Helicobacter pylori infection has been linked to the occurrence of several non-gastrointestinal disorders which might have health consequences in the long term( Reference Roubaud Baudron, Franceschi and Salles 1 ). Because the infection is mainly acquired during childhood and persists throughout life unless treated, its association with micronutrient deficiencies and growth impairment, along with other extra-gastric conditions, has been postulated( Reference Akcam 2 , Reference Queiroz, Rocha and Crabtree 3 ). Although several cross-sectional and cohort studies have demonstrated an association between H. pylori infection and growth retardation( Reference Richter, Richter and List 4 – Reference Mera, Bravo and Goodman 8 ), other investigations have not supported this relationship( Reference Leandro Liberato, Hernandez Galindo and Torroba Alvarez 9 – Reference Chimonas, Baggett and Parkinson 12 ). Interpretation of the results appears to be complex, because socio-economic factors, sanitary conditions, age, diet and other co-infections are all variables which are independently associated with growth variation( Reference Vilchis, Duque and Mera 6 , Reference Sood, Joshi and Akobeng 13 – Reference Quinonez, Chew and Torres 16 ). According to the guidelines of the North American and European Societies for Pediatric Gastroenterology, Hepatology and Nutrition( Reference Koletzko, Jones and Goodman 17 ) and the Canadian Helicobacter Study Group Consensus Conference( Reference Bourke, Ceponis and Chiba 18 ), there is not sufficient evidence to establish a causative role of H. pylori infection for growth retardation.
H. pylori colonisation has also been postulated to influence another aspect related to the modulation of appetite and its consequences in energy homeostasis and body weight. It has been proposed that gastric secretion of ghrelin, an orexigenic hormone produced mainly in the oxyntic mucosa of the stomach( Reference Date, Kojima and Hosoda 19 ), could be affected by H. pylori infection. A systematic review of the literature published in 2011 concluded that circulating ghrelin levels are lower in H. pylori-infected individuals( Reference Nweneka and Prentice 20 ); however, the impacts of H. pylori infection and its eradication on body weight are still controversial. Moreover, studies evaluating dietary intake in H. pylori-infected and H. pylori-uninfected individuals, particularly children, are scarce. In addition, body weight could also influence the values obtained from the 13C-urea breath test (13C-UBT) for H. pylori infection diagnosis. 13C-UBT results are usually expressed in terms of breath 13CO2 enrichment related to the baseline values (delta over baseline, DOB), and this depends not only on the quantity of exhaled 13CO2 but also on the endogenous CO2 production as well. In this sense, a certain quantity of exhaled 13CO2 could result in a higher DOB value for a person who has a lower CO2 production rate (smaller body size) as compared to a person with a higher CO2 production rate (larger body size). Alternatively, the urease activity expressed by the H. pylori burden may be independent of body size. In order to normalise these results by the endogenous CO2 production, patients' surface area and BMR should be taken into account along with their DOB values when calculating their urea hydrolysis rates (UHR). 13C-UBT has been considered a semi-quantitative method for H. pylori diagnosis because DOB values have been related to bacterial load( Reference Ellenrieder, Glasbrenner and Stoffels 21 ). It could be speculated that higher UBT results (normalised as UHR values) are related to lower ghrelin production and consequently to a lower body weight.
The present retrospective cross-sectional study aimed to evaluate the association between H. pylori infection and the dietary and anthropometric indicators of nutritional status in a paediatric population with gastrointestinal symptoms from Buenos Aires, Argentina.
Patients and methods
Patients and ethics
A total of 525 children aged 4 to 16 years who were referred to the gastroenterology unit of the Superiora Sor Maria Ludovica Children's Hospital in La Plata, Argentina, were included in the present study. The hospital is a tertiary-level health care referral institution with the highest clinical complexity for attending children in Buenos Aires province. Inclusion criteria were the presence of upper gastrointestinal signs and symptoms, such as gastroesophageal reflux, symptoms of oesophagitis, dyspepsia and abdominal pain, and exclusion criteria were the use of antibiotics or acid suppressants during the previous month.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving patients were approved by the ethics committee of the Superiora Sor Maria Ludovica Children's Hospital. Written informed consent was obtained from parents or legal guardians after they read the protocol information. Results on H. pylori infection status and the other measured variables were sent to the responsible health facilities, where children received appropriate treatment.
13C-urea breath test
The 13C-UBT is a highly accurate test, with values of sensitivity and specificity of more than 95 % for the diagnosis of H. pylori infection( Reference Leal, Flores and Fuentes-Panana 22 ). The test consisted of the following: after at least 6 h of fasting, children were instructed to exhale air into two hermetically sealed containers (Labco Limited) to determine basal 13C:12C ratios. Then, 150 ml of reconstituted, powdered, non-fatty milk containing 50 mg of 13C-urea (Cambridge Isotope Laboratories, Inc.) was taken by each patient, and two more breath samples were collected, one at 30 min and another at 45 min after the ingestion of the labelled solution. Each sample of exhaled air was measured in an isotope ratio mass spectrometer coupled to a gas chromatograph (Finnigan MAT GmbH, Thermo Fisher Scientific). A change of >3·5 ‰ in the DOB values was considered positive( Reference Leal, Flores and Fuentes-Panana 22 ). In order to normalise these results by endogenous CO2 production, the UHR was calculated( Reference Slater, Preston and Weaver 23 ) considering the DOB values of each patient and using the following formula:
VCO2 was estimated from body surface area using the Schofield equation, which provides an accurate estimate in children( Reference Rodriguez, Moreno and Sarria 24 ). Results expressed in terms of UHR could then be compared independent of age, sex or body size.
Dietary assessment
Energy and macronutrient intakes were assessed with 24 h dietary recalls administered to the parent or guardian of each child. A book of picture charts was used to aid respondents in portion size estimation( Reference Avila, Chiappe, Avila and Chiappe 25 ). Data analysis was performed using the food composition database compiled in 2007 by the Argentine Ministry of Health( 26 ). Information for two non-consecutive days of nutrient intake was obtained from a subsample of 160 children selected by systematic random sampling( 27 , Reference Hoffmann, Boeing and Dufour 28 ). Epidemiological characteristics of the subsample did not differ statistically from the main sample, which meant that it was representative of the entire cohort. Adjustment of nutrient intake was made by calculating within-person and between-person variances using variance analyses (ANOVA) according to the methodology described by the National Research Council, the US National Academy of Sciences( 27 ) and Slater et al. ( Reference Slater, Marchioni and Fisberg 29 ) in order to partially remove variation in within-person consumption and to obtain the estimated usual intake distribution for each nutrient. Transformation was applied to nutrient intakes that did not have a normal distribution in order to remove asymmetry, and data were back-transformed to the original scale. The prevalence of inadequate intake was estimated by calculating the percentage of the group with usual nutrient intake that was less than the estimated average or the energy requirements recommended by the Food and Nutrition Board of the Institute of Medicine( 30 , 31 ). Those 24 h dietary recalls in which energy intake was lower than 2092 kJ/d (500 kcal/d) or higher than 15 062·4 kJ/d (3600 kcal/d) were considered to under- or over-report energy intake, respectively, and were consequently excluded from the adjustment( Reference Willett and Willet 32 ).
Epidemiological questionnaire
An epidemiological questionnaire was administered to the parent or guardian of each child in order to obtain information about possible predictive variables for H. pylori positivity. The questionnaire was focused on ethnicity, socio-demographic factors and sanitary conditions. Unsatisfied basic needs were defined according to the guidelines of the National Institute of Statistics and Census of Argentina( 33 ), taking into account the following variables: family crowding, type of housing, type of flooring, type of toilet, source of water, education levels of the children, the head of the family and the mother and the number of family members who depend economically of the household head.
Anthropometric indicators
At enrolment, body weight and height were obtained from each of the participating children in duplicate by the same researcher. Height was recorded using a stadiometer (Stanley) to the nearest 0·1 cm, and weight was measured with a portable mechanical scale (CAM) to the nearest 100 g. Height-, weight- and BMI-for-age were expressed as Z scores, and underweight, stunting, overweight and obesity were defined relative to the age- and sex-appropriate standards in the WHO Growth Charts( 34 , Reference de Onis, Onyango and Borghi 35 ). Anthropometric techniques were previously standardised( Reference Habicht 36 ), and z scores were calculated by the Anthro and AnthroPlus programmes.
Statistical analysis
An χ2 test was used to analyse dependency between H. pylori positivity and other categorical variables. The normality of the data was assessed using the Shapiro–Wilk test. A Student's t test was used when it was proven that variances were homogeneous; if not, the nonparametric Mann–Whitney U test was applied. A logistic regression was performed to estimate the association between socio-demographic variables and H. pylori status, and crude and adjusted OR were obtained with 95 % CI. Linear regression was used to evaluate the association of H. pylori infection status with anthropometric indicators and nutrient intakes, and β coefficients were obtained with 95 % CI. Variables associated with H. pylori infection which could affect nutrient intake and anthropometric indicators were considered as confounding factors for these parameters. Significance levels were set at α < 0·05. Statistical analyses were performed using SPSS software version 17.0 (IBM SPSS).
Results
Epidemiology
The present study included 525 children (55·6 % females) with a mean age of 10·1 years (95 % CI 9·8, 10·3). The response rate was 100 %. A total of 132 patients were found to be H. pylori-positive by means of the 13C-UBT, which correlated to a prevalence of 25·1 % (95 % CI 21·5, 29·5) in this symptomatic population. All of the patients completed the epidemiological questionnaire. There were no statistically significant differences in terms of demographic variables, such as mean age (P= 0·24) and age groups (P= 0·60), sex (P= 0·12) and education level of the children (P= 0·18), between H. pylori-positive and H. pylori-negative patients (Table 1).
Table 1 Socio-demographical and sanitary data of the children included in the present study (Mean values and standard deviations; number of children; percentages)

BGE, basic general education; Great BA, Great Buenos Aires (suburban areas); BA province, Buenos Aires province (inner city); UBN, unsatisfied basic needs.
* Percentages were significantly different between the groups (P< 0·05; χ2 test).
† Percentages were significantly different between the groups (P< 0·05; Mann–Whitney U test).
‡ For children aged 6 to 15 years.
§ For children aged 15 to 18 years.
The variables which proved to be associated to H. pylori infection included ethnicity (P< 0·001), with a higher prevalence in South American Indians, place of residence (P= 0·02), a higher number of siblings (P< 0·01) and family members (P< 0·01) in the household, a lower education level of the mother and the head of the family (P< 0·01), unsatisfied basic needs (P< 0·01) (Table 1) and poorer sanitary conditions, as denoted by the type of flooring (P< 0·01) and the type of toilet (P< 0·01), where a cement or soil floor and a pit latrine toilet were associated with H. pylori infection. After adjusting for confounding factors, the remaining predictive variables for H. pylori positivity were ethnicity (OR 2·08, 95 % CI 1·09, 3·97), number of siblings (OR 1·21, 95 % CI 1·07, 1·38), education level of the mother (OR 0·53, 95 % CI 0·33, 0·86) and type of flooring (OR 1·99, 95 % CI 1·27, 3·12).
Food intake
Dietary recalls were collected from all of the participating children. We excluded from the analysis six 24 h recalls due to under-reporting and fifteen recalls due to over-reporting of energy intake. The prevalence of inadequate energy intake was 42 % in the whole population, with no significant differences according to H. pylori infection or age group (P= 0·34). Table 2 summarises the macronutrient and energy intakes according to H. pylori status in the different age groups. Energy, carbohydrate, protein and fat intakes were not associated with H. pylori infection either by age or by sex. The association between H. pylori infection status alone and infection status adjusted by confounders and nutrient intake is shown in Table 3. Fat intake was observed to be affected by H. pylori infection (P= 0·02); however, this result was no longer significant after adjustment (P= 0·08).
Table 2 Nutrient intake in the different age groups according to Helicobacter pylori infection (Mean values and standard deviations)
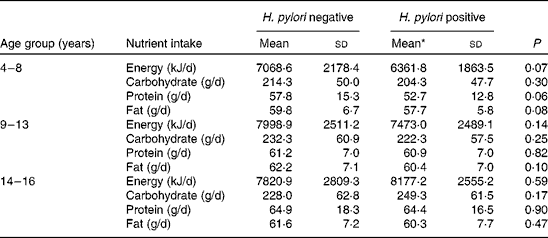
* Mean values were not significantly different from those of the H. pylori-negative group (P>0·05; Mann–Whitney U test).
Table 3 Crude and adjusted β coefficients for nutrient intake and anthropometric indicators according to Helicobacter pylori infection (β Coefficients and 95 % confidence intervals)
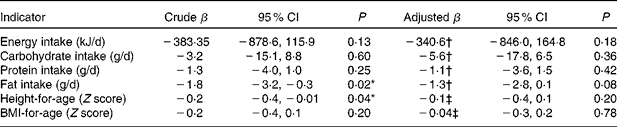
* Crude β coefficients were significantly different between the groups (P< 0·05; linear regression).
† Adjustment by number of siblings, unsatisfied basic needs, ethnicity, sex and age.
‡ Adjustment by number of siblings, unsatisfied basic needs, ethnicity and protein intake.
Anthropometric indicators
When analysing the anthropometric indicators as quantitative variables, height-for-age Z score values and BMI-for-age Z score values were lower within the infected children ( − 0·38, 95 % CI − 0·55, − 0·19 and − 0·02, 95 % CI − 0·24, − 0·20) than they were in the uninfected group ( − 0·17, 95 % CI − 0·26, − 0·06 and 0·12, 95 % CI − 0·01, 0·25). Results for the anthropometric indicators showed a significantly lower height-for-age Z score in H. pylori-infected children (Table 3), but these scores were not significant after multiple variable analyses.
Table 4 summarises the anthropometric nutritional status of the children according to H. pylori infection. The prevalence of underweight, normal weight, stunting, overweight and obesity did not differ significantly between the infected and the uninfected children.
Table 4 Anthropometric indicators of nutritional status according to Helicobacter pylori infection* (Percentages and 95 % confidence intervals)

* Percentages were not significantly different between the groups (P>0·05; χ2 test).
13C-urea breath test results and anthropometric nutritional status
13C-UBT results were normalised by endogenous CO2 production through calculation of the UHR. In the H. pylori positive group, UHR values were significantly higher in children with underweight or normal weight (P= 0·02), but in the H. pylori negative group, no significant difference in UHR could be found between children between underweight–normal weight and overweight–obesity (P= 0·66). Neither DOB nor its normalised variable UHR differed in terms of their association with the anthropometric indicators of nutritional status.
Discussion
It has been postulated that H. pylori infection could affect dietary intake; however, studies that have investigated this relationship are limited. We aimed to evaluate the association between H. pylori positivity and the dietary and anthropometric indicators of nutritional status in children with gastrointestinal symptoms. The nature of the sample, which was represented exclusively by symptomatic children, should be taken into consideration as a limitation of the present results. In addition, sources of measurement error in the 24 h dietary recall, the method we used for dietary assessment in the present epidemiological study, should also be considered; in particular, the parents or guardians of the children could have unintentionally misreported dietary intakes( Reference Willett and Willet 32 ).
We found a higher prevalence of inadequate energy intake in the present population than that reported by the Argentine National Questionnaire of Nutrition and Health( 37 ), in which 24 h dietary recalls were also used for dietary assessment. Because a lower mean energy intake has been described for dyspeptic children regardless of the presence of H. pylori infection( Reference Soylu and Ozturk 38 ), the lower intake could have been related to the presence of gastric symptoms in all of the children, representing a population bias. After a quantitative analysis of the dietary intakes in the present study, we did not find any significant differences in the mean values of the energy, carbohydrates, proteins and fat intakes between the H. pylori-infected and the H. pylori-uninfected children for any of the evaluated age groups (Table 2). However, a tendency towards lower intakes for all of the evaluated nutrients could be evidenced by the β coefficients described in Table 3. Moreover, fat intake was significantly lower in H. pylori-positive children, although this was no longer significant after adjustment for confounders. Sample size could have been another variable that limited the present results. In order to evaluate whether the observed tendency towards lower dietary intakes in H. pylori-positive children results in a significant difference, a higher number of subjects should be studied to increase the power of the study; in the present study, a 40 % power was obtained from a retrospective power calculation based on mean differences in energy intake. On the other hand, the present results showed that anthropometric indicators of nutritional status did not differ significantly between infected and uninfected children with digestive symptomatology (Table 4). Future studies should be conducted to evaluate the anthropometric indicators of nutritional status in asymptomatic children with or without the infection.
It has been postulated that values from the 13C-UBT are related to H. pylori bacterial load( Reference Ellenrieder, Glasbrenner and Stoffels 21 ). It could be speculated that higher UBT results (normalised as UHR values) may be associated with lower ghrelin production and consequently with lower body weight. In the present study, we investigated whether UHR could be associated with anthropometric indicators of nutritional status and obtained significantly higher UHR values for the H. pylori-positive children with underweight or normal weight (P= 0·02). Nevertheless, measurements of circulating ghrelin levels as well as histological affection of the gastric mucosa should be evaluated in order to confirm these results. Because the H. pylori genotype and virulence factors, such as the presence of the cag pathogenicity island, are related to the severity of gastric disease( Reference Rick, Goldman and Semino-Mora 39 ), these variables should also be evaluated.
Growth is a complex phenomenon that is influenced by multiple factors. In the present study, we did not find an association between H. pylori infection and growth-related anthropometric indicators; however, we did observe a tendency towards lower Z scores for the anthropometric indicators evaluated in the H. pylori-infected children. Moreover, significant differences that were obtained for height-for-age Z scores in the H. pylori-positive group were no longer significant after adjustment for confounders, and this is consistent with findings from other cross-sectional studies( Reference Leandro Liberato, Hernandez Galindo and Torroba Alvarez 9 – Reference Chimonas, Baggett and Parkinson 12 ). Cohort studies evaluating the effect of H. pylori on growth retardation have reported controversial results( Reference Mera, Correa and Fontham 5 , Reference Goodman, Correa and Mera 7 , Reference Chimonas, Baggett and Parkinson 12 , Reference Bravo, Mera and Reina 40 – Reference Thomas, Dale and Bunn 42 ). Although growth velocity has been found to be impaired in H. pylori-positive infants and children from Colombia and Ecuador( Reference Mera, Correa and Fontham 5 , Reference Goodman, Correa and Mera 7 , Reference Bravo, Mera and Reina 40 , Reference Egorov, Sempertegui and Estrella 41 ), reports on children from other areas have not found the same effect( Reference Chimonas, Baggett and Parkinson 12 ). Evaluation of the long-term effects on growth after H. pylori infection has been resolved suggests that the growth of children benefits when they are treated for H. pylori infection( Reference Mera, Bravo and Goodman 8 ). However, it has been reported that a BMI decrease is also related to the presence of recurrent abdominal pain associated with gastric lesions, independent of the presence of H. pylori infection( Reference Gulcan, Ozen and Karatepe 43 ).
In conclusion, predictive variables for H. pylori positivity included ethnicity, number of siblings, education level of the mother and type of flooring. H. pylori infection was not associated with dietary intake or with anthropometric indicators in children with gastrointestinal symptoms; however, an increased sample size would be needed to confirm the observed tendencies towards lower dietary intake and anthropometric indicators in H. pylori-infected children.
Acknowledgements
We thank Professor Dr Tom Preston from the Stable Isotope Biochemistry Laboratory, The University of Glasgow, for kindly reviewing the final version of the present manuscript.
The present work was supported by the University of Buenos Aires (UBA) (C. G. G., Project UBACyT 20020100100837) and the International Atomic Energy Agency (IAEA) (C. G. G., Coordinated Research Programme ARG-16746). UBA and IAEA had no role in the design, analysis or writing of the present article.
The authors' contributions are as follows: M. B. Z., J. R. B. and C. G. G. formulated the research questions and designed the study; M. A. J., P. M., E. C. R., N. B., G. Z. d. P. and M. C. conducted the research; M. A. J. and P. M. performed the statistical analysis; M. A. J., P. M. and C. G. G. wrote the article. All authors have read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.







