Dietary lipids are important sources of energy and essential fatty acids(1). Linoleic acid (LA, 18 : 2n-6) is an essential fatty acid for mammals. Unlike most mammals, cats have low Δ-6 desaturase activity and cannot efficiently convert LA to arachidonic acid (AA)(Reference Rivers, Sinclair and Crawford2–Reference Bauer5). Thus, LA and AA must be supplied through dietary intake in all phases of life. Δ-6 desaturase is also needed for the synthesis of DHA (22 : 6n-3) and EPA (20 : 5n-3) from α-linolenic acid (18 : 3n-3). Kittens must be supplemented with these n-3 fatty acids(Reference Pawlosky, Denkins and Ward6). For adult cats, n-3 fatty acids are considered non-essential, and there is no consensus on the recommended levels(1,7) .
The beneficial effects of n-3 fatty acids as nutraceuticals, particularly as immunomodulatory nutrients, have been reported in the literature(Reference Lenox and Bauer8–Reference Echeverría, Valenzuela and Hernandez-Rodas10). Both n-3 and n-6 fatty acids are precursors of PG and other eicosanoids, such as leukotrienes, thromboxanes and prostacyclins. Enriched diets with n-3 fatty acids can promote the synthesis of less inflammatory PGE3 and inhibit the production of pro-inflammatory PGE2, derived from n-6(Reference Bagga, Wang and Farias-Eisner11–Reference Calder14). n-3 Fatty acids can partially inhibit leucocyte chemotaxis, adhesion molecule expression, leucocyte–endothelial adhesive interactions, eicosanoid production from n-6 fatty acids, inflammatory cytokine production and T-cell reactivity(Reference Ajmone-Cat, Salvatori and Simone13–Reference Calder15). The mechanisms underlying these anti-inflammatory effects include modification of phospholipid fatty acid composition in the cell membrane, disruption of lipid rafts, inhibition of the activation of the pro-inflammatory transcription factor NF-κB and consequent reduction in the expression of inflammatory genes(Reference Calder15). Eicosanoid production depends on the oxidation of lipid molecules by cyclo-oxygenases (COX), lipoxygenases and epoxygenases(Reference Trebble, Wootton and Miles16–Reference Xia, Zhang and Zheng18), showing that there is a correlation between inflammation and oxidation in the body. Thus, immunomodulatory ingredients may help prevent oxidative stress.
Marine algae such as Schizochytrium spp. (Thraustochytriaceae) are promising alternative sources of n-3 fatty acids. These organisms can be grown heterotypically, their cultivation is sustainable and they are commercially available as dried products(Reference Li, Robinson and Tucker19). Hadley et al.(Reference Hadley, Bauer and Milgram20) supplemented beagle dogs with Schizochytrium spp. and observed higher deposition of DHA in plasma phospholipids compared with a control. Since DHA has an inhibitory effect on the production of PGE2(Reference Ajmone-Cat, Salvatori and Simone13) and consequently, on the inflammatory response, it is expected that the use of ingredients as a source of this nutrient, such as the microalgae Schizochytrium spp., will have immunomodulatory benefits. In the present study, we evaluated the effects of Schizochytrium spp. supplementation as a DHA source on the inflammatory responses of 11-month-old female and male cats following neutering. A dose–response model was used to associate DHA supplementation with inflammatory (PGE2, acute-phase proteins, 12-hydroxyeicosatetraenoic acid (12-HETE) and wound healing) and oxidative markers (total thiols, thiobarbituric acid reactive substances (TBARS) and total antioxidant capacity (TAC)) in the blood and fatty acid deposition and n-6:n-3 ratio in the gonads of cats.
Materials and methods
The present study was conducted according to the ethical principles of animal experimentation and under the approval of the Ethics Committee on Animal Use of the State University of Maringá (protocol number 4982281114/2014), Brazil. Authors ensure that the manuscript complied with the ‘ARRIVE Guidelines for Reporting Animal Research’ summarised at www.nc3rs.org.uk.
Animals and diets
Thirty-seven mixed-breed young cats (males, n 21; females, n 16) with 3·46 (sd 0·56) kg of body weight and 11·5 (sd 0·5) months of age were used in the study. All animals were clinically healthy.
A control diet was formulated to meet nutritional recommendation for growing cats without EPA and DHA sources (Table 1), according to the European Pet Food Industry Federation(7). The diet was extruded using a twin-screw extruder (POLYtwin™ 125, Bühler AG Appenzell) and dried to 5–7 % moisture. On the basis of the control diet, four additional diets were designed and supplemented with DHA-rich microalgae (dehydrated Schizochytrium spp. Biomass; All-G Rich, Alltech do Brasil) at 4·0, 8·0, 12·0 and 16·0 g/kg, on DM basis, corresponding to DHA concentrations of 239·3, 442·0, 643·4 and 1278·4 mg/kg of diet, respectively. The control diet contained 31·4 mg of DHA/kg (on DM basis). Chemical composition of the biomass and analysed concentrations of other fatty acids were performed (Table 2). The control diet was coated with 40 g/kg of poultry fat and 20 g/kg of liquid palatant (C’sens, SPF Palatability). DHA-supplemented diets (4·0, 8·0, 12·0 and 16·0 g/kg of diet) were additionally coated with microalgae to partially substitute the poultry fat. However, fat content was similar among the five diets. The microalgal ingredient was applied after coating with poultry fat, but before liquid palatant, to ensure its adherence to the kibble.
Table 1. Ingredients, chemical composition and energy content of the control diet (without n-3 fatty acid supplementation)

* Minimum guaranteed levels of vitamins and minerals (per kg of product): vitamin A, 3375 μg; vitamin D3, 26·025 μg; vitamin K, 0·20 mg; vitamin B12, 25 μg; vitamin B6, 5·00 mg; riboflavin (vitamin B2), 5·00 mg; pantothenic acid, 6·25 mg; niacin, 83 mg; folic acid, 1·40 mg; thiamin (vitamin B1), 6·25 mg; choline, 3200 mg; Mn, 24 mg; Cu, 26 mg; iodine, 1·59 mg; Se, 0·46 mg; Zn, 140 mg; and Fe, 270 mg.
† Determined by the total faeces collection method and corrected for energy lost in urine(21).
Table 2. n-6 and n-3 Fatty acid concentration in the experimental diets*
* Chemical composition per kg of biomass: moisture 37·0 g/kg; crude protein 192·2 g/kg; total ash 36·7 g/kg; crude fat 500 g/kg (palmitic acid 54·69 %; DHA 27·2 %); crude fibre 9 g/kg and carbohydrates 248·8 g/kg.
† Dehydrated Schizochytrium spp. biomass (All-G Rich; Alltech Inc.).
Pre-experimental procedures
Prior to the experiment, the coefficient of total tract apparent digestibility of nutrients and metabolisable energy of the control diet were determined using the total faeces collection method, without urine collection, according to AAFCO(21). Feed was weighed daily and separated into two equal portions given to the animals at 08.00 and 14.00 hours. Leftover feed was weighed daily, and food intake was calculated. On the first day of faecal collection, all faeces produced before 8.00 hours were removed and discarded. Faeces excreted thereafter were collected for five consecutive days. Faecal samples were weighed, placed in separate plastic bags and stored in a freezer (−15°C). After the end of the collection period, faeces were thawed, homogenised and dried in a forced-air oven (MA035, Marconi) at 55°C for 72 h. Samples were milled through a 1 mm Wiley mill screen (MA340, Marconi) and homogenised for analysis. Feeds were subjected to the same milling procedure.
DM (method 930.15), crude protein (method 954.01) and mineral matter (method 942.01) in faeces samples were determined according to the AOAC(22). The above-mentioned parameters, acid-hydrolysed ether extract (method 954.02) and crude fibre (method 962.09) were determined in feeds(22). The gross energy of feed and faeces was determined using an adiabatic calorimetric pump (6200 Isoperibol calorimeter; Parr Instrument Company).
Following the determination of metabolisable energy, all cats (n 37) were fed the control diet for 15 d prior to the beginning of the experiment to allow them to adapt to the feeding routine in individual cages.

Fig. 1. Average increment in serum PGE2 levels after neutering of cats fed diets supplemented with different levels of DHA-rich Schizochytrium spp. biomass. Values are means, with standard deviations represented by vertical bars. The dashed diagonal line indicates the tendency line. PGE2 = −11·305 × (algae inclusion) + 43·437; R 2 0·87; n 37.
Animal management, experimental design and analysis
Cats were randomly assigned to blocks based on sex. The control group consisted of five males and four females, and the treatment groups were composed of four males and three females each. The maintenance energy requirements of cats were calculated by using the standard National Research Council equation(1) for adult lean cats: Metabolisable energy (kJ) = 418·4 × kg BW0·67, where BW is the body weight. It was not necessary to adjust the amount of feed provided throughout the experiment, as cats were in growing phase (11 months old). The daily intake for each cat was determined by weighing the amount of feed supplied and the amount of leftover feed, using a digital scale (Prix 3 fit, Toledo do Brasil). These data were used to calculate the fatty acid intake per treatment.
Each group received one of five diets for 62 d. Animals were housed in individual cages (1·0 × 1·0 × 0·5 m) twice per d for 1 h intervals for feeding (08.00–09.00 and 14.00–15.00 hours). During the remaining time, cats were housed separately (males and females) in collective rooms with 49 m2 of total area with water ad libitum. The environmental temperature was 23·0 (sd 4·6)°C and the natural daily rhythm was 12 h light–12 h dark cycle. To maintain the females in anoestrus until neutering, as they were reaching puberty, an oestrous cycle blocker was administered (Covinan®; MSD Saúde Animal) when the animals achieved 8 months old and before the beginning of the experiment, at 11 months old. All the animals were surgically sterilised on day 58 of the experiment, and the gonads were used for analysis of fatty acid deposition.
Collection procedures
Blood collection was performed at the beginning of the experiment immediately before the first feeding (day 0) for the determination of basal parameters. Blood samples were also collected on days 58 (immediately before surgery) and 62 (4 d after surgery). The haematological profile and levels of markers of inflammation and oxidative status were determined. Aliquots (5 ml) were collected from the external jugular vein by venipuncture. Prior to collection, animals were anaesthetised intramuscularly with a solution of 0·01 mg/kg acepromazine and 0·2 mg/kg tiletamine + zolazepam. A 4 ml aliquot of blood was immediately added to a tube containing coagulation activator and centrifuged at 3000 rpm for 10 min. The serum was frozen at –80°C until analysis. The remaining 1 ml of blood was placed in a tube containing 7·2 mg (4 ml) of EDTA dipotassium salt for haemogram analysis.
On day 58, the same blood collection procedure was performed, followed by injection of 1 ml of 2 % lidocaine by the epidural route as anaesthetic for the sterilisation procedure. Gonads were removed and immediately placed in screw-cap bottles, saturated with N2 gas and frozen at –80°C. After surgery, tramadol hydrochloride was administered orally at 1 mg/kg three times per d for 3 d as an analgesic. The surgical wound was cleaned with a chlorine-based antiseptic solution twice per d for 14 d.
Blood analysis
Serum acute-phase proteins (ceruloplasmin, haptoglobin, α-1-acid glycoprotein, albumin and transferrin) were quantified by electrophoresis. Concentrations of acute-phase proteins were expressed as a ratio of the total protein concentration(Reference Laemmli23). Serum samples were subjected to electrophoresis on SDS-PAGE. Protein fractions were determined by computerised densitometry using molecular weight protein markers of 29, 45, 66, 116 and 205 kDa.
Complete blood and platelet counts were obtained using an automated haematological counter (BC-2800 Vet; Mindray). Serum PGE2 (MBS074706, MyBioSource) and 12-HETE (EIA-ADI-900-050; Enzo Life Sciences) concentrations were determined using commercial ELISA kits. The optical density was read at 450 nm after sample preparation, and values were compared with those of a standard curve. Results are expressed as pg/ml of serum.
Thiol analysis was used to determine serum reduced glutathione (GSH) levels, according to the method proposed by Costa et al. (Reference Costa, Santos and Lima24). Blood serum and a Tris-EDTA solution were used for the first spectrophotometric reading (BIO 2000; Bio plus) at 412 nm. Then, the dithionitrobenzoic acid solution was added for the second reading at 412 nm. A standard curve of GSH was constructed and used to determine the concentration of sulphydryl groups.
TBARS were determined according to the colorimetric method of Payá et al. (Reference Payá, Halliwell and Hoult25). A solution containing 15 % TCA, 0·275 % thiobarbituric acid and 0·25 m hydrochloric acid was used to measure hydroxyl radical (OH•) levels. Briefly, PBS (pH 7·4) and blood serum were mixed, heated to 100°C, cooled on ice and centrifuged at 1200 g for 15 min each. Absorbance of the supernatant was read at 532 nm (BIO 2000; Bio plus). Results were expressed as nmol of TBARS/ml of serum against a standard curve of 1,1,3,3-tetramethoxypropane (malondialdehyde, CAS no. 108383; Sigma–Aldrich).
The TAC of serum was determined by the colorimetric method of Miller et al. (Reference Miller, Rice-Evans and Davies26). An antioxidant assay kit (CS0790; Sigma–Aldrich) was used. Working solutions of Trolox, myoglobin and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) were added to the microplates at room temperature. After 2 min, the reaction was stopped, and the absorbance of the microplates was read at 405 nm using a tunable microplate reader (Versa Max Plus ROM; Thermo Fisher Scientific). The standard curve was calculated according to the manufacturer’s recommendations, and results were expressed as equivalents of Trolox (mmol/l).
The fatty acid profile of reproductive tissues (testicles and ovaries) was determined by the method of Hartman & Lago(Reference Hartman and Lago27), as modified by Figueiredo et al. (Reference Figueiredo, Claus and Santos Junior28). All the testicles and all the ovaries from each treatment were pooled, separately, for analysis due to the small amount of gonadal tissue. First, 100 mg of sample was subjected to basic catalysis (sodium hydroxide and methanol) for 5 min. Subsequently, acid catalysis (sulphuric acid and methanol) was carried out for 5 min in an ultrasonic bath (Eco Sonics; Ultronique). Isooctane was added, and the supernatant was pipetted off. Fatty acid methyl esters were separated using a GC equipped with a flame ionisation detector (TRACE GC Ultra; Thermo Fisher Scientific) and a fused silica capillary column (100 m × 0·25 mm internal diameter, 0·25 μm cyanopropyl; CP-7420, Select FAME). The flow rates were 1·2 ml/min for the carrier gas (H2), 30 ml/min for the auxiliary gas (N2) and 35 and 350 ml/min for H2 and synthetic air, respectively, for the detector flame. The injection volume was 2·0 μl with a split ratio of 1:80. Injector and detector temperatures were 240°C. The column was held at 165°C for 7 min, heated to 185°C at 4°C/min for 4·67 min and ramped at 6°C/min to 235°C for 5 min, totalling 30 min of analysis. Retention times and peak areas of fatty acids in reproductive tissues were determined using ChromQuest version 5.0 (Thermo Fisher Scientific). Fatty acids were identified by comparison of their retention times with those of standards of known composition (Sigma–Aldrich). Quantification of fatty acids was performed by internal standardisation using tridecanoic acid (23:0) methyl ester (2433-97-8; Sigma–Aldrich). The amount of fatty acids was expressed as mg of fatty acid/100 g of sample, calculated using the equation M x = (A x M p F CT × 100)/A p M A F CEA, where M x is the concentration of fatty acid x, A x is the peak area of fatty acid x, A p is the peak area of the internal standard, M p (mg) is the mass of the internal standard, M A (g) is the mass of the sample, F CT is the theoretical correction factor for the flame ionisation detector and F CEA is the conversion factor of methyl ester to fatty acid.
Thermographic analysis of the surgical wound was performed using a thermographic camera (Thermography Ti110; Fluke Biomedical) with an emissivity of 0·98 and a transmissivity of 100 %. The skin surface of the ventral and caudal region was photographed in females and males, respectively. Thermographic photographs were taken in duplicate 24 h after surgery and consecutively for 4 d, always at the same time, under controlled room temperature.
Statistical analysis
Each individual cat was considered as the experimental unit. To calculate the sample size (n), the PGE2 data were used: mean of 60·0 pg/ml of PGE2, sd of 15·0, Δ value as 20·0 pg/ml, level of significance was set at P < 0·05 and test power was set at ≥0·80. Thus, the minimum sample size was estimated in seven cats (n 7) by using the following procedure by using the R statistical software for one-sample t test power calculation:

The experiment was carried out in a randomised block design, considering sex as the block, in a 5 × 2 factorial experiment, with five microalgae addition levels and two collection periods (58 and 62 d). Data were subjected to Anderson–Darling and Shapiro–Wilk tests at the 5 % significance level to test the normality assumption. The effects of diet, period and their interaction were tested using the function PROC MIXED from SAS version 9 (Statistical Analysis System). The covariance structure was compound symmetry, which considered the repeated measurements in animals within treatments. The blood parameters from sampling prior to the beginning of the trial were included as a covariate, within the respective groups. The means were compared by Tukey’s test at P < 0·05. Polynomial contrasts, as a function of treatments, were performed to test for linear or quadratic effects of microalgal concentration on fatty acids deposition in the gonads, by sex.
Results
The n-6:n-3 ratio decreased from 15·3:1·0 (control) to 5·04:1·0 with the addition of DHA-rich microalgae to the diet (Table 2). There was no difference (P > 0·05) in DM intake when cats were fed the control diet or diets supplemented with microalgae (4·0, 8·0, 12·0 and 16·0 g/kg). However, the DHA intake increased (P < 0·0001) following increasing levels of microalgae supplementation. The means of DM intake and DHA intake, for treatments control, 4·0, 8·0, 12·0 and 16·0 g/kg DHA-rich microalgae, were, respectively, 25·9, 28·3, 27·5, 25·1, 28·7 g/kg BW0·67, and 0·81, 6·97, 11·78, 16·02, 36·05 mg/kg BW0·67.
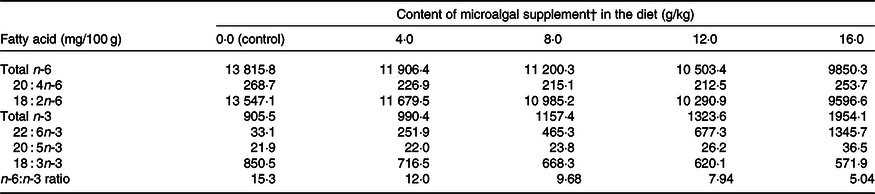
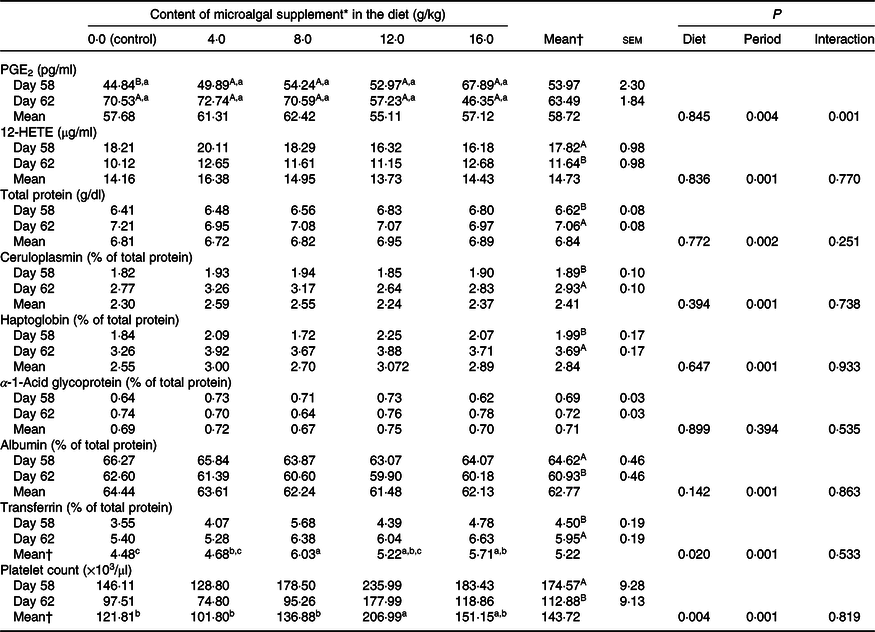
There was treatment and diet interaction (P = 0·001) on PGE2 levels. However, diet alone had no effect on this parameter, and PGE2 levels were higher (P < 0·05) after surgery only in the control group (Table 3). Serum total protein and ceruloplasmin, haptoglobin and transferrin concentrations increased after surgery (P < 0·001), whereas albumin concentration decreased (P < 0·05) when evaluated for period effect (Table 3). Protein levels were not influenced by microalgal supplementation (Table 3). There was a linear decrease (R 2 0·87; PGE2 = −11·3x + 43·44) on PGE2 increment (difference of the concentration after and before surgery) following the inclusion of microalgae in the diet (Fig. 1). 12-HETE decreased after surgery (P < 0·0001), and no treatment effect was observed (P = 0·836).
Table 3. Concentration of serum inflammatory markers immediately before neutering (day 58) and 96 h after neutering (day 62) in cats (males and females) fed the experimental diets for 62 d
(Mean values with their standard errors)
12-HETE, 12-hydroxyeicosatetraenoic acid.
a,b,c Mean values within a row with unlike lowercase superscript letters differ by Tukey’s test (P < 0·05).
A,B Mean values in a column within a marker with unlike uppercase superscript letters differ by Tukey’s test (P < 0·05).
* Dehydrated Schizochytrium spp. biomass (All-G Rich, Alltech Inc.).
† Letters included in the general means (period or treatment) indicate that there was no interaction between them (P > 0·05).
There was a reduction (P = 0·001) in platelet count in the postoperative period, and diet had a significant effect (P = 0·004) on this parameter. The highest platelet count was observed in cats fed diets containing 12 g/kg microalgae, which did not differ from that observed in cats fed diets supplemented with 16 g/kg microalgae (Table 3).
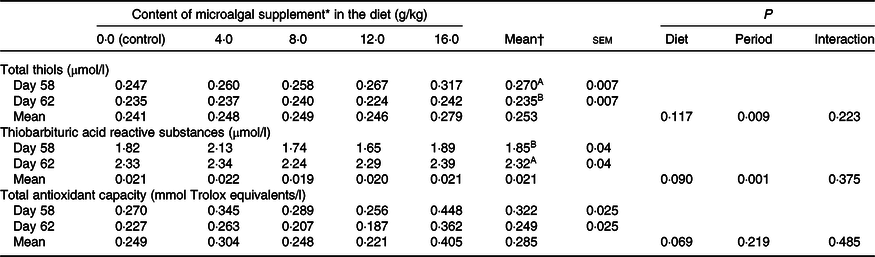
There was a reduction in serum thiol concentration (P = 0·009) and an increase (P = 0·001) in TBARS levels after surgery, showing the effect of neutering on oxidative status (Table 4). TAC did not vary significantly (P > 0·05). None of these parameters was influenced by diet (P > 0·05).
Table 4. Serum oxidative markers immediately before surgery (day 58) and 96 h after surgery (day 62) in cats (males and females) fed the experimental diets for 62 d
(Mean values with their standard errors)
A,B Mean values in a column within a marker with unlike superscript letters differ by Tukey’s test (P < 0·05).
* Dehydrated Schizochytrium spp. biomass (All-G Rich, Alltech Inc.).
† Letters included in the general means (period or treatment) indicate that there was no interaction between them (P > 0·05).
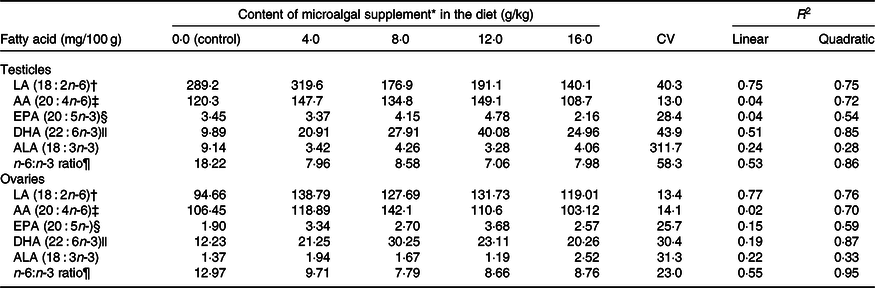
LA levels in testicles (R 2 0·75) and ovaries (R 2 0·77) reduced linearly with microalgal concentration (Table 5). On the other hand, DHA (testicles, R 2 0·85; ovaries, R 2 0·87), EPA (testicles, R 2 0·54; ovaries, R 2 0·59) and AA levels (testicles, R 2 0·72; ovaries, R 2 0·70) showed positive quadratic responses to microalgal concentration. It was estimated that the highest DHA deposition could be obtained using 10·93 and 9·26 g/kg microalgae in the diet for males and females, respectively. Microalgal concentration had a negative quadratic effect on n-6:n-3 ratio (testicles, R 2 0·86; ovaries, R 2 0·95). Modelling showed that the lowest n-6:n-3 ratio could be obtained by supplementing diets with 11·03 g/kg microalgae for males and 10·74 g/kg microalgae for females.
Table 5. n-6 and n-3 Fatty acid accumulation in the testicles and ovaries of cats fed an unsupplemented diet or a diet supplemented with different concentrations of microalgal biomass
(Mean values and coefficients of variation)
LA, linoleic acid; AA, arachidonic acid; ALA, α-linolenic acid.
* Dehydrated Schizochytrium spp. biomass (All-G Rich; Alltech Inc.).
† The relationship between microalgal concentration and LA accumulation in testicles is described by the equation LA = –10·67x + 308·78 and in ovaries by LA = –1·38x + 143·32.
‡ The relationship between microalgal concentration and AA accumulation in testicles is described by the equation AA = –0·483x 2 + 7·19x + 101·02 and in ovaries by AA = –0·422x 2 + 6·38x + 105·71.
§ The relationship between microalgal concentration and EPA accumulation in testicles is described by the equation EPA = –0·0233x 2 + 0·3443x + 3·07 and in ovaries by EPA = –0·0155x 2 + 0·2906x + 2·00.
|| The relationship between microalgal concentration and DHA accumulation in testicles is described by the equation DHA = –0·2103x 2 + 4·5977x + 8·158 and in ovaries by DHA = –0·178x 2 + 3·2966x + 12·13.
¶ The relationship between microalgal concentration and n-6:n-3 ratio in testicles is described by the equation n-6:n-3 ratio = 0·097x 2 − 2·14x + 19·03 and in ovaries by n-6:n-3 ratio = 0·044x 2 − 0·945x + 13·87.
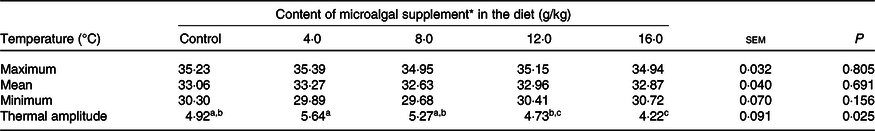
There was no difference in skin surface temperature (maximum, P = 0·805; mean, P = 0·691; minimum, P = 0·156) between treatments (Table 6). However, the thermal amplitude was lower (P = 0·025) in cats fed diets containing 16 g/kg microalgae. The correlation between surface temperature and PGE2 serum levels was high in the postoperative period (R 2 0·912; P < 0·0001).
Table 6. Surface temperature of surgical wounds of cats (males and females) fed the experimental diets
(Mean values with their standard errors)
a,b,c Mean values within a row with unlike superscript letters differ by Tukey’s test (P < 0·05).
* Dehydrated Schizochytrium spp. biomass (All-G Rich; Alltech Inc.).
Discussion
Marine microalgae of the genus Schizochytrium have high PUFA synthase activity and are cultivated commercially as a source of DHA(Reference Hauvermale, Kuner and Rosenzweig29). As an essential component of cell membranes, DHA modulates barrier properties and has a regulatory role in cell signalling. This long-chain PUFA is abundant in the retina and brain grey matter(Reference Fedorova-Dahms, Marone and Bauter30). Both DHA and EPA can modulate T-helper-cell responses and inhibit delayed hypersensitivity reactions and T-cell proliferation(Reference Fedorova-Dahms, Marone and Bauter30,Reference Chapkin, Kim and Lupton31) . Three mechanisms of action have been proposed to explain the immunomodulatory properties of DHA and EPA: (i) regulation of nuclear receptor activation, including suppression of NF-κB activity; (ii) inhibition of AA metabolism and reduction of PGE2 production by COX and (iii) alteration of cell membrane lipid rafts, which might alter the function of toll-like receptors and the recruitment of T-lymphocytes to effector sites(Reference Chapkin, Kim and Lupton31).
There is no recommended daily intake of EPA and DHA for adult cats(7). Nevertheless, these fatty acids have been widely used in commercial diets because of their nutraceutical properties. During early growth and reproduction, cat diets should provide between 4·5 and 7·5 mg/kg BW0·67 of EPA and/or DHA(7).
In the present study, diets supplemented with 0·81, 6·97, 11·78, 16·02 or 36·05 mg of DHA/kg BW0·67 altered the serum response of PGE2 of the cats after neutering, causing a concentration-dependent linear reduction (R 2 0·865) in this eicosanoid in the postoperative period. PGE2 is synthesised from AA molecules in the sn-2 position of membrane phospholipids by the action of COX, mainly COX-2, which has higher pro-inflammatory and mitogenic action than other COX isoforms(Reference Funk32,Reference Wall, Ross and Fitzgerald33) . As the presence of AA in cell membranes is essential for the synthesis of PG, limiting the availability of this fatty acid can modulate PGE2 synthesis and decrease inflammatory responses(Reference Bagga, Wang and Farias-Eisner11). Accordingly, incorporation of n-3 fatty acids into the cell membrane was shown to decrease PGE2 synthesis in favour of the production of PGE3, a less inflammatory PG(Reference Broughton, Bayesa and Culver34). Bagga et al. (Reference Bagga, Wang and Farias-Eisner11) found that PGE3 is less efficient in inducing COX-2 gene expression in NIH 3T3 fibroblasts and production of pro-inflammatory cytokines in RAW 264·7 macrophages, such as IL6, and has lower mitogenic activity than PGE2. The findings of the present study do not allow drawing conclusions about PGE3 production in postoperative cats, as this PG was not quantified. However, the linear decrease in PGE2 levels produced by microalgal supplementation and the significant increase in PGE2 concentrations observed in control cats in the postoperative period support the in vivo modulatory effects of n-3 fatty acids on inflammatory responses mediated by PGE2. As the DHA is not directly a precursor of the PGE3 formation, the observed effect on the PGE2 reduction after this fatty acid supplementation was probably related to the competitive deposition of the n-3 fatty acids, instead of than the absolute concentration of the n-6 fatty acids into the cell membrane(Reference Bagga, Wang and Farias-Eisner11,Reference Broughton, Bayesa and Culver34) .
Another eicosanoid investigated in the present study was 12-HETE. There was no difference in serum 12-HETE levels between treatments, but a significant decrease was observed in all groups in the postoperative period. This metabolite is produced from AA by 12-lipoxygenase, an enzyme expressed preferentially in platelets and implicated in local eicosanoid synthesis. Although its effects are not fully understood, 12-HETE, similarly to AA, seems to induce platelet activation and aggregation and cell chemotaxis(Reference Kühn and O’Donnell35–Reference Porro, Songia and Squellerio37). Moreover, 12-HETE plays an important role in angiogenesis and tissue regeneration by stimulating the proliferation of endothelial cells(Reference Nie, Tang and Diglio38,Reference Burzaco, Conde and Parada39) . Gomolka et al. (Reference Gomolka, Siegert and Blossey40) measured, in blood samples from human and mouse, metabolites of n-6 (AA) and n-3 (EPA and DHA) fatty acids in Ca ionophore-activated plasma and observed an increase in 12-lipoxygenase products, including AA-derived 12-HETE and n-3-derived 14-HDHA. These results contrast with our findings of decreased serum 12-HETE levels post-surgery. The low 12-HETE levels observed may be related to reduced platelet counts, as platelet count and serum 12-HETE concentration are highly correlated(Reference Katoh, Ikeda and Murohara41), as observed in dogs.
Platelets can induce vascular constriction, local inflammation and the release of chemokines in response to tissue damage(Reference Klinger and Jelkmann42,Reference Harrison43) . A diet rich in n-6 fatty acids increases AA concentration in the cell membrane, and AA is not only a precursor of 12-HETE but also of thromboxane A2, which has a pro-aggregatory effect on platelets and contributes to the formation of thrombi. By increasing DHA intake and decreasing the n-6:n-3 ratio of the diet, the concentration of DHA in cell membranes increases, favouring the production of thromboxane A3, a less pro-aggregatory eicosanoid, to the detriment of thromboxane A2 synthesis(Reference Bagga, Wang and Farias-Eisner11,Reference Saker, Eddy and Thatcher44,Reference Calder45) . Saker et al. (Reference Saker, Eddy and Thatcher44) found that long-term supplementation of cat diets with fish oil significantly reduced platelet aggregation and increased bleeding time, but these effects were observed at a very low n-6:n-3 ratio (1·2:1). In the present study, we found a linear increase in platelet counts post-neutering with increasing DHA intake. This effect is probably related to the reduced pro-aggregatory activity of AA and increased anti-aggregatory activity of DHA, resulting in a greater number of circulating platelets in n-3-supplemented cats. The high correlation (R 0·651) between DHA intake and the number of circulating platelets seems to reinforce this hypothesis.
Acute-phase proteins are defined as proteins whose plasma concentration increases (positive acute-phase proteins) or decreases (negative acute-phase proteins) by at least 25 % during inflammation(Reference Gitlin, Colten, Pick and Landy46–Reference Schrödl, Büchler and Wendler49). Haptoglobin, α-1-acid glycoprotein and amyloid A are the most studied positive acute-phase proteins in cats. These inflammation markers increase 2–4 times 24 h after an inflammatory stimulus(Reference Gruys, Toussaint and Upragarin50–Reference Paltrinieri, Marchini and Gelain52). In the present study, different acute-phase proteins were quantified to confirm that the surgical procedure was effective in inducing an inflammatory response. Ceruloplasmin and haptoglobin showed a moderate increase of 55 and 85·4 %, respectively, whereas α-1-acid glycoprotein levels did not change after surgery. Albumin decreased in the postoperative period, as expected(Reference Jain, Gautam and Naseem47,Reference Anziliero, Bassi and Pain48) . Transferrin levels increased by 32·2 % after surgery. This protein appears to be a positive acute-phase protein in cats as well as birds, but the reasons for this are still unclear(Reference Gruys, Toussaint and Upragarin50). The results confirmed the occurrence of an inflammatory response to surgery. No difference was observed in acute-phase markers between control cats and cats fed n-3-enriched diets.
An increase in the production of cytokines and activation of oxidative enzymes, such as COX, lipoxygenase and epoxygenases, to produce eicosanoids, are inflammatory responses that modify the oxidative status of the body. Increased production of free radicals, mainly of reactive oxygen species, occurs during this process. Chronic inflammation is associated with oxidative stress and can potentially lead to degenerative diseases(Reference Halliwell53–Reference Siti, Kamisah and Kamsiah55). In the present study, we measured markers of oxidative stress in postoperative cats to investigate their correlation with the inflammatory response. There was no association between oxidative markers and DHA supplementation. Thiol levels decreased in the postoperative period, whereas TBARS levels increased. TAC was not significantly influenced by the surgical procedure. Thiols are antioxidant molecules that may be consumed during inflammation, TBARS is as an indicator of lipoperoxidation and TAC represents the redox status of the blood after a stressful event. The surgical procedure was effective in modifying the oxidative status, but DHA supplementation had no effect on oxidative parameters. It is known that hydrogen peroxide activates NF-κB, which in turn stimulates the production of inflammatory cytokines(Reference Coppo and Ghezzi17). Thiols, present in GSH, have antioxidant action and inhibit NF-κB. Thus, GSH acts as a regulator of the inflammatory process by capturing free radicals and serving as a signalling molecule. DHA has a positive effect on GSH gene expression(Reference Casañas-Sánchez, Pérez and Fabelo56). However, according to Ghezzi(Reference Ghezzi57), inflammatory processes stimulate the production of acute-phase proteins, resulting in the depletion of GSH. This is a possible explanation for the reduction in thiol levels after castration and the lack of effect of diet observed in the present study.
TBARS are important secondary lipid peroxidation compounds that are more stable and easily measured in tissues and biological fluids than primary compounds(Reference Birben, Sahiner and Sackesen58). Inflammation may favour oxidative stress, as evidenced by the TBARS levels in the postoperative period. An increase in TBARS levels might have occurred as a result of lipase- and lipoxygenase-mediated lipid oxidation. These enzymes are released by degranulation of immune cells and tissue damage(Reference Lim, Raftery and Goyette59). However, no differences in TBARS were observed between DHA-supplemented cats. Some studies in companion animals found a higher concentration of TBARS when using diets with higher levels of n-3 fatty acids(Reference Vaagenes, Muna and Madsen60–Reference Verbrugghe, Janssens and Van de Velde62). DHA controls the expression of genes that regulate peroxisomes, directly affecting these organelles responsible for storing enzymes that catalyse reactive oxygen species generation. An in vitro study shows that the main product of DHA oxidation, 4-hydroxyhexenal aldehyde, positively regulates the Nrf2/HO-1 antioxidant signalling pathway. Nrf2 also modulates the expression of protein-coding genes with antioxidant and anti-inflammatory function(Reference Di Nunzio, Valli and Bordoni63). It should be noted that cats tolerate higher amounts of fat in the diet and are more resistant to diet-induced inflammation and oxidative stress than other mammals(1).
PUFA are important cell constituents and play an important role in the fertility. DHA is found in high levels in human semen and has been associated with high sperm quality and male fertility status(Reference Caesar, Manieri and Kelder64,Reference Wang, Wu and Wen65) . Martinez-Soto et al. (Reference Martínez-Soto, Landeras and Gadea66) reported that DHA is positively correlated with sperm antioxidant capacity, viability and motility after freezing/thawing. The same authors in another study(Reference Martínez-Soto, Domingo and Cordobilla67) analysed the effect of dietary supplementation with 1500 mg/d of fish oil (n 32 men) or sunflower oil (n 25 men) for 10 weeks in fifty-seven men. There was no difference in traditional sperm parameters or lipid composition of the spermatozoon membrane after treatments. However, the authors observed an increase in DHA and n-3 fatty acids content in seminal plasma, followed by an improvement in antioxidant status and a reduction in the percentage of spermatozoa with DNA damage. The antioxidant effects of DHA in seminal plasma improve fertility in men, mainly in asthenozoospermic patients(Reference González-Ravina, Aguirre-Lipperheide and Pinto68). In females, PG have a regulatory influence on ovulation. EPA and DHA decrease PGE2 synthesis, stimulating ovulation. Broughton et al. (Reference Broughton, Bayesa and Culver34), studying the effects of EPA and DHA supplementation in rats, found that these fatty acids were primarily deposited in membrane phospholipids of the ovarian tissue, which increased the number of ova released, probably as a result of reduced PGE2 synthesis. These authors also observed a reduction in AA content in the ovarian tissue.
Van Niel & Beynen(Reference Van Niel and Beynen69) found strong correlations between the intake of LA (R 0·99), ALA (R 0·99), EPA (R 0·94) and DHA (R 0·91) and their deposition in cutaneous adipose tissue in cats. Dietary concentrations of n-3 fatty acids were shown to determine their deposition in the female reproductive system (follicular fluid, granulosa cells, cumulus–oocyte complex and ovarian tissues) in cattle, ewes, mice and rats, but the biological significance of these findings remains controversial(Reference Zarezadeh, Mehdizadeh and Leroy70). It has been suggested that the enhancement of ovulation and fertility promoted by n-3 PUFA might be due to a shift in the biosynthesis of PGE2 towards the production of less active PGE3, thereby preventing the anti-ovulatory effects of excessive PGE2(Reference Zarezadeh, Mehdizadeh and Leroy70). In the present study, a higher intake of DHA was accompanied by increased deposition of this fatty acid in the reproductive tissue of both females and males. However, cats probably have limited capacity to accumulate DHA in gonads. Although the quadratic model has shown a good fit, it seems that from the lower inclusion of microalgae in the diet, there was a tendency towards deposition stability, which could also be explained by the broken line model, suggesting limited ability to deposit DHA in this tissue by this specie. Maroufyan et al.(Reference Maroufyan, Kasim and Ebrahimi71) supplemented diets for broilers with tuna oil (as n-3 fatty acids source) and observed results similar to our study. At 42 d, n-3 fatty acids in plasma and breast were lower when birds were fed the lowest n-3 fatty acids concentration in the diet (8·0 % of the total fatty acids), but had limited additional DHA deposition following increased dietary DHA concentration (11·0 and 16·5 % of the total fatty acids). There are different methods for modelling experiments with nutrient levels, which are classified as linear and non-linear. The most used linear model is the broken line, where a linear response is estimated following an increase of a nutrient in the diet, which subsequently reaches a plateau. However, responses can also be adjusted to curvilinear or even sigmoid models, as demonstrated by other studies(Reference Maroufyan, Kasim and Ebrahimi71–Reference Mercer, May and Dodds73). The deposition of DHA in other tissues of the cats was not measured. Furthermore, the reduced amount of experimental units used in the present study limits the discussion about these findings. However, mathematical modelling showed that the maximum DHA deposition was 33·29 mg/100 g for males and 27·39 mg/100 g for females and could be achieved with microalgal concentrations of 10·93 and 9·26 g/kg, respectively. Similar results were obtained for the n-6:n-3 ratio. The lowest ratio was estimated at 11·03 for males and 10·74 for females, achieved with 7·23 and 8·80 g/kg microalgae, respectively. The correlation between DHA deposition in gonads and reproductive parameters was not investigated in the present study because cats were only 11 months old and there was not sufficient gonadal material to perform the analyses. The present study was limited by the small gonadal sample size, which only allowed the analysis of the total fatty acid profile. Thus, it was not possible to correlate DHA deposition and functional aspects, such as histological and seminal or follicular quality evaluation. The fatty acid deposition in the tissues does not ensure their biological effect as verified in human cells by other authors(Reference Hawcroft, Loadman and Belluzzi74). Thus, further studies are needed to define whether there is an association between n-3 fatty acids deposition and reproductive functions in cats. Further studies are needed to define whether there is an association between n-3 fatty acids deposition and reproductive functions in cats.
n-3 Fatty acids can alter the expression of pro-inflammatory cytokine genes and suppress endothelial inflammatory activity by inhibiting the production of nitric oxide and PGE2, thereby affecting wound healing(Reference Oliveira and Nunes-Pinheiro75,Reference Soleimani, Hashemdokht and Bahmani76) . We found that dietary supplementation with DHA-rich microalgae caused a decrease in surgical wound temperature in healthy cats. PGE2 and leukotriene B4 are AA-derived inflammatory mediators known to induce fever, pain, vasodilation, swelling, redness, neutrophil chemotaxis and extravasation of vascular fluids, all of which result in increased temperature at the site of tissue injury(Reference James, Gibson and Cleland77). In the present study, the reduction in wound temperature may be explained by the decreasing effect of DHA intake on serum PGE2 levels. More specific analyses are needed to confirm this hypothesis.
Conclusions
The findings of the present study suggest that DHA from Schizochytrium spp. modifies inflammatory responses and haemodynamic mechanisms of wound healing in cats subjected to surgical castration without altering markers of oxidative status. Cats can store DHA in reproductive tissues, but this ability appears to be limited and its effects on reproduction need to be elucidated.
Acknowledgements
The authors thank the collaboration of Professor Luiz Paulo Rigolon, Andressa Francisca Silva Nogueira and Michelle Campano de Souza.
The authors are grateful for the financial support provided by Alltech do Brasil for laboratory analyses. The scholarship of Suellen Scheibel was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Alltech do Brasil and CAPES have no role in the design, analysis or writing of this article.
The authors’ contributions were as follows: S. S., C. A. L. O., J. V. V. and R. S. V. contributed to the study design and data analyses; S. S., M. A. B. and L. C. P. contributed to the study design, subject briefings and data collection; D. M. R., M. R. L. B., F. C. and R. R. H. contributed to the study design and carried out data analyses; S. S., L. B. R. and R. S. V. contributed to the study, revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.










