Infants born preterm (<37 weeks’ gestation), particularly those born <29 weeks’ gestation, are known to be at higher risk of neurodevelopmental impairments compared with infants born at term(Reference Anderson and Doyle1-Reference Wilson-Ching, Molloy and Anderson3). Higher-order cognitive skills are governed by the frontal lobes(Reference Bell and Richard4) and appear to be particularly impaired in this population(Reference Wilson-Ching, Molloy and Anderson3,Reference Anderson and Doyle5) . Attention is one of the higher-order cognitive skills that is assessable in infancy and early childhood(Reference Colombo, Kannass and Shaddy6). Measures of attention in infancy have been associated with later outcomes of traditional psychometric assessments of intelligence and language(Reference Bornstein and Sigman7,Reference Colombo8) .
DHA is an n-3 long-chain PUFA (LCPUFA) that is thought to be essential for the development of the fetal and infant brain(Reference Innis9). DHA accretion into the brain occurs at the highest rate during the last trimester of pregnancy(Reference Clandinin, Chappell and Leong10), at a time of peak brain growth, particularly of the frontal lobes(Reference Volpe and Volpe11). This region of the brain is responsible for higher-order cognitive skills including attention(Reference Bell and Richard4). Infants born preterm miss out on this critical period of in utero accretion, and while they receive some DHA from breast milk and/or preterm infant formula, the amount is thought to be insufficient to match the in utero DHA accretion rates(Reference Lapillonne, Groh-Wargo and Gonzalez12). Animal studies of severe n-3 fatty acid deprivation during pregnancy have demonstrated reduced concentration of brain (particularly frontal lobe) DHA and deficits in offspring abilities that reflect the functioning of the frontal lobes(Reference Moriguchi, Greiner and Salem13-Reference Champoux, Hibbeln and Shannon15).
DHA deficit in infants born preterm is therefore hypothesised to contribute to the poorer neurodevelopmental outcomes common in this population, although evidence from DHA supplementation randomised controlled trials (RCTs) has been mixed. Early RCTs of DHA supplementation in stable formula-fed preterm infants compared infants fed formula with added DHA to infants fed formula devoid of DHA(Reference Moon, Rao and Schulzke16). Visual development showed at least some improvement in infants fed formula with added DHA(Reference Birch, Birch and Hoffman17-Reference O’Connor, Hall and Adamkin20) following which, DHA was added to all commercially available preterm formula. However, no clear benefits to neurodevelopment were shown(Reference Moon, Rao and Schulzke16,Reference Smithers, Gibson and McPhee21) . Subsequent RCTs were inclusive of breast milk feeding and preterm infants representative of the usual clinical profile and compared standard DHA breast milk and/or formula with higher DHA breast milk and/or formula(Reference Makrides, Gibson and McPhee22,Reference Henriksen, Haugholt and Lindgren23) . Assessments of neurodevelopment in these later DHA RCTs not only revealed some benefits of supplementation during infancy(Reference Makrides, Gibson and McPhee22,Reference Henriksen, Haugholt and Lindgren23) but also suggested that these benefits do not persist to early childhood(Reference Almaas, Tamnes and Nakstad24,Reference Collins, Gibson and Anderson25) . It is difficult to draw clear conclusions regarding the efficacy of DHA supplementation to improve neurodevelopmental outcomes in preterm populations for a number of reasons: (1) Previous trials were underpowered to determine an effect on the smallest and most at-risk preterm infants; (2) DHA was provided through breast milk (achieved through either maternal DHA supplementation(Reference Makrides, Gibson and McPhee22) or directly adding to expressed breast milk(Reference Henriksen, Haugholt and Lindgren23)) or preterm infant formula, and therefore infants did not receive the full dose of DHA until they were able to tolerate all enteral feeds (typically at least 1–2 weeks or more after birth); (3) The concentration of DHA in breast milk is dependent on maternal biological variability and compliance(Reference Smithers, Markrides and Gibson26) and (4) Most previous trials assessed neurodevelopmental outcomes using global measures, which may limit the ability to detect differences in those specific domains hypothesised to be most sensitive to DHA in the neonatal period, that is, the higher-order cognitive functions(Reference Wainwright and Colombo27). Recently in the N3RO (n-3 fatty acids for improvement of Respiratory Outcomes) trial, infants born <29 weeks’ gestation were supplemented with DHA or a control through an enteral emulsion within the first days of birth to ensure direct enteral delivery of DHA at a dose sufficient to meet the estimated in utero accretion rate(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29) . Although the N3RO trial was designed to assess the effect of the intervention on the incidence of bronchopulmonary dysplasia, it offers an ideal opportunity to determine the effect on neurodevelopment. The aim of this study, therefore, was to assess the early development of the higher-order cognitive function, attention, in a subset of the N3RO trial infants to evaluate the effect of DHA intervention on neurodevelopment.
Participants and methods
This was a follow-up at 18 months’ corrected age of a subset of infants who participated in the N3RO multicentre RCT(Reference Collins, Makrides and McPhee29). Corrected age (rather than chronological age) takes into account prematurity and is used by clinicians working with preterm patients to accurately assess age-appropriate growth and development(Reference Engle30). Corrected age is also recommended for use in research as there is bias in cognitive test scores if they are not corrected for prematurity(Reference Doyle, Anderson and Battin31,Reference Rickards, Kitchen and Doyle32) . Corrected age is calculated by subtracting the number of weeks of prematurity from the chronological age. The N3RO trial protocol and results have been published(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29) as well as the protocol for this current follow-up(Reference Gould, Colombo and Collins33). The aim of this follow-up was to determine whether DHA supplementation in infants born <29 weeks’ gestation can improve areas of the brain associated with frontal lobe function, namely attention and distractibility.
Initial randomised controlled trial – the N3RO trial
Between 2012 and 2015, 1273 infants born <29 weeks’ gestation from thirteen neonatal centres in Australia, New Zealand and Singapore were randomised, within 3 d of their first enteral feed, to receive an enteral emulsion containing either DHA (providing 60 mg/kg per d of DHA) or no DHA (soya-oil control) until 36 weeks’ post menstrual age(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29) . Infants were excluded from the N3RO trial if they had a major congenital or chromosomal abnormality, were participating in another fatty acid study, receiving intravenous lipid emulsions containing fish oil or if their mother was breast feeding and taking supplements containing >250 mg/d of DHA(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29) . Infants were randomised according to a computer-generated randomisation schedule by an independent statistician who was not otherwise involved with the trial or data analysis. Randomisation was stratified for sex, study centre and gestational age (<27 completed weeks and 27 to <29 completed weeks). Multiple births were randomised individually(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29) .
Current study – follow-up of a subset of N3RO children in early childhood
Children were eligible for this follow-up if they were enrolled in the N3RO trial at either the Women’s and Children’s Hospital (WCH) or the Flinders Medical Centre, Adelaide, South Australia, and were between 15 and 30 months’ corrected age between March 2015 and June 2016(Reference Gould, Colombo and Collins33). Children who were over 30 or under 15 months’ corrected age during the time of the follow-up study or had a medically diagnosed major pathology (e.g. blindness or cerebral palsy) that was likely to invalidate performance on the attention assessment were excluded from the follow-up study.
An information sheet and consent form were posted to the parents of eligible children, and appointments scheduled via a follow-up phone call. Parent–child pairs were invited to attend an appointment at either the WCH or Flinders Medical Centre when the child was 18 months’ corrected age (target window 18 ± 3 months, accepted range 15–30 months). Written informed consent was obtained from all parents of participating children. Participants and their families, clinicians and researchers remained blinded to group allocation until the completion of the attention assessments.
These children are included in routine clinical follow-up care which includes a Bayley Scales of Infant and Toddler Development, third edition (Bayley-III) assessment at approximately 2 or 3 years’ corrected age. Consent also included the consent to access the Bayley-III results from the medical records.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Women’s and Children’s Health Network Human Research Ethics Committee, Adelaide, Australia (HREC/14/WCHN/50). The N3RO trial and this follow-up are registered on the Australia and New Zealand Clinical Trial Registry (ANZCTR: ACTRN12612000503820).
Outcomes
The primary outcome for this follow-up study is the average time to be distracted when attention is focused on a toy (i.e. the main outcome of the Distractibility task from the attention assessment as described below). Secondary outcomes include other aspects of attention, and (where possible) an assessment of cognition, language and motor development with the Bayley-III.
Attention assessments
All attention tasks were completed by a single investigator and took place in a neutrally decorated room with children seated on their parent’s lap at a desk. Parents were asked to avoid interacting with their child or influencing their behaviour during attention tasks. Attention and distractibility assessments were carried out according to a previously detailed method(Reference Colombo, Kannass and Shaddy6,Reference Gould, Colombo and Collins33,Reference Kannass, Colombo and Carlson34) . Children were given a series of toys to freely play with in three separate tasks measuring different aspects of attention (1. Single-object task, 2. Multiple-object task, and 3. Distractibility task) administered in the same order for all children, while their eye movements were recorded by a digital video recorder (GZ-MS120; JVC) for subsequent review and data extraction(Reference Gould, Colombo and Collins33). The published protocol includes a supplementary manual for conducting the attention assessment(Reference Gould, Colombo and Collins33).
Single-object task
The child was given a single, complex toy with multiple buttons and functions (My Discovery House, LeapFrog) to play with freely for 5 min. The single-object task measures the child’s ability to attend to a toy in the absence of competition or distraction. Outcomes of the single-object task are the proportion of time spent looking at the toy, duration of time spent looking at a toy, number of episodes of looking at a toy, average duration of an episode of looking at a toy and longest duration of a look to a toy.
Multiple-object task
The child was given five toys (squeaky rubber frog, airplane with wheels and a button for sound, turtle with see-through shell containing beads, alligator with rattle and three stackable Fisher-Price blocks) to play with freely for 5 min. The multiple-object task measures the child’s ability to sustain attention to a single toy in the presence of four other toys competing for the child’s attention. Outcomes of the multiple-object task are the number of times the child shifted their attention between toys, duration of time spent looking at toys, number of episodes of looking away from toys, average duration of looking at toys, proportion of time spent looking at toys and longest look duration to the toys.
Distractibility task
The child is provided with four toys (plastic turtle with four detachable mini turtles, set of stackable rings, shape sorter and plastic train with buttons and removable blocks), one at a time, to play with for 3 min each in the presence of a distractor. A television (SONIQ E23Z13AT2 23-inch HD LED LCD TV with integrated DVD player) positioned about 1 m away at a 45° viewing angle played a DVD consisting of 7 s distractor segments (segments of various children’s programmes) with pseudorandom 5–25 s intervals of blank screen. The child’s state of attention at the onset of each distractor segment was coded as focused (looking at the toy and engaged in active learning), casual (looking at the toy but not engaged in active learning) or other (not looking at the toy) on the basis of the child’s facial expression and behaviour(Reference Colombo, Kannass and Shaddy6). Children were excluded from the data analysis of the latency to turn to the distractor outcome if there was not at least one episode each of casual and/or focused attention during the entire task. A zero value could not be assigned to these cases as it would depict that the children turned to the distractor instantly, thus giving rise to a different average time of latency to turn to the distractor.
The main outcome of the Distractibility task is the average latency to turn to the distractor when the child’s attention was focused on the toy (i.e. how long the child takes to look at the television if a distractor segment started when the child’s attention was focused on the toy, averaged across the four toys) and this forms the primary outcome for the study.
Other outcomes from the distractibility task are the proportion of turns to the distractor when the child’s attention was focused, latency to turn to a distractor when the child’s attention was casual, proportion of turns to the distractor when the child’s attention was casual, and duration of looking to the distractor when the TV was on, and when the TV was off.
Data extraction
Video recordings of each task were viewed with a built-in timer and a shuttle jog (Contour ShuttleXpress) for frame-by-frame (twenty-five frames per second) viewing to determine the exact timing of the child’s eye movements(Reference Gould, Colombo and Collins33). Any episode of looking at the toy or looking away from the toy was coded as attention or inattention, respectively. An episode of attention or inattention was only included in the single-object task if it was >1 s in duration(Reference Colombo, Kannass and Shaddy6,Reference Gould, Colombo and Collins33) , and only included in the multiple-object task if it was >0·5 s in duration(Reference Gould, Colombo and Collins33,Reference Kannass, Colombo and Carlson34) . Any episodes of interruptions that may have influenced the child’s behaviour during free-play were coded as interference and excluded from the assessment data.
In order to ensure the reliability of the results, 25 % of the assessments were coded independently by a second investigator. The data extracted by the two investigators were closely correlated (r ≥ 0·99), and there were no differences in the values obtained by the two coders for any of the attention outcomes.
Bayley-III
The Bayley-III is administered as part of the routine clinical follow-up of infants born very preterm at many neonatal units and provided an opportunity for an exploratory investigation of the effect of the enteral DHA emulsion on global neurodevelopment. At the WCH, children are assessed at 2 years’ corrected age, and at the Flinders Medical Centre, children are assessed at 3 years’ corrected age. Results of the Bayley-III assessment were accessed by study personnel upon consent from parents. The Bayley-III is a psychometric global developmental test for infants with cognitive, motor and language scale scores that are age standardised (according to corrected age for infants born preterm) to a mean of 100 (sd 15)(Reference Bayley35). Scores less than 85 are indicative of developmental delay.
Sociodemographic, clinical and baseline information
Sociodemographic and baseline clinical data (including sex, birth weight and gestational age) were collected for infants and their families at the time of enrolment into the N3RO trial(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29) .
Baseline heel prick blood samples from newborns were collected for fatty acid analysis on specialised dried blood spots (PUFAcoatTM) with superior ability for stabilising LCPUFA(Reference Collins, Gibson and Makrides28,Reference Collins, Makrides and McPhee29,Reference Liu, Muhlhausler and Gibson36) . According to a modified Folch method, total lipids from blood spots were extracted using chloroform–methanol (2:1, v/v), transmethylated to fatty acid methyl ester with 1 % sulphuric acid in methanol at 70°C for 3 h and extracted with heptane for analysis by GC (Hewlett-Packard)(Reference Folch, Lees and Sloane Stanley37).
Information regarding the breast/formula feeding within the first 12 months’ age, type of infant formula used and the consumption of DHA-rich foods or supplements by the child during the previous week were collected at the time of the attention assessment. Parents were also asked to complete the Home Screening Questionnaire for children aged 0–3 years, which assesses the child’s home environment including parental involvement and responsivity, availability of stimulating play materials, educational stimulation and provision of opportunities for exploration, as well as the length of time the television is on in the home and length of time the child typically spends watching television(Reference Frankenburg and Coons38).
All assessments and procedures were conducted in accordance with the study protocol(Reference Gould, Colombo and Collins33).
Sample size and statistical analysis
A sample size of seventy-two children (thirty-six per group) was required to detect a difference of 0·8 (sd 1) s, with 85 % power, between treatment groups in the mean child’s latency to turn to the distractor during focused attention. The calculation includes a 19 % inflation factor for clustering due to multiple births according to a previous study in a similar population(Reference Makrides, Gibson and McPhee22). As the clinical meaning of performance on this assessment is currently unknown, the effect size is an estimate based on a previous study that found an average difference of 1·1 (sd 0·7–1·7) s in latency at 12 and 18 months of age in a term sample dichotomised into high and low maternal DHA level at birth(Reference Colombo, Kannass and Shaddy6). In the present sample, we expected a lower difference in latency as the age at assessment was more homogenous and analysis was conducted according to randomised allocation of DHA.
The baseline characteristics of the subset of N3RO trial participants who participated in this study, as well as home and dietary characteristics collected at 18 months’ corrected age, were compared between the DHA and control groups using independent t tests, Mann–Whitney U tests or χ 2 tests. Group differences in baseline variables were tested for the follow-up participants as only a small subset of the N3RO trial cohort was included. The baseline characteristics of children who participated in the follow-up study were also compared with those who were eligible but did not participate in the follow-up study, as well as to the remaining infants in the N3RO trial cohort who were not eligible.
Analyses followed a pre-specified statistical analysis plan and were performed on an intention-to-treat basis according to the infant’s allocation to the treatment or control intervention. Un-blinding of the follow-up study investigators only occurred after all analyses were completed. The primary analysis was performed using linear regression models. Generalised estimating equations with an independent working correlation structure were used to account for clustering due to multiple births. Both unadjusted and adjusted analyses were performed, with adjustment for the stratification variables of the original N3RO trial (sex, gestational age category and study centre). For outcomes that did not follow a normal distribution, analyses relied on the central limit theorem and post hoc sensitivity analyses were performed to compare the treatment groups using Wilcoxon rank sum tests.
As smoking and parental education have been shown to be associated with neurodevelopment(Reference Julvez, Ribas-Fito and Torrent39,Reference Patra, Greene and Patel40) , additional post hoc sensitivity analyses were performed using the same method as for the primary analysis, with additional adjustment for smoking during pregnancy and maternal tertiary education. The post hoc sensitivity analyses were also adjusted for the concentration of EPA in infants’ whole blood, due to the baseline differences between intervention groups in the subset of N3RO trial infants in the follow-up study. Due to the wide age range of infants at the time of the follow-up study assessment, further post hoc sensitivity analyses were conducted for the attention outcomes using the same method as the primary analysis, additionally adjusting for corrected age of child at assessment. This adjustment was not required for the Bayley-III outcomes, as these are age standardised.
Baseline comparisons were conducted using IBM SPSS Statistics version 24, and all other statistical analyses were conducted using SAS version 9.3. A P ≤ 0·05 was considered statistically significant in all analyses. No adjustment was made for multiple comparisons as the study has two treatment groups and a single primary outcome.
Results
Participants
A total of 192 infants were randomised to the initial N3RO trial at the included neonatal centres (WCH n 133, Flinders Medical Centre n 59), and although 120 were eligible to participate in the current follow-up study (Fig. 1), recruitment ceased once the sample size needed for statistical power was reached. A total of seventy-seven children completed the assessment, thirty-seven from the DHA group and forty from the control group. Adequate data for analysis of the primary outcome (the average latency to turn to the distractor when the child’s attention was focused on the toy) were available for seventy-three participants (95 %). A similar number of families from the DHA (n 19) and the control groups (n 19) did not participate in the follow-up study. Some participants declined to participate (n 25) due to the difficulty attending the appointment or being too busy. There were thirteen potentially eligible participants who were not contacted about the follow-up study at the request of clinicians undertaking the routine follow-up of these children.

Fig. 1. Flow of children in the N3RO (n-3 fatty acids for improvement of Respiratory Outcomes) trial through the attention follow-up study at 18 months’ corrected age (CA). * Children did not turn 15 months’ age during the time period of the follow-up study. † Participants were not contacted due to rural location as requested by the Growth and Development Unit of the hospital. ‡ Children excluded due to the lack of an episode of focused attention during distractor onset.
Of the seventy-seven children who participated in this follow-up study, sixty-seven had parental consent to access their Bayley-III assessment results. At the time of completing this study, fifty-six of these children had completed their Bayley-III assessment (twenty-seven in the DHA group and twenty-nine in the control group), and one child in the DHA group did not have a language score.
Baseline characteristics
The majority of the baseline sociodemographic characteristics in the subset of N3RO families who participated in this follow-up were similar between the DHA and control group. There were small but statistically significant differences in maternal age with mothers of children in the DHA group being older (32·5 (sd 4·5) v. 30·2 (sd 5·3) years, P = 0·04) (Table 1) and infant blood EPA concentration higher in the DHA group (0·6 (sd 0·4) v. 0·3 (sd 0·2) %, P = 0·008) at the time of enrolment into the N3RO trial (Table 1).
Table 1. Characteristics of children and their parents at birth, randomisation and 18-month assessment (Mean values and standard deviations; numbers and percentages; medians and interquartile ranges (IQR))

LCPUFA, long-chain PUFA; Apgar, Appearance, Pulse, Grimace, Activity and Respiration score; CRIB, clinical risk index for babies.
* Data missing for one child in the control group.
† Data missing for three children in the control group.
‡ Data missing for two children in the DHA group and three in the control group.
§ Data missing for three children in the DHA group and one in the control group.
|| Data missing for three children in the DHA group and two in the control group.
¶ Data missing for one child in the DHA group.
** Data missing for one child in the DHA group and five in the control group.
†† Data missing for two participants in the DHA group and six in the control group.
‡‡ Data missing for six children in the DHA group and seven in the control group.
Baseline characteristics of children included in the follow-up study were somewhat different to the remainder of the N3RO trial cohort (online Supplementary Table S1). Similarly, the baseline characteristics differed between eligible N3RO children who did and did not participate in the 18-month assessment (online Supplementary Table S2). For instance, the parents of children who participated in the follow-up study were more educated, and mothers were less likely to have smoked during pregnancy compared with parents of eligible children who did not participate.
Participant characteristics at 18 months’ corrected age
The majority of child characteristics at the time of the 18-month attention assessment, such as age, Home Screening Questionnaire score (including average time per d spent watching TV), were similar between the DHA and control groups (Table 1). However, reported fish consumption in the week prior to the attention assessment was lower (54·1 v. 77·5 %, P = 0·03) in the DHA group compared with the control group (Table 1).
Attention
There was no evidence of a difference between the DHA and control groups in the primary outcome latency to turn to the distractor when the child’s attention was focused on the toy during the distractibility task (adjusted mean difference 0·08 s, 95 % CI –0·81, 0·97; P = 0·86, Table 2). The proportion of time the children spent looking at the toy in the single-object task (adjusted mean difference –0·02, 95 % CI –0·08, 0·04; P = 0·51) and the number of times children shifted attention between toys in the multiple-object task (adjusted mean difference 3·16, 95 % CI –1·97, 8·30; P = 0·23) did not significantly differ between groups. There were also no significant differences in any other attention outcomes assessed in the three tasks between the DHA and control group, in either unadjusted or adjusted analyses.
Table 2. Outcomes of assessments of attention (18 months’ corrected age) and Bayley-III scores (2–3 years’ corrected age) by treatment groups (Mean values and standard deviations; 95 % confidence intervals)
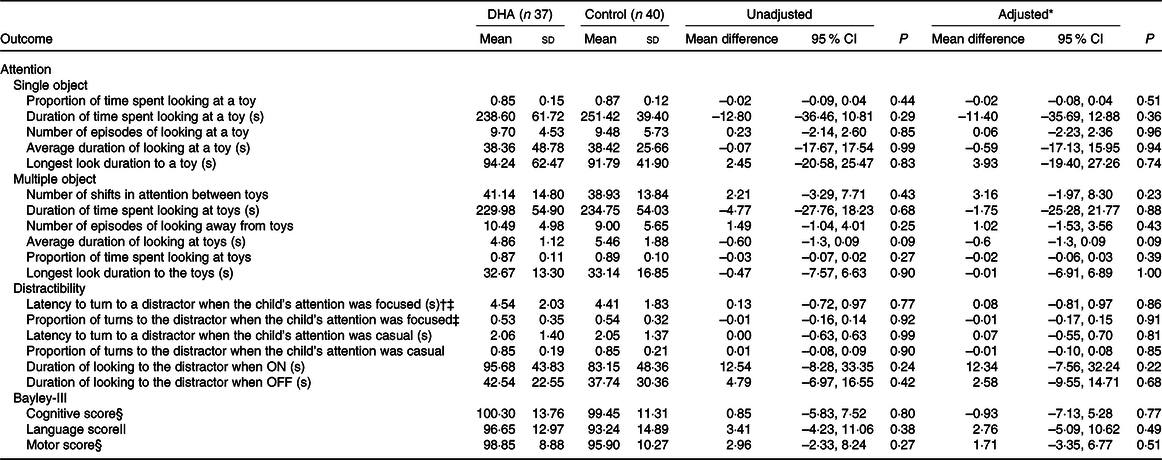
* Adjusted for stratification variables of the N3RO trial (sex, gestational age category (<27 weeks or 27 to <29 weeks) and centre (Flinders Medical Centre and Women’s and Children’s Hospital)).
† Primary outcome of the attention assessment.
‡ Data missing for two children in each group.
§ Data missing for ten children in the DHA group and eleven children in the control group.
|| Data missing for eleven children in each group.
Nor was there evidence of a difference in the majority of the attention outcomes between the DHA and control groups in the post hoc sensitivity analyses adjusting for maternal smoking during pregnancy, maternal further education and concentration of EPA in infants’ blood at baseline (online Supplementary Tables S3 and S4). The exception was that the children in the control group had a higher average duration of look at the toys during the multiple object task compared with the DHA group (adjusted mean difference –0·93 s, 95 % CI –1·66, –0·19; P = 0·01). Attention outcomes were similar between the groups in the post hoc sensitivity analyses adjusting for corrected age of child at assessment (online Supplementary Table S3).
Bayley-III
The cognitive score in the Bayley-III assessment did not significantly differ between the DHA and control groups (adjusted mean difference −0·93, 95 % CI −7·13, 5·28; P = 0·77) (Table 2). Similarly, the motor and language scores did not significantly differ between the treatment groups in either unadjusted or adjusted analyses. Impaired performance (composite score < 85) on the cognitive scale was present in four (out of fifty-six, DHA n 2, control n 2), whilst impaired language performance was present in ten (out of fifty-five, DHA n 4, control n 6) and impaired motor performance was detectable in six children (out of fifty-six, DHA n 1, control n 5). Two children in the control group had language scores <70, suggesting delayed language development.
Discussion
We followed up a subset of infants who participated in the N3RO RCT to determine the effect on attention at 18 months’ corrected age of a DHA intervention v. control. The intervention was administered via an enteral emulsion to maximise control over the dose and timing in preterm infants born <29 weeks’ gestation. The results obtained from the primary outcomes of the N3RO trial were contrary to the hypothesis that DHA supplementation would reduce the incidence of bronchopulmonary dysplasia at 36 weeks’ corrected age(Reference Collins, Makrides and McPhee29). In fact, the results of the N3RO trial showed a small but significant increase in the incidence of bronchopulmonary dysplasia in the DHA supplemented group(Reference Collins, Makrides and McPhee29). In the present follow-up, we used a specialised measure of early childhood attention, considered to provide a more sensitive indicator of higher-order cognitive functioning and frontal lobe development than global neurodevelopment assessments(Reference Wainwright and Colombo27). We found no evidence of benefit of the DHA intervention on attention in at 18 months’ corrected age.
The assessment measure we used is an experimental measure and is currently without standardised administration, calculated outcomes or clinical interpretation. Whilst infant measures of attention have previously been linked to later intelligence and language abilities(Reference Bornstein and Sigman7,Reference Colombo8) , the validity of the assessment is yet to be established. However, it is one of very few published tools available for assessing attention in early childhood and is capable of detecting differences in performance between two groups. Variations of the assessment have been applied in several observational and nutritional intervention studies(Reference Gould, Makrides and Colombo41-Reference Westerberg, Schei and Henriksen47). In an earlier RCT in very low birth weight infants (<1500 g) fed DHA (mean daily intake of 59 mg/kg per d in the intervention group and 32 mg/kg per d in the control group) and arachidonic acid (mean daily intake of 47 mg/kg per d in the intervention group and 22 mg/kg per d in the control group) supplemented human milk from 1 week after birth until discharge from hospital (9 weeks on average) and then underwent a variation of the attention assessment at 20 months chronological age(Reference Westerberg, Schei and Henriksen47). As with our study, the majority of the group comparisons of attention were null, although the supplemented group had an improved ability to sustain attention towards the toys at 1 min (but not at 2 or 3 min)(Reference Westerberg, Schei and Henriksen47).
Other studies that have used another variation of the specialised measure of attention to determine efficacy of nutritional interventions have had mixed results. In one study, the trajectory of the development of attention between 12 and 18 months of age in healthy term children was positively associated with maternal blood DHA levels at delivery(Reference Colombo, Kannass and Shaddy6,Reference Kannass, Colombo and Carlson34) . However, a RCT of DHA supplementation in pregnancy found no group differences using the same attention measure at 27 months in healthy term children, and no association between attention and cord blood DHA at delivery(Reference Gould, Makrides and Colombo41). A small RCT supplementing preterm infants with sphingomyelin (a lipid found in high abundance in neural membranes) found improved attention at 18 months’ corrected age compared with control infants(Reference Tanaka, Hosozawa and Kudo45). It is difficult to directly compare the performance of our sample on the attention assessment with the performance in other studies due to differences in the attention assessment procedure, assessed outcomes and ages of the samples. Our sample appears to have wide standard deviations for the attention outcomes, suggesting that there is considerable variation in outcomes of the attention measure within extremely preterm samples.
Other (age-appropriate) assessments of attention after DHA interventions in preterm samples have been administered to school-age children. In one of the earlier trials comparing preterm infant formula devoid of DHA to formula supplemented with DHA (0·5 % total fatty acids, about 30 mg/kg per d), there were no differences in attention, as assessed with the Test of Everyday Attention for Children, at 10 years’ corrected age(Reference Isaacs, Ross and Kennedy48). Similarly, in a larger more recent RCT, preterm infants receiving high-dose DHA (about 1 % total fatty acids, approximately 50 mg/kg per d) did not display any benefits on the Test of Everyday Attention for Children at 7 years’ corrected age when compared with infants receiving the standard (low) dose of DHA (about 0·3 % total fatty acids, approximately 20 mg/kg per d)(Reference Collins, Gibson and Anderson25). Although there are important differences in the type of intervention, dose of DHA, assessment of attention and age of the children, these studies suggest there is no long-term benefit of DHA supplementation for the development of attention in children born preterm.
The majority of RCTs involving DHA supplementation in preterm infants which have undertaken neurodevelopment assessments have used global measures, such as the Bayley-II or III cognitive score to assess the effects of the intervention. In the current study, the global assessment did not suggest an effect of DHA on this outcome, although the sample was considerably underpowered to detect a clinically meaningful effect. Meta-analyses of global assessments in previous trials have shown little evidence of benefit(Reference Moon, Rao and Schulzke16,Reference Smithers, Gibson and McPhee21) , although two RCTs that have compared standard (low) dose DHA (about 0·3 % total fatty acids, approximately 20 mg/kg per d) to high-dose DHA (about 1 % total fatty acids, approximately 50 mg/kg per d) have shown some short-term neurodevelopmental benefits to infants born preterm assessed using Bayley-II at 18 months’ corrected age and Ages and Stages Questionnaire at 6 months of age (parental reported screen of developmental milestones)(Reference Makrides, Gibson and McPhee22,Reference Henriksen, Haugholt and Lindgren23) . Subsequent assessments of the same children in early childhood show that these benefits do not persist long-term(Reference Almaas, Tamnes and Nakstad24,Reference Collins, Gibson and Anderson25) . This is may be due to an overwhelming effect of the family and environment on neurodevelopmental outcomes, and as a result any benefits of DHA seen at a younger age may wane as the child grows older(Reference Wong and Edwards49). Overall, there is little evidence to suggest DHA supplementation benefits neurodevelopment in preterm samples, although it is still unclear whether DHA can improve outcomes in infants born extremely preterm.
Performance on the Bayley-III was within the normal (average) range for the majority of the children in our study, with <10 % scoring lower than 1 sd below the mean. While this may reflect an underestimation of delay by the Bayley-III(Reference Anderson, De Luca and Hutchinson50), our preterm sample performed similarly in cognitive, motor and language abilities to other preterm samples(Reference Anderson, De Luca and Hutchinson50,Reference Spencer-Smith, Spittle and Lee51) .
Strengths of this study include the use of high-dose DHA (60 mg/kg per d) to match the estimated in utero accretion during the last trimester of pregnancy(Reference Lapillonne, Groh-Wargo and Gonzalez12) that was delivered directly to infants within the first few days after birth, ensuring that infants received the full DHA dose as early as possible. This study was conducted in a subset of preterm infants who would be expected to be at high risk of DHA deficit in the early postnatal period, and at risk of deficits such as developmental delay in childhood(Reference Anderson and Doyle1,Reference Anderson, De Luca and Hutchinson50,Reference Anderson and Doyle52,Reference Anderson53) . Thus, the N3RO trial sample offers an ideal opportunity to detect an effect of DHA on early attention, if there is one. In addition, the attention measure used is considered to be sensitive to subtle changes in development as a result of a nutritional intervention(Reference Colombo, Kannass and Shaddy6,Reference Colombo and Carlson54) .
One key limitation of the study is the potential selection bias, as the baseline characteristics of the follow-up study children were different to the original N3RO trial cohort. The families who participated were largely restricted to those residing in metropolitan Adelaide, since the nature of the assessment meant that it was not possible to offer home visits. The socio-economic status of the families who participated in the follow-up study was higher than those who were eligible but did not participate. It is therefore possible that the findings of the study would have been different had it been undertaken in families of lower education and, if home visits were feasible, possible attrition bias could have been reduced. It is also possible that the parents of children without developmental deficits were more likely to participate in our follow-up assessments. Bayley-III scores were accessed opportunistically, and group comparisons were not powered to detect a difference in scores. There was a wide window for outcome assessments, although this is unlikely to have significantly impacted on results of the assessment given the stability of the development of attention and distractibility across this stage of development(Reference Kannass, Oakes and Shaddy55) and the Bayley-III is age standardised. Results of supplementary analyses adjusted for age at assessment did not differ. There may be some infants who respond differently to supplementation or who benefit from the intervention more than others, such as those with low DHA status at birth. Future studies with appropriately large samples should consider exploring this. DHA intake after term may be beneficial for ongoing brain development but was not captured in sufficient depth to ascertain DHA from other dietary sources, such as breast milk. A higher fish consumption was noted in the control group compared with the DHA group prior to the follow-up assessment; however, this is unlikely to have influenced the findings as there is very limited evidence of an effect of DHA intake during infancy and accumulation in neural tissue. Additionally, a recent Cochrane review has found no neurodevelopmental benefits of feeding LCPUFA-rich infant formula to term infants during infancy compared with formula without LCPUFA(Reference Jasani, Simmer and Patole56). In conclusion, DHA supplementation in infants born <29 weeks’ gestation had no effect on early development of attention. However, it will be important to follow-up the N3RO children at older ages to assess whether there are any longer-term effects of DHA supplementation on neurodevelopmental outcomes.
Acknowledgements
We thank the families and their children for their participation in the study; the N3RO steering committee (Assoc/Prof Carmel Collins, Prof Robert Gibson, Prof Maria Makrides, Dr Andrew McPhee and Dr Thomas Sullivan) for reviewing study protocols and statistical analysis plans; the Growth and Development units at both the Women’s and Children’s Hospital and Flinders Medical Centre.
This project was supported by a Women’s and Children’s Hospital Foundation Project Grant. The original N3RO trial was funded by the National Health and Medical Research Council (NHMRC) (grant ID 1022112). The treatment and control emulsion for the original N3RO trial were donated by Clover Corporation. The funding bodies had no role in the study design or conduct; in the data collection, management, analysis or interpretation; or in the preparation, review or approval of any manuscripts arising from this study. E. H. is supported by an Australian Postgraduate Award scholarship from the University of Adelaide. J. F. G. is supported by a Women’s and Children’s Hospital Foundation MS McLeod Research Postdoctoral Fellowship. M. M. is supported by a NHMRC Senior Research Fellowships ID: 1061704. B. S. M. is supported by a Career Development Award from the NHMRC of Australia (1083009). C. T. C. is supported by a NHMRC Translating Research Into Practice (TRIP) Fellowship (ID 1132596). L. N. Y. is supported by a NHMRC Early Career Fellowship (ID 1052388). J. C. is supported by US NIH grant U54-HD090216-02.
J. F. G., M. M., C. T. C., L. G. S. and J. C. designed the study (project conception, development of overall research plan and study oversight); J. F. G. trained and supervised E. H. in the administration and data extraction of assessments and performed the data extraction reliability check and has primary responsibility for final content; J. C. provided essential assessment techniques necessary for the research; E. H. conducted research (hands-on conduct of research, data collection and processing), performed baseline statistical analyses and wrote the manuscript under the supervision of J. F. G., C. T. C. and B. S. M.; L. N. Y. designed and performed statistical analyses and all authors contributed to the scientific interpretation of the analysis, commented on drafts of the manuscript and accepted final responsibility for the manuscript.
Honoraria have been paid to J. F. G.’s institution to support conference travel by the Nestlé Nutrition Institute and Fonterra. M. M. reports serving on scientific advisory boards for Nestlé and Fonterra. Associated honoraria for M. M. are paid to her institution to support conference travel and continuing education for postgraduate students and early career researchers. B. S. M. serves on the advisory board for the Nestlé Nutrition Institute and has given talks for Danone Nutricia and Aspen Nutrition. All associated honoraria are paid directly to her host organisation and used to support travel and professional development for students and early career researchers. J. C. serves on the scientific advisory boards for Nestlé and Fonterra and receives honoraria.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520002500






