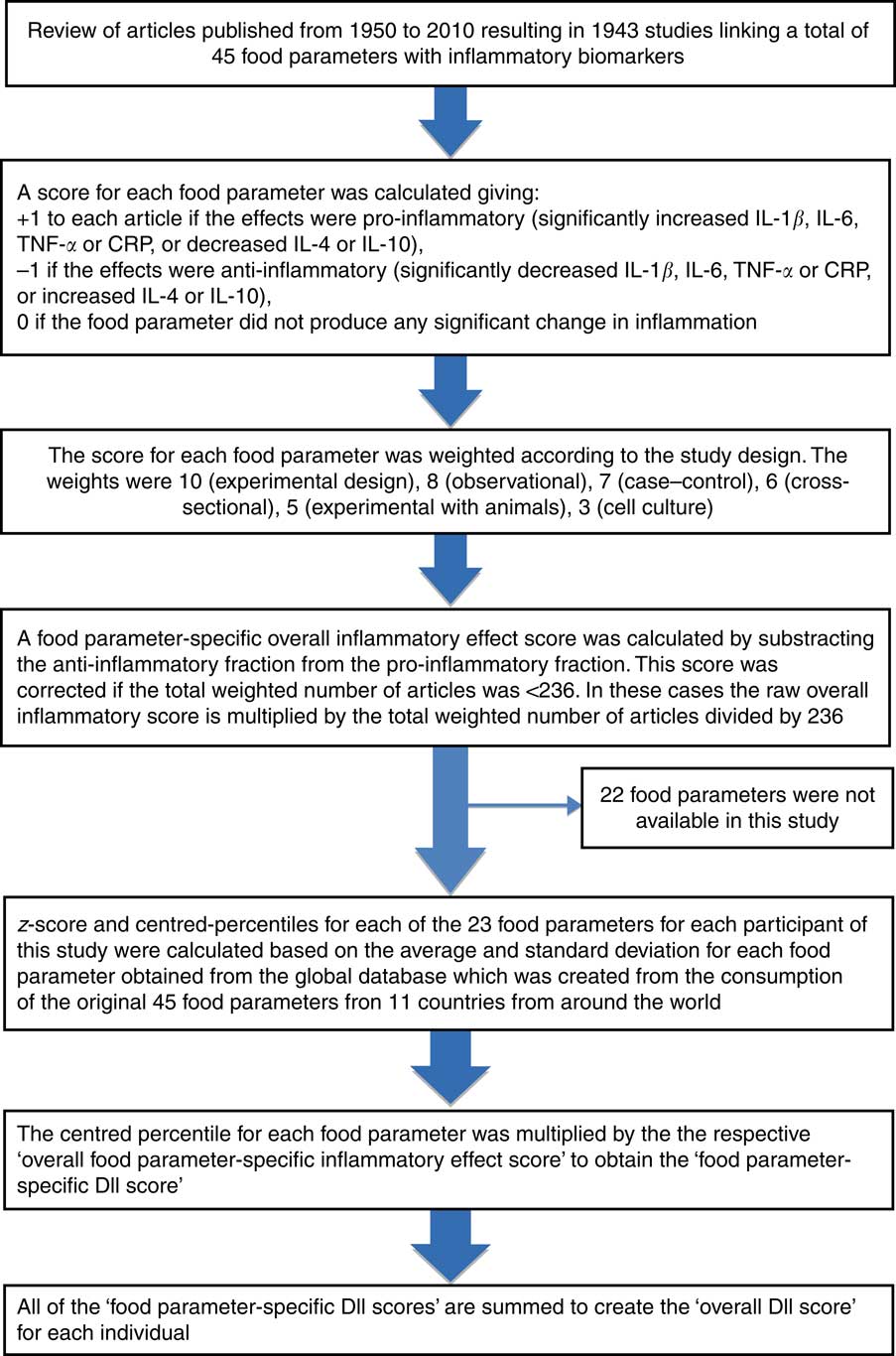
Fig. 1 Sequence of steps in creating the dietary inflammatory index (DII) in the Chinese oesophageal cancer case–control study. CRP, C-reactive protein.
Statistical analysis
The DII score was categorised into quartiles with cut-off points based on the control distribution. The χ 2 test was used for categorical variables. Either the Student’s t test or the Mann–Whitney U test was used for continuous variables to compare the sample characteristics and DII scores between case and control groups. χ 2 and one-way ANOVA tests were next conducted to test four differences in participant characteristics across quartiles of the DII score by case and control status.
Unconditional logistic regression analyses were then performed to ascertain the association between DII score and the oesophageal cancer risk, with the lowest level of DII score being the reference category. Potential confounding variables considered were age (years), sex, education level (none/primary, secondary, tertiary), BMI (5 years ago, kg/m2), total energy intake (kJ/d (kcal/d)), smoking status (never, ever), alcohol drinking (never/seldom, often) and family history of cancer in first-degree relatives (no, yes). These variables were either established or plausible risk factors based on findings in the peer-reviewed literature. All of the variables were included in the multivariable logistic regression model.
In addition to reporting crude and adjusted OR and corresponding 95 % CI, dose–response relationships were assessed by tests for linear trend. All statistical analyses were performed using the SPSS® package version 22 (IBM Corp.).
Results
Table 1 compares characteristics and DII scores of the sample by case–control status. The case and control participants were, on average, 61·0 (sd 11·4) years old with a mean BMI of 24·1 (sd 3·7) kg/m2. Approximately, 72 % of cases were male, 54 % smoked and 46 % regularly drank alcoholic beverages. Compared with the controls, patients with oesophageal cancer tended to belong to an ethnic minority group (as compared with the Han majority), have lower education level and have a family history of oesophageal cancer. DII scores in this study ranged from −4·72 (most anti-inflammatory score) to 4·18 (most pro-inflammatory score). The median DII score among oesophageal cancer patients (−0·35, interquartile range (IQR) −2·25, 1·86) was significantly higher than that of their control counterparts (−1·41, IQR −3·07, 0·40).
Table 1 Comparison of participant characteristics and dietary inflammatory index between case and control groups, Xinjiang, China, January 2008–December 2009 (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))
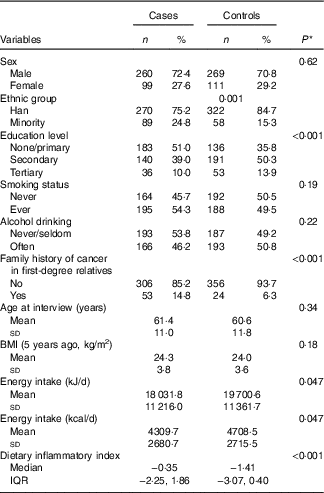
* χ 2, Student’s t test or Mann–Whitney U test for difference between cases and controls.
Study characteristics across quartiles of DII are provided in Table 2. Participants who consumed more pro-inflammatory diets (i.e. with higher DII scores) were older, more likely to belong to a minority ethnic group (as opposed to Han) and to smoke.
Table 2 Participant characteristics across quartiles (Q) of dietary inflammatory index (DII) among all participants, Xinjiang, China, January 2008–December 2009 (Numbers and percentages; mean values and standard deviations)
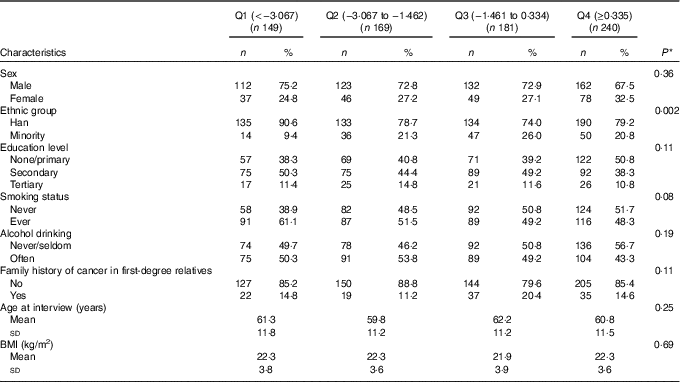
* χ 2 Test or one-way ANOVA for difference among quartiles of DII.
The results of logistic regression analyses are shown in Table 3. A higher DII score (indicating a more pro-inflammatory diet) was associated with an increased risk of oesophageal cancer, with a significant dose–response relationship (P trend<0·001). The adjusted OR was 2·55 (95 % CI 1·61, 4·06) for participants in the highest quartile of DII scores compared with those in the lowest quartile. Belonging to an ethnic minority group (adjusted OR 1·86; 95 % CI 1·24, 2·80), having a family history of oesophageal cancer (adjusted OR 2·74; 95 % CI 1·60, 4·66), an education level lower than secondary school (adjusted OR 1·82; 95 % CI 1·09, 3·06) and smoking (adjusted OR 1·59; 95 % CI 1·07, 2·36) also were significantly and independently associated with an elevated risk of oesophageal cancer. None of their interactions with DII was found to be significant.
Table 3 Oesophageal cancer risk for dietary inflammatory index in Xinjiang, China, January 2008–December 2009 (Numbers and percentages; odds ratios and 95 % confidence intervals)
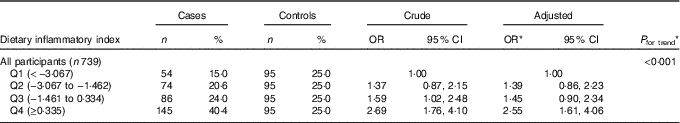
Q, quartile.
* From logistic regression model adjusting for age (years), sex, ethnic group (Han, minority), education level (none/primary, secondary, tertiary), BMI (5 years ago, kg/m2), total energy intake (kJ/d (kcal/d)), smoking status (never, ever), alcohol drinking (never/seldom, often) and family history of cancer in first-degree relatives (no, yes).
Discussion
Findings from this case–control study in Northwestern China suggest that consuming a more pro-inflammatory diet, as evidenced by higher DII scores, is associated with an increased risk of oesophageal cancer. This is the first study in China to explore the association between DII scores and oesophageal cancer. Our findings are generally in line with the results obtained from a population-based case–control study from Sweden, in which the significant associations with DII were observed for oesophageal squamous cell carcinoma (ORQuartile4v.1 4·35; 95 % CI 2·24, 8·43), EAC (ORQuartile4v.1 3·59; 95 % CI 1·87, 6·89) and gastrooesophageal junctional adenocarcinoma (ORQuartile 4 v. 1 2·04; 95 % CI 1·24, 3·36). Significant trends across quartiles of DII were observed for all oesophageal cancer subtypes( Reference Lu, Shivappa and Lin 6 ). Results from the current study also are consistent with those of two previous studies undertaken in Iran( Reference Tabung, Steck and Zhang 24 ) and Italy( Reference Shivappa, Zucchetto and Serraino 31 ), both of which showed that a pro-inflammatory diet is associated with a higher risk of oesophageal squamous cell carcinoma. In addition to these studies (ORdii>1·20v.≤1·20=8·24; 95 % CI 2·03, 33·47 and ORquartile5v.1 2·46; 95 % CI 1·40, 4·36; P trend<0·001, respectively), the DII also has been shown to be associated with increased odds of reflux oesophagitis (OR 1·87; 95 % CI 0·93, 3·73), significantly increased odds of Barrett’s oesophagus (OR 2·05; 95 % CI 1·22, 3·47) and EAC (OR 2·29; 95 % CI 1·32, 3·96)( Reference Shivappa, Hebert and Anderson 34 ).
One of the possible mechanisms behind the observed positive association between the DII and oesophageal cancer may be mediated by increasing levels of pro-inflammatory cytokines such as vascular endothelial growth factor, CRP and IL-8. These cytokines form an important component of the oesophageal tumour micro-environment. As such, they play a key role in cancer development, growth and progression( Reference Liu, Li and Cui 41 ) through various processes including promoting proliferation, inducing angiogenesis and by inhibiting the recruitment of immune cells to the tumour site( Reference Bonomi, Patsias and Posner 42 ). Hypoxia, a common state of low O2 levels in inflamed tissues, causes DNA damage and induces tumorigenic factors that further result in progression of cancer( Reference Bonomi, Patsias and Posner 42 ). Inflammation also alters the extracellular matrix and provides structural support to developing tumours( Reference Bonomi, Patsias and Posner 42 ). Resveratrol, which is an important component of grapes, has been found to be a natural cyclo-oxygenase-2 (COX-2) inhibitor that is involved in the anti-inflammatory pathway( Reference Dommels, Haring and Keestra 43 ). Another phytochemical, curcumin, which is present in large quantities in turmeric, can down-regulate inflammation, and has been demonstrated to be capable of preventing activation of a pro-inflammatory chemical – NF-κB( Reference Chung, Lim and Lee 44 ). n-3 Fatty acids, which are abundant in marine fish that are fairly rarely eaten in this population, can stimulate anti-inflammatory signalling molecules that have been associated with oesophageal cancer protection( Reference Fietkau, Lewitzki and Kuhnt 45 ).
This study adds to the body of research that clearly indicates a role of diet-associated inflammation in oesophageal carcinogenesis. Unlike the effect of tobacco, which appears to be stronger for squamous cell cancers( Reference Ahsan, Neugut and Gammon 4 , Reference Kabat, Shivappa and Hebert 5 ), the effect of diet appears to affect both histopathologic subtypes( Reference Shivappa, Hebert and Anderson 34 , Reference Lin, Lagergren and Lu 46 – Reference Terry, Lagergren and Wolk 48 ). This finding is reinforced by findings from our study in Sweden( Reference Lu, Shivappa and Lin 6 ), which found nearly identical increased odds of 4·35 and 3·59, respectively, for ESCC and EAC.
Some mechanisms may manifest irrespective of specific histopathological type. For example, both CRP and certain interleukins such as IL-6 appear to affect both ESCC and EAC( Reference Groblewska, Mroczko and Sosnowska 49 ). These cytokines regulate processes associated with inflammation that occur along carcinogenic pathways that appear to be operative irrespective of histopathological subtype. Both histopathologic subtypes are well known to be associated with inflammation. The effect of chronic inflammation in the growth of ESCC is well-established( Reference Shivappa, Zucchetto and Serraino 31 , Reference Blank, Nienhuser and Dreikhausen 50 – Reference Shivappa, Hebert and Rashidkhani 52 ). Similarly, the influence of inflammation in the process of malignant transformation in the sequence from Barrett’s oesophagus to frank adenocarcinoma is particularly well defined( Reference Colleypriest, Ward and Tosh 53 – Reference Kavanagh, O’Sullivan and O’Hanlon 55 ), including the effect of oxidative stress, which is intimately associated with both diet and inflammatory response, in relation to reflux oesophagitis( Reference Song, Han and Kim 56 ).
Some exposures, such as consuming beverages at very high temperatures, will logically affect the proximal oesophagus, where most ESCC occur( Reference Maghsudlu and Farashahi Yazd 57 ). In addition, within China some putative environmental causes have been suggested to affect ESCC in particular( Reference Tang, Chen and Lin 58 ).
Certain processes are emerging for specific cancer subtypes. However, the specificity of the effect is hard to determine given the infancy of the field. For example, the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) may play a unique role in ESCC by encouraging cross talk between NF-κB and COX-2, which are well known to link inflammation to tumorigenesis. Blocking the JAK/STAT3 pathway in ESCC cell lines appears to regulate cell growth and inhibit angiogenesis( Reference Fang, Chu and Li 59 ). With the rapid rise of EAC, there also has been an interest in sorting out inflammation-related pathways for this cancer. These include local immune response, tissue micro-environment, metabolic profile, intracellular signalling and microRNA( Reference O’Sullivan, Phelan and O’Hanlon 12 ). Given the current state of knowledge, there is nothing compelling the view that these mechanisms are exclusive to a particular histopathological type of tumour. For example, although dysregulation of cytokines in ESCC is known and members of VEGF family may play an important role in ESCC( Reference Diakowska 60 ), this same process may apply to EAC. Similarly, while the effect of the diet on the microbiome has been investigated in EAC( Reference Neto, Whitaker and Pei 61 ), these effects may not be specific to histology of the cancer. However, it must be borne in mind that adenocarcinomas occur in the distal oesophagus where the influence of microbiome could be different from that in the proximal oesophagus. This potential lack of specificity may help to put in perspective the results of this Chinese study in which it is not been possible to distinguish subtype (although there are many more squamous cell cancers than adenocarcinomas in this population).
Limitations of this study include the retrospective nature of the case–control design. Although exposure information was collected with an instruction to report on exposures in the past, true temporal sequence cannot be established with certainty. This limits inferences regarding the causal relationship between pro-inflammatory diet and oesophageal cancer risk. We also cannot rule out selection bias (i.e. towards healthier cases), as patients who died from oesophageal cancer in the last 12 months were not included in the study. Nevertheless, the use of four hospitals reduced selection bias to some extent, as these hospitals serve the entire catchment region. Therefore, the participants are probably representative of the target population of Xinjiang Uyghur Autonomous Region. Although the recall of habitual food and beverage consumption should not be affected by case–control status, dietary assessment was made on the basis of self-report, which probably introduced some recall error in the response of participants, especially because the recall period of dietary intake was set at 5 years before the interview. Face-to-face interviews were thus conducted in the presence of next-of-kin to help improve the accuracy of their answers( Reference Liang, Binns and Lee 37 ). Furthermore, information bias was minimised by binding all participants to the study hypothesis; i.e. the idea that pro-inflammatory diets are associated with increased risk of oesophageal cancer in the north-west of China was not commonly held at the time of interview. Alcoholic beverage intake also represents an area of weakness as status was categorised as never/seldom v. often, instead of daily intake of alcohol. Because this variable was used in the multivariable logistic regression model, some residual confounding may remain. Finally, information on the histologic subtypes of oesophageal cancer was not available to enable subgroup analyses of oesophageal tumours. However, the vast majority of cancers in this study are, no doubt, ESCC.
Conclusion
Consumption of pro-inflammatory diets appears to be associated with an increased risk of oesophageal cancer in Northwestern China. Although further prospective cohort studies are required to confirm the findings, discouraging the intake of more pro-inflammatory foods, such as processed meat and red meat, may be a strategy to protect against oesophageal cancer in this high-risk area of China.
Acknowledgements
The authors would like to thank nurses for conducting patient interviews. The authors acknowledge patients and their families for their time and willingness to contribute to this study.
N. S. and J. R. H. were supported by grant no. R44DK103377 from the US National Institute of Diabetes and Digestive and Kidney Diseases. The funder did not have any role in the design, analysis or writing of this article.
F. X. and C. W. B. conceived and designed the study; L. T. and N. S. drafted the manuscript; N. S. calculated the DII scores; L. T. and A. H. L. performed the data analysis; and J. R. H., F. X. and C. W. B. provided suggestions and critically revised the manuscript. All the authors have read and approved the final version of the manuscript.
J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the DII from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. N. S. is an employee of CHI. The subject matter of this paper will have no direct bearing on the work of CHI, nor has any CHI-related activity exerted any influence on this project. None of the authors has any conflicts of interest to declare.







