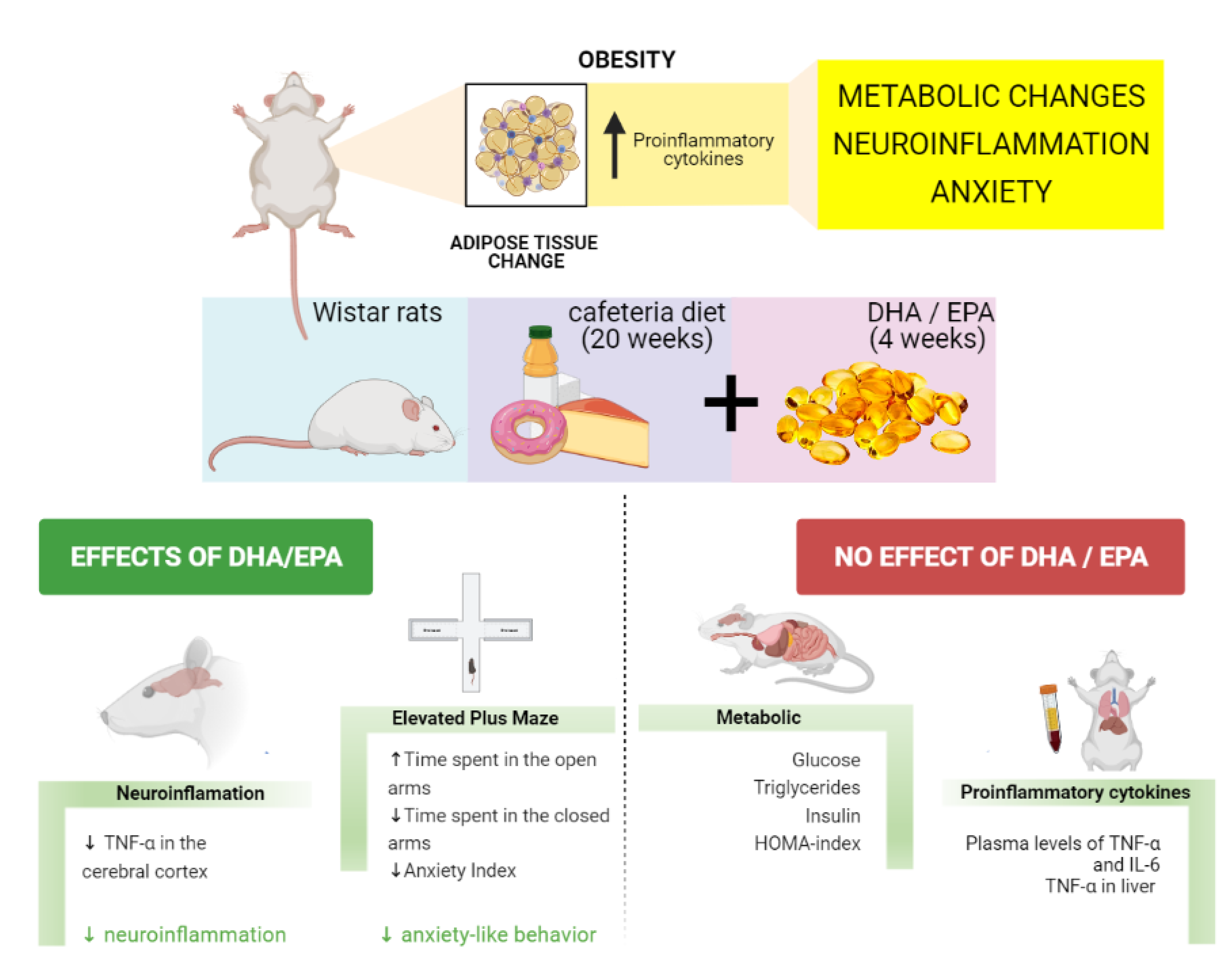
The global prevalence of obesity has risen over the years representing a major health issue nowadays(Reference Blüher1). Obesity increases the risk of developing several chronic diseases such as diabetes mellitus, cardiovascular diseases, some types of cancer and non-alcoholic fatty liver disease(Reference Chooi, Ding and Magkos2,Reference Polyzos, Kountouras and Mantzoros3) . More recently, increased pathogenicity to infectious diseases has also been linked to excessive body fat(Reference Maffetone and Laursen4), seen by higher mortality rates in patients with obesity in the COVID-19 pandemic(Reference Klang, Kassim and Soffer5,Reference Palaiodimos, Kokkinidis and Li6) .
Treatments available for obesity are not efficient for many patients since they are based on (1) lifestyle interventions, which commonly result in weight regain; (2) pharmacological treatments, but most of them are not well tolerated because of side effects; and (3) bariatric surgery, which is associated with several risks and it should be carefully recommended(Reference Blüher1). Considering the harmful effects of obesity, the search for new therapeutic strategies is urgent. In this perspective, it has been shown that the consumption of omega-3 (n-3) polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA) is associated with a significant reduction in plasma levels of triglycerides(Reference Natto, Yaghmoor and Alshaeri7,Reference Chen, Yu and Shao8) and reduced fasting glucose levels(Reference García-López, Arriaga and Nájera Medina9). In this regard, n-3 supplementation is recognised for reducing the risk of coronary heart disease mortality and related events(Reference Abdelhamid, Brown and Brainard10). Its anti-inflammatory properties are demonstrated in several animal models of inflammatory diseases(Reference Calder11). Moreover, n-3 may be related to anxiolytic and anti-depressant effects(Reference Larrieu and Layé12), although these results are controversial and need further investigations(Reference Appleton, Sallis and Perry13,Reference Deane, Jimoh and Biswas14) .
It is worth mentioning that obesity is related to a low-grade chronic inflammatory profile originating from adipose tissue’s immune signals. These signals activate the NF-κB signalling pathway in the cells, followed by an increase in the transcription of proinflammatory cytokines such as interleukin 6 (IL-6) and tumour necrosis factor-alpha (TNF-α)(Reference Zatterale, Longo and Naderi15). The increased levels of inflammatory mediators appear not only in the periphery but also in the brain, establishing a neuroinflammatory state(Reference Lainez and Coss16). Pathological brain conditions may be initiated or intensified due to neuroinflammation such as cognitive decline and neurodegenerative diseases, characterised by impaired memory and attention(Reference Kaur, Sharma and Deshmukh17,Reference Fourrier, Singhal and Baune18) , and psychiatric diseases, such as depression and anxiety(Reference Fung, Olson and Hsiao19). In this regard, it is shown that obesity and metabolic dysfunction are correlated with cognitive dysfunction(Reference Farruggia and Small20) and anxiety symptoms(Reference Amiri and Behnezhad21).
Among different protocols to induce obesity in animal models, the cafeteria diet (CAF) is beneficial since it mimics the human population’s Western diet. CAF is composed of ultra-processed foods that are characterised by their high palatability. Therefore, it can lead to a hyperphagia state similar to the human pattern of food consumption(Reference Leigh, Kendig and Morris22). Another advantage of animal studies using CAF is the presence of several food additives and the lack of vitamins and minerals(Reference Bortolin, Vargas and Gasparotto23). Consequently, this diet induces metabolic changes related to obesity such as hepatic steatosis, increased visceral adiposity, glucose intolerance(Reference Maeda Júnior, Constantin and Utsunomiya24) and is also capable of inducing neuroinflammation in regions such as the hippocampus(Reference Gomez-Smith, Karthikeyan and Jeffers25), which is essential for memory consolidation and neurogenesis(Reference Anacker and Hen26).
Although DHA/EPA benefits in metabolic dysfunction are largely studied, there are still controversial findings. In addition, DHA/EPA effects on the brain are also debated. To elucidate the beneficial properties of DHA/EPA in the management of obesity and obesity-related neurological outcomes, we evaluated whether 4 weeks of DHA/EPA supplementation were able to change metabolic and neuroinflammatory parameters and anxiety-like behaviour in rats fed with CAF.
Materials and methods
Animals
Forty-eight adult male Wistar rats were obtained from the animal facility of the Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA) and housed in plastic cages at 23 (sd 1)°C with a 12/12 h of light/dark cycle. This project was approved by the Institutional Animal Care and Use Committee of UFCSPA under the protocol Nº. 570/18. The procedures were designed to minimise the number and the suffering of animals following the international laws for laboratory animals’ care. Only male rats were employed to avoid female hormonal fluctuations, which may impact the results of this study.
Experimental groups and diet
Animals (n=12/group) were randomly allocated into four groups: Control diet (CT); CT + DHA/EPA; CAF; and CAF + DHA/EPA. Animals received normal chow diet Nuvilab® CR-1 (Nuvital®) or normal chow diet plus CAF for 20 weeks with DHA/EPA supplementation starting at the 16th week. Groups supplemented with DHA/EPA received Mega DHA® (Vitafor®) in a high concentration of EPA (10 %) and DHA (50 %) at the dose of 500 mg/kg every day by gavage. The other groups received water (0.5 ml/rat) instead of DHA/EPA. The sample size was chosen based on previous studies using a similar methodology(Reference Sampey, Vanhoose and Winfield27,Reference Demers, Roy and Machuca-Parra28) .
Cafeteria groups were fed with three distinct menus interchanged every 2 d to maintain novelty and stimulate consumption. Menus were composed of standard chow plus palatable human food as cookies, wafer, sausage, bologna and white chocolate. They also received orange-based soft drink besides water ad libitum. Every component’s leftovers were weighed every 2 d, including soft-drink consumption, to determine food intake per cage.
The regular chow diet’s total energy content was 14·22 kJ/g (3·4 kcal/g, 63 % carbohydrates, 26 % protein, 11 % fat). The CAF energy content was calculated based on the manufacturer’s information and provided 18·8 kJ/g (4·5 kcal/g) distributed in 42 % carbohydrates, 16 % protein and 42 % lipids. Detailed nutritional information on the components of the diet is shown in Table 1. Animals were weighed weekly to determine weight gain.
Table 1. Nutritional information of cafeteria diet*

* Values obtained from food labels considering a portion of 100 g of food or 100 ml of soft drink.
Elevated plus maze test
Elevated plus maze was used to assess anxiety-like behaviour in rats. The test was conducted on the 20th week of the experiment and was applied as described by ref.(Reference Serova, Tillinger and Alaluf29). The apparatus consisted of four arms 50 cm long and 10 cm wide. Two of those are open, and the other two arms are closed with walls (40 cm high) on the sides. The maze was 50 cm above the floor. The rats were placed on the central platform facing an open arm and freely explored the maze for 5 min. The entrance was defined when the four paws touched one of the arms. More time spent in the closed arm and a decrease in risk behaviour (head dipping) could indicate anxious-like behaviour(Reference Ruthschilling, Albiero and Lazzari30,Reference Martins De Almeida, Giovenardi and Charchat31) .
The following parameters were analysed in the test: number of head dipping, the number of entries in the open and the closed arms, total time spent in the open and closed arms. Subsequently, it was measured the percentage of open arm entries (100 × open arms entries/total arms entries; PEOA) and the percentage of time spent in the open arm (100 × time in the open arms/total time; PTOA)(Reference Ruthschilling, Albiero and Lazzari30,Reference Martins De Almeida, Giovenardi and Charchat31) . Also, the anxiety index was calculated using the formula: anxiety index = 1 – ((open arm time/5 min) + (open arm entry/total entry))/2(Reference Huynh, Krigbaum and Hanna32). Results vary from 0 to 1; values closer to 1 indicate high anxiety.
Tissue and blood collection
In the 20th week of the experiment, the rats were euthanised by decapitation after fasting for 6 h. Trunk blood was collected and, after 30 min, it was centrifuged at 3500 rpm for 10 min. Plasma obtained was aliquoted and stored at −80°C for further analysis. Brains were quickly removed. The prefrontal cortex was manually dissected and immediately frozen in liquid nitrogen. Samples were kept at −80°C until further processing.
Plasma metabolic parameters
Plasma levels of glucose and triglycerides were measured using commercial enzymatic colourimetric kits (Labtest©). Insulin levels were assessed by ELISA (Sigma©). Tests were conducted following the manufacturer’s instructions.
Insulin resistance-homeostasis model assessment index
Insulin resistance-homeostasis model assessment (IR-HOMA) index was used to evaluate insulin resistance(Reference Matthews, Hosker and Rudenski33). The IR-HOMA index was calculated using the formula: fasting blood glucose levels × fasting blood insulin levels/22.5(Reference Salgado, De Carvalho and Oliveira34).
Pro-inflammatory cytokines
Levels of inflammatory cytokines in plasma (IL-6 and TNF-α), liver (IL-6 and TNF-α) and prefrontal cortex (TNF-α) were quantified by ELISA (Invitrogen©), following the manufacturer’s instructions.
Statistical analysis
Main effects and interactions were analysed with two-way ANOVA and differences between groups with Tukey post-hoc test. The results of two-way ANOVA are shown as the main effects (diet or DHA/EPA supplementation) and the interaction between diet and supplementation. Weight gain per week was analysed by two-way repeated-measures ANOVA. Rout test was used to remove outliers. All statistical analysis was conducted using Graphpad Prism 8 (GraphPad Software, Inc.). The results were expressed as mean values with their standard error of the mean. The results were considered statistically significant at P < 0.05.
Results
Weight gain, adiposity and metabolic profile
Weight gain was significantly increased in CAF groups (diet effect: F(1,42) = 114.2, P < 0.0001) regardless of DHA/EPA supplementation (DHA/EPA effect: F(1,42) = 3.92, P = 0.054; interaction: F(1,42) = 0.05, P = 0.81), as shown in Fig. 1(a). Weight gain was significantly different between CAF and CT groups from the 8th week onwards (Fig. 1(b)). Similarly, the visceral fat deposition was higher in CAF-fed groups (diet effect: F(1,42) = 140.3, P < 0.0001) with no effect of DHA/EPA (DHA/EPA effect: F(1,42) = 1.72, P = 0.19; interaction: F(1,42) = 0.07, P = 0.78) as demonstrated in Fig. 1(c).

Fig. 1. DHA/EPA supplementation did not change weight gain or visceral adiposity in cafeteria diet (CAF)-fed rats. (a) Weight gain at the end of the experiment. (b) Body weight over time. (c) Visceral fat mass at the end of the experiment. Arrow indicates the beginning of DHA/EPA supplementation. Significant differences showed by two-way ANOVA regarding effects of diet (CAF and CAF + DHA/EPA v. CT and CT + DHA/EPA), EPA/DHA treatment (CAF and CT v. CAF + DHA/EPA and CT + DHA/EPA) and diet × DHA/EPA interactions are indicated in the text boxes. Multiple comparisons were performed by Tukey post-hoc test and are indicated as follows: *P < 0.0001 comparing CAF v. CT or CT + DHA/EPA; CAF + DHA/EPA v. CT or CT + DHA/EPA. n=11–12 animals/group.
Metabolic effects of CAF and DHA/EPA were analysed by measuring plasma levels of glucose, triglycerides, insulin and HOMA-index calculation. A diet effect (F(1,35) = 26.75, P < 0.0001) was found in plasma glucose, which was increased in CAF groups. Two-way ANOVA did not show a significant effect of DHA/EPA supplementation (DHA/EPA effect: F(1,35) = 2.6, P = 0.11; interaction: F(1,35) = 1.8, P = 0.18). In the multiple comparisons, the CAF group was significantly different from the CT and CT + DHA/EPA groups (P < 0.001) (Fig. 2(a)). Also, CAF increased triglycerides levels (diet effect: F(1,41) = 45.39, P < 0.0001) and DHA/EPA supplementation did not mitigate it (DHA/EPA effect: F(1,41) = 2.35, P = 0.13; interaction: F(1,41) = 0.35, P = 0.55) (Fig. 2(b)). Following that, insulin levels were also affected by the diet (F(1,38) = 12.62, P = 0.001) but not by the DHA/EPA treatment (DHA/EPA effect: F(1,38) = 1.25, P = 0.27; interaction: F(1,38) = 0.10, P = 0.75) (Fig. 2(c)). Insulin resistance, assessed by the HOMA-index, also showed a CAF effect (F(1,38) = 12.28, P = 0.0012), although the supplementation did not impact it (DHA/EPA effect: F(1,38) = 0.49, P = 0.48; interaction: F(1,38) = 0.0009, P = 0.97). However, Tukey post-hoc test showed a significant difference between CAF and CT + DHA/EPA group (P < 0.05) for insulin levels and HOMA-index (Fig. 2(d)).

Fig. 2. DHA/EPA supplementation did not change glycaemic control and triglycerides levels following cafeteria diet (CAF). (a) Fasting plasma glucose. (b) Triglycerides levels. (c) Plasma insulin levels. (d) HOMA-Index. Significant differences showed by two-way ANOVA regarding effects of diet (CAF and CAF + DHA/EPA v. CT and CT + DHA/EPA), EPA/DHA treatment (CAF and CT v. CAF + DHA/EPA & CT + DHA/EPA) and diet × DHA/EPA interactions are indicated in the text boxes. Multiple comparisons were performed by Tukey post-hoc test and are indicated as follows: glucose: *P < 0.001 comparing CAF v. CT or CT + DHA/EPA; triglycerides: *P < 0.01 comparing CAF v. CT or CT + DHA/EPA; CAF + DHA/EPA v. CT or CT + DHA/EPA; insulin, HOMA: # P < 0.05 comparing CAF v. CT + DHA/EPA. n=9–12 animals/group.
Inflammatory markers
We also evaluated the levels of inflammatory cytokines TNF-α and IL-6 in plasma and liver, and TNF-α in the prefrontal cerebral cortex. Plasma TNF-α (diet effect: F(1,25) = 0.014, P = 0.9; DHA/EPA effect: F(1,25) = 1.21, P = 0.28; interaction: F(1,25) = 0.14, P = 0.70) and IL-6 (diet effect: F(1,22) = 0.22, P = 0.63; DHA/EPA effect: F(1,22) = 0.75, P = 0.39; interaction: F(1,22) = 0.69, P = 0.41) were affected neither by diet nor by supplementation (Fig. 3(a) and (b), respectively). However, in the liver, TNF-α levels were increased following CAF (diet effect: F(1,24) = 31.55, P < 0.0001), with no effect of DHA/EPA (DHA/EPA effect: F(1,24) = 0.19, P = 0.66; interaction: F(1,24) = 0.93, P = 0.34), as shown in Fig. 3(c). Interestingly, IL-6 in the liver was not affected by diet (diet effect: F(1,21) = 0.02, P = 0.88; interaction: F(1,21) = 0.50, P = 0.48), but DHA/EPA supplementation diminished it in both CT and CAF groups (DHA/EPA effect: F(1,21) = 6.003, P = 0.0231), although post-hoc analysis did not show differences among groups (Fig. 3(d)). In the prefrontal cerebral cortex, TNF-α level showed an interaction between diet and supplementation (F (1,19) = 6.121, P = 0.023; diet effect: F(1,19) = 0.74, P = 0.39; DHA/EPA effect: F(1,19) = 2.56, P = 0.12). Post-hoc test also evidenced that DHA/EPA supplementation decreased TNF-α in the CAF + DHA/EPA compared with CAF (Fig. 3(e)), showing the ability of n-3 in reducing this pro-inflammatory cytokine in the cerebral cortex of obese animals.

Fig. 3. DHA/EPA supplementation did not modify TNF-α and IL-6 levels in the plasma and liver, but it reduced TNF-α in the prefrontal cortex of cafeteria diet (CAF)-fed rats. (a) Plasma levels of TNF-α. (b) Plasma levels of IL-6. (c) TNF-α levels in the liver. (d) IL-6 levels in the liver. (e) TNF-α levels in the prefrontal cerebral cortex. Significant differences showed by two-way ANOVA regarding effects of diet (CAF and CAF + DHA/EPA v. CT and CT + DHA/EPA), DHA/EPA treatment (CAF and CT v. CAF + DHA/EPA and CT + DHA/EPA) and diet × DHA/EPA interactions are indicated in the text boxes. Multiple comparisons were performed by Tukey post-hoc test and are indicated as follows: TNF-α liver: *P < 0.0001 comparing CAF v. CT or CT + DHA/EPA; CAF + DHA/EPA v. CT or CT + DHA/EPA. TNF-α cortex: *P < 0.05 comparing CAF v. CAF + DHA/EPA. n=5–8 animals/group.
Evaluation of anxiety-like behaviour
Figure 4 shows results regarding the elevated plus maze task, which was chosen to evaluate anxiety-like behaviour. The number of head dipping (NHD), which assesses risk behaviour, was measured every time the rats inclined the head towards the lower arm; its increase is related to an anxiolytic manifestation(Reference Martins De Almeida, Giovenardi and Charchat31). Regarding NHD, we found a significant interaction between diet and supplementation (interaction: F(1,33) = 5.544, P = 0.024; diet effect: F(1,33) = 0.2620, P = 0.61; DHA/EPA effect: F(1,33) = 1.316, P = 0.25), although Tukey post-hoc test did not show any difference among groups (Fig. 4(a)). Considering the number of entries in the open arms (NEOA, Fig. 4(b)) and the number of entries in the closed arms (NECA, Fig. 4(c)), there was a diet effect in both parameters, showing that rats fed with CAF had a reduced number of entries (NEOA – diet effect: F(1,29) = 6.96, P = 0.013; interaction: F(1,29) = 0.83, P = 0.36; DHA/EPA effect: F(1,29) = 0.83, P = 0.36; NECA – diet effect: F(1,32) = 6.13, P = 0.018; interaction: F(1,32) = 0.32, P = 0.57; DHA/EPA effect: F(1,32) = 0.32, P = 0.57). Furthermore, considering the percentage of entries in the open arms (PEOA, Fig. 4(d)), we found a significant interaction between diet and supplementation (interaction: F(1,32) = 6.27, P = 0.017; diet effect: F(1,32) = 0.63, P = 0.43; DHA/EPA effect: F(1,32) = 0.004, P = 0.94), although Tukey post-hoc did not show differences. Another analysis we conducted was the time spent in the open arms (TSOA, Fig. 4(e)) and time spent in the closed arms (TSCA, Fig. 4(f)), which were altered by DHA/EPA supplementation (TSOA – DHA/EPA effect: F(1,33) = 6.51, P = 0.015; interaction: F(1,33) = 3.89, P = 0.056; diet effect: F(1,33) = 0.15, P = 0.69; TSCA – DHA/EPA effect: F(1,33) = 6.51, P = 0.015; interaction: F(1,33) = 3.89, P = 0.056; diet effect: F(1,33) = 0.15, P = 0.69). Also, Tukey post-hoc test indicated that CAF-fed animals showed a significant reduction in TSOA (P = 0.013) and, consequently, increased TSCA (P = 0.013) in comparison with the CAF + DHA/EPA group. These findings point to a decrease in anxiety-like behaviour in obese rats supplemented with DHA/EPA since higher time spent in the open arms indicates reduced anxiety-like behaviour. These results were also seen in the percentage of time in the open arms (PTOA, Fig. 4(g)), which, besides the effect of DHA/EPA, we also found an interaction between diet and supplementation (DHA/EPA effect: F(1,34) = 5.52, P = 0.024; interaction: F(1,34) = 4.90, P = 0.033; diet effect: F(1,34) = 0.031, P = 0.86). These findings were corroborated by the post-hoc test, which demonstrated a significant reduction of PTOA in the CAF group compared with the CAF + DHA/EPA group (P = 0.014). In addition, the anxiety index was calculated (Fig. 4(h)). Two-way ANOVA showed an effect of DHA/EPA supplementation (DHA/EPA effect: F(1,32) = 12.59, P = 0.0012; interaction: F(1,32) = 1.26, P = 0.26; diet effect: F(1,32) = 0.96, P = 0.33), and post-hoc test showed a higher score in CAF compared with the CAF + DHA/EPA (P = 0.0088) and CT + DHA/EPA (P = 0.0238) groups, showing a higher anxiety level in obese rats. Taken together, these results corroborate the effect of DHA/EPA in reducing anxiety-like behaviour.

Fig. 4. DHA/EPA decreased anxiety-like behaviour in cafeteria diet (CAF)-fed rats. (a) Number of head dipping. (b) Number of entries in the open arms (NEOA). (c) Number of entries in the closed arms (NECA). (d) Percentage of entries in the open arms (PEOA). (e) Time spent in the open arms (TSOA). (f) Time spent in the closed arms (TSCA). (g) Percentage of time in the open arms (PTOA). (h) Anxiety index. Significant differences showed by two-way ANOVA regarding effects of diet (CAF and CAF + DHA/EPA v. CT and CT + DHA/EPA), DHA/EPA treatment (CAF and CT v. CAF + DHA/EPA and CT + DHA/EPA) and diet × DHA/EPA interactions are indicated in the text boxes. Multiple comparisons were performed by Tukey post-hoc test and are indicated as follows: CT, control group. TSOA, TSCA, PTOA: *P < 0.05 comparing CAF v. CAF + DHA/EPA; anxiety index: *P < 0.05 comparing CAF v. CT + DHA/EPA, **P < 0.01 comparing CAF v. CAF + DHA/EPA. n=7–10 animals/group.
Discussion
The effects of n-3 PUFA in brain function have been extensively studied(Reference Ahmmed, Ahmmed and Tian35,Reference Layé, Nadjar and Joffre36) ; however, the results are controversial. Here, we showed an anti-inflammatory effect of DHA/EPA supplementation in the brain in an experimental model of obesity, the CAF. In addition, we showed the ability of DHA/EPA in decreasing anxiety-like behaviour in obese rats. Otherwise, despite the evidence showing the role of n-3 in the improvement of metabolic profile(Reference Hossain, Kendig and Wild37,Reference Martín-González, Palacios and Rodríguez38) , we found that DHA/EPA was not able to revert weight gain, adiposity, lipidic profile and hepatic levels of TNF-α in CAF-fed rats. Thus, using CAF, a highly obesogenic diet, we are showing for the first time that DHA/EPA beneficial effects might depend on the severity of the obesity. On the other hand, DHA/EPA still provides neuroprotection, irrespective of the metabolic dysfunction.
DHA/EPA does not ameliorate metabolic dysfunction and inflammation in severe obesity
A CAF leads to a more pronounced obesity phenotype in rodents than other diet protocols such as a high-fat diet (HFD)(Reference Sampey, Vanhoose and Winfield27,Reference Buyukdere, Gulec and Akyol39,Reference Zeeni, Dagher-Hamalian and Dimassi40) . It has been shown that CAF is more efficient in inducing hyperglycaemia, glucose intolerance and insulin resistance compared with HFD(Reference Sampey, Vanhoose and Winfield27,Reference Higa, Spinola and Fonseca-Alaniz41) . We tested whether DHA/EPA would abrogate the metabolic disruption triggered by CAF. Our study demonstrated that 20 weeks of CAF increased weight gain, adiposity, blood glucose, triglycerides and insulin levels, and insulin resistance, providing an efficient model to mimic Western diet-associated obesity in Wistar rats. However, 4 weeks of DHA/EPA administration (500 mg/kg) did not ameliorate any of these parameters. In a previous study, 4 weeks of n-3 supplementation (400 mg/kg) also failed to decrease weight gain after 6 weeks of HFD in mice, but it reduced adipose tissue storage(Reference de Mello, de Bona Schraiber and de Souza Goldim42). Although it was an interesting finding, 6 weeks of HFD may be a short period to investigate chronic manifestations of obesity. In another study, 16 weeks of HFD lead to increased weight and adipose tissue deposition with no effect of fish oil supplementation at a dose of 0.7 mg/kg for 5 weeks(Reference Demers, Roy and Machuca-Parra28). Thus, results about the effects of DHA/EPA supplementation are controversial. However, we can suppose that in cases of more severe obesity, it may not be sufficient to revert metabolic dysregulation, as shown in the present study.
Most of the studies about the effects of PUFA in metabolism are conducted in males. When sexual differences were investigated following CAF and a HFD, no differences were found in fatty acid metabolism regarding sex, including depletion of EPA content in male and female rats(Reference Mašek, Barišić and Micek43). In addition, the effects of DHA/EPA supplementation have already been evidenced in female rats in different experimental models. In female rats with polycystic ovary syndrome, DHA/EPA reduced levels of triglycerides, insulin, blood glucose and weight gain(Reference Komal, Khan and Imran44). The present study used only male rats, but it would be important to include females to evaluate differential responses to DHA/EPA in obesity.
In obesity, the accumulation and expansion of adipocytes lead to increased inflammatory cytokine secretion, promoting a sustained inflammatory state related to the onset of other chronic diseases(Reference Karczewski, Śledzińska and Baturo45). Here, we assessed the systemic inflammation caused by CAF by measuring IL-6 and TNF-α levels in the plasma and liver. Although we did not find diet or supplementation effects on circulating levels of IL-6 and TNF-α, evidence has shown that DHA/EPA may play an anti-inflammatory role in the periphery. Souza et al. showed that 8 weeks of n-3 supplementation decreased IL-6 and TNF-α levels after HFD(Reference de Souza, da Silva Pieri and Comim46). Also, Candido et al. showed a protective effect of n-3 in decreasing IL-6 in rats fed with HFD after 2 months of supplementation(Reference Candido, Figueiredo and Silva47). In the liver, obesity increases inflammatory markers, driving hepatic dysfunction such as non-alcoholic fatty liver disease and hepatocellular carcinoma(Reference Park, Lee and Yu48). In the present study, we found increased hepatic levels of TNF-α after CAF, which was already expected. However, we did not find the same increase in IL-6 levels. Nonetheless, in obesity, the increase in TNF-α leads to the production of IL-6 in the liver(Reference Park, Lee and Yu48), suggesting that this could be a subsequent event as the disease develops. Importantly, we found a statistically significant effect of DHA/EPA in reducing IL-6 hepatic basal levels, although it could not mitigate the TNF-α increase. Worthwhile to note, we started supplementation after 16 weeks of CAF, while most studies start this intervention simultaneously with the diet protocol. Thus, we may speculate that the time point for the beginning of the supplementation may be a determining factor in the protective effect of n-3, at least in the liver. This idea is corroborated by a study in which supplementation of n-3 decreased TNF-α levels, but, in this case, the supplementation started concomitantly with the high-fat and high-sucrose diet(Reference Méndez, Muñoz and Miralles-Pérez49). Lionetti et al. also found lower levels of TNF-α after 6 weeks of HFD rich in fish oil, demonstrating a protective effect of n-3 in the liver(Reference Lionetti, Mollica and Donizzetti50). Although previous studies demonstrate a protective role of n-3 on hepatic TNF-α levels, it seems that the beneficial effect may not be seen under chronic conditions. Regarding the effects of n-3 in reducing hepatic IL-6, Schmöcker et al. found a significant decrease in IL-6 mRNA in mice treated with n-3 in a model of hepatitis, contributing to the reduction of inflammation(Reference Schmöcker, Weylandt and Kahlke51). It was already demonstrated that EPA and DHA interact with peroxisome proliferator-activated receptor alpha (PPAR-α), preventing NF-κB activation(Reference Zúñiga, Cancino and Medina52). Nevertheless, it is suggested that the anti-inflammatory properties of n-3 may depend on the pathogenesis and the strength of the inflammation(Reference Inoue-Yamauchi, Itagaki and Oda53). Thus, our protocol of CAF may have elicited such intense liver inflammation that DHA/EPA could not completely reverse.
There is a close relationship between systemic inflammation triggered by obesity and neuroinflammation(Reference Williams54,Reference Grillo, Woodruff and Macht55) . In the neuroinflammatory process, microglia and astrocytes carry out an inflammatory response that can result in psychiatric manifestations(Reference Noronha, Lima and Campos56,Reference Moazzami, Lima and Sullivan57) . Here, we showed a decrease in TNF-α levels in the prefrontal cortex of CAF-fed rats supplemented with DHA/EPA compared with the CAF group with no supplementation, suggesting a neuroprotective effect of DHA/EPA. In a previous study, we demonstrated that DHA/EPA supplementation decreased IL-6 and TNF-α in the prefrontal cortex of obese rats after HFD(Reference de Andrade, da Cruz Fernandes and de Fraga58). The anti-inflammatory effect of DHA/EPA in the central nervous system may be mediated by inhibiting the activation of intracellular phospholipase A2, an enzyme that cleaves plasma membrane phospholipids to become available for metabolism to lipid mediators(Reference Smesny, Milleit and Hipler59). In this context, these PUFA can also compete with arachidonate for the enzymes 5-LOX and COX2, blocking the formation of pro-inflammatory products(Reference Fischer and Weber60,Reference Serhan, Clish and Brannon61) . Moreover, in an in vitro study, DHA administration to hypothalamic neurons reduced NF-κB pathway activation and TNF-α production upon inflammatory challenge in a G protein-coupled receptor 120 (GPR120)-dependent way(Reference Wellhauser and Belsham62).
DHA/EPA influences brain function in obesity by decreasing anxiety-like behaviours
Obesity is considered a risk factor for anxiety disorders, but the pathophysiological mechanisms linking these conditions are still unclear. There is evidence showing a stronger association between severe obesity (defined as a BMI ≥ 35) and anxiety disorders compared with moderate obesity (BMI 30–35)(Reference Zhao, Ford and Dhingra63,Reference Becker, Margraf and Türke64) . Besides, chronic conditions related to obesity can increase anxiety risk(Reference Vink, Aartsen and Schoevers65,Reference Sareen, Cox and Clara66) . On the other hand, anxiety disorders may lead to weight gain by deregulation of the hypothalamic–pituitary–adrenal axis that alters the appetite and leads to subsequent weight gain in stressed individuals(Reference Torres and Nowson67,Reference Dallman, Pecoraro and La Fleur68) . The symptoms of anxiety disorders can increase appetite and stimulate the desire for foods rich in sugar and fat(Reference Torres and Nowson67,Reference Canetti, Bachar and Berry69,Reference Nieuwenhuizen and Rutters70) . To better clarify the relationship between obesity and anxiety, Gariepy et al. performed a systematic review with meta-analysis, including sixteen studies with a total of 346289 individuals, suggesting (moderate evidence) that obesity is positively associated with anxiety disorders in adults(Reference Gariepy, Nitka and Schmitz71).
Since neuroinflammatory processes are directly linked to the aetiology of behavioural disorders, n-3 supplementation benefits emotional states through its anti-inflammatory actions in the central nervous system(Reference Capuron and Miller72,Reference Rosenblat, Cha and Mansur73) . It was shown elsewhere that CAF drives an anxiety-like behaviour(Reference Ferreira, Castro and Andrade74), while n-3 may be efficient to improve mood(Reference Robinson, Gallego and John75) and attenuate anxiety symptoms in humans(Reference Kiecolt-Glaser, Belury and Andridge76). Studies also demonstrated that an adequate supply of n-3 could protect against cognitive decline and neurodegenerative diseases development by supporting adequate synaptic function and plasticity(Reference Freitas, Ferreira and Trevenzoli77,Reference Denis, Potier and Vancassel78) . These findings are in agreement with our results, which also show a neuroprotective DHA/EPA effect in CAF-fed rats. In female rats, n-3 fatty acids also showed an anxiolytic effect(Reference Pusceddu, Kelly and Ariffin79,Reference Borsonelo, Vieira and Galduróz80) . However, to our knowledge, no study addressed the behavioural effects of DHA/EPA in obese female animals, which would be essential to investigate.
Here, we assessed anxiety-like behaviour using the elevated plus maze test. The consumption of a CAF for 20 weeks was sufficient to trigger anxiety-like behaviour in our study. CAF-fed rats supplemented with DHA/EPA showed a consistent reduction of anxiety parameters evaluated in the elevated plus maze test. It was shown elsewhere that chronic activation of GPR120 through an intracerebroventricular infusion of its agonist had an anxiolytic effect but failed to affect energy balance in HFD mice(Reference Auguste, Fisette and Fernandes81). n-3 fatty acids are also GPR120 ligands and therefore were demonstrated to inhibit cytokine production in cultured neurons upon inflammation, evidencing its anti-inflammatory properties regarding central nervous system cells(Reference Wellhauser and Belsham62). In the HFD model, a study found that fish oil enriched with DHA/EPA (0.7 mg/kg) could protect against behaviour abnormalities, glial activation and increased neuroinflammation compared with the non-treated obese counterparts(Reference Demers, Roy and Machuca-Parra28). In agreement with our current data, Demers et al. also demonstrated that n-3 could improve behaviour even without changing body weight(Reference Demers, Roy and Machuca-Parra28). However, we could speculate that although supplemented rats in our study did not benefit from significant metabolic improvements, DHA/EPA was efficient to protect obese rats against the anxiogenic manifestations observed in the obese non-treated group. This is a relevant finding considering the potent effect of CAF in inducing obesity, and even in this condition, DHA/EPA was able to improve the behaviour. Since psychiatric symptoms such as anxiety are prevalent in patients with obesity, n-3 may be indicated as a strategy for this population. It is worth mentioning that n-3 supplementation is a low-cost treatment, and it does not have any side effects, reinforcing the importance of its usage.
Limitations and conclusions
Here we showed that DHA/EPA supplementation decreased anxiety-like behaviour in a preclinical model of obesity-induced after a CAF. It is worth mentioning that the dose of 500 mg/d used in rats in the present study is higher than n-3 consumed by humans, even by supplementation. In human studies, the dose of DHA/EPA ranges from 1 to 4 g/d(Reference Watanabe and Tatsuno82). Thus, it can be a limitation to translate our findings to the human population. However, our findings are important to highlight the potential of these PUFA in exerting neuroprotection. Based on these beneficial results, more studies should be encouraged to develop new formulations of DHA/EPA with alternative routes to facilitate brain delivery, such as intranasal. On the other hand, the absence of robust metabolic effects indicates that DHA/EPA supplementation might not be a useful treatment in severe obesity, which was induced in the present study by CAF. Thus, DHA/EPA supplementation may exert an important neuroprotective effect in obesity, but further investigations are needed to elucidate effective doses of DHA/EPA.
Financial Support
This work was supported by the Research Support Foundation of Rio Grande do Sul (FAPERGS), the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Authors Contributions
JN and JJ, investigation, formal analysis, writing - original draft; SO, MFB, LFSC and BFD, investigation; JCFM, MG and MP, formal anlaysis, writing - review & editing; RPG, conceptualization, funding acquisition, supervision, writing - review & editing. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.