Atherosclerosis is a multi-factorial disease characterised by lipid deposition, cellular infiltration, proliferation of the smooth muscle cells and intimal thickening with subsequent thrombus formation(Reference Ross1). ApoE deficiency causes defects in lipolysis, and it has an important role in the transport of TAG and cholesterol by VLDL and chylomicrons(Reference Mahley and Rall2). Thus, apoE knockout (apoE − / − ) mice have high lipid levels in their plasma and cholesterol-rich β-VLDL and spontaneously develop atherosclerosis(Reference Mahley and Rall2), being an accepted model for the study of the modulation of atherosclerotic lesions by dietary factors(Reference Osada, Joven and Maeda3).
The transendothelial migration of monocytes is an important early step in atherosclerosis and is mediated by endothelial adhesion molecules. Selectins are involved in the primary monocyte–endothelium interaction (tethering and rolling)(Reference Lasky4). Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are responsible for the secondary monocyte–endothelium interaction (firm adhesion)(Reference Adams and Shaw5), and their expression is up-regulated in atherosclerotic lesions of hypercholesterolaemic mice(Reference Nakashima, Raines and Plump6). NF-κB is a redox-sensitive inducible transcription factor that is activated in response to various extracellular stimuli and regulates the mRNA expression of endothelial adhesion molecules(Reference Barnes and Karin7).
An inverse association between flavonoids(Reference Hertog, Kromhout and Aravanis8), red wine(Reference Renaud and de Lorgeril9) and beer(Reference Brenner, Rothenbacher and Bode10) intake and CHD development has been demonstrated. Polyphenols have antioxidant activity in vitro (Reference Rice-Evans, Miller and Paganga11) and ex vivo (Reference Lopez, Pavelkova and Gallova12, Reference Whitehead, Robinson and Allaway13), which is related to cardiovascular protection(Reference Hertog, Kromhout and Aravanis8). There is a long list of beverages and foods with a high phenolic content, and beer provides more antioxidants per day than wine(Reference Vinson, Mandarano and Hirst14) as it is consumed in larger amounts than wine in the USA(Reference Klatsky, Armstrong and Friedman15) and Europe(Reference Pyorala16). It is a popular beverage that has been produced for over 4000 years, and a moderate consumption of beer is now related to a decreased plasma C-reactive protein concentration, a biomarker for vascular inflammation in early stages of atherosclerosis, mediated by the alcohol present in beer(Reference Sierksma, van der Gaag and Kluft17). However, the presence of polyphenols in beers raises the question of whether health benefits may be associated with the non-alcoholic components of beers.
We hypothesise that the phenolic content reduces the progression of atherosclerosis by modulating one important initial step in the disease, the transmigration of monocytes to the intima. Thus, we studied the beneficial effect of polyphenols from beers on the development of atheroma plaques by supplementing the diet of apoE − / − mice with two different types (lager (LB) and dark (DB)) of dealcoholised beers. As the internalisation of monocytes through the endothelium is regulated by endothelial adhesion molecules, we analysed their expression in the aorta of apoE − / − mice and NF-κB involvement.
Materials and methods
Chemicals
Mineral and vitamin mixes of diets were purchased from ICN Biomedicals (Aurora, OH, USA). 4-Hydroxyhippuric acid (>99 %) was obtained from PhytoLab GmbH & Co. KG (Vestenbergsgreuth, Germany). Vanillic acid, m-coumaric acid, tyrosol, hydroxytyrosol, 4-O-methylgallic acid and taxifolin (>90 %) were purchased from Extrasynthèse (Genay, France). HPLC-grade methanol, acetonitrile and formic acid were from Scharlau (Barcelona, Spain). The NucleoSpin kit for RNA extraction was purchased from Macherey-Nagel (Düren, Germany), and the kit for the retrotranscription was from Roche Diagnostics (Mannheim, Germany). The oligonucleotides for the PCR were obtained from Bonsai Technologies Group (Madrid, Spain). The optical cutting temperature was from Tissue-Tek (Dublin, Ireland). Rabbit anti-mouse P-selectin antibody was obtained from Chemicon Immunostains (Hampshire, UK), rat anti-mouse VCAM-1 was from eBioscience (San Diego, CA, USA), goat anti-mouse ICAM-1 was from R&D Systems (Abingdon, UK) and rabbit anti-mouse NF-κB-p65 (Ser 276) was obtained from Thermo Fisher Scientific (Chesire, CT, USA). The secondary antibodies for immunofluorescence (donkey anti-rabbit 647, donkey anti-rat 488 and donkey anti-goat 546) were purchased from Molecular Probes (Poort Gebouw, The Netherlands). All other standards for analyses of beers and plasma, components of diets and reagents used were obtained from Sigma-Aldrich-Fluka (St Louis, MO, USA).
Animals and diets
Male C57BL/6J apoE − / − mice (Charles River, L'Arbresle, France) were housed in temperature (21–23°C) and humidity (40–60 %)-controlled rooms and exposed to 12 h light–12 h dark cycles. At 4 weeks of age, they were placed randomly into three groups of fourteen mice each and were fed one of the following semi-purified diets for 20 weeks: (1) a control diet; (2) a dealcoholised lager beer (DLB)-rich diet; (3) a dealcoholised dark beer (DDB)-rich diet (Table 1). The semi-purified diet was prepared weekly. Solid components of the diet were mixed with 210 ml/kg water (control diet) or with 210 ml/kg beer, which replaced the water in treated mice. The diet mixes were air dried for 18 h and stored at − 20°C to prevent oxidation and loss of antioxidants. The dose of dealcoholised beers that mice ingested with the diet is 42 ml beer/kg body weight/d which is equivalent to about 500 ml of daily consumption for humans, as mice have more surface area per unit of weight than humans(Reference Pinkel18). All diets were supplemented with 0·15 % cholesterol to accelerate the development of atherosclerosis. The quantity was restricted to 5–6 g/d to avoid differences in the diet and cholesterol ingestion. Food was provided and removed daily. All the mice were weighed weekly and examined after fasting overnight. Eight mice were used to evaluate the plasma lipid profile and the atherosclerotic lesions, and the other six were used to measure the phenols in the plasma; the adhesion molecules were measured by RT-PCR and immunofluorescence assays. The procedures and care of the animals were performed according to the University of Barcelona Ethical Committee for Animal Experimentation, following European Union guidelines.
Table 1 Composition of semi-purified diets
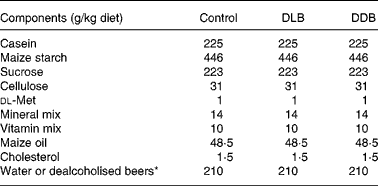
DLB, dealcoholised lager beer; DDB, dealcoholised dark beer.
* The 210 ml DLB or DDB replaced the 210 ml water to compact the diet.
Spanish commercial LB and DB were dealcoholised in a rotary evaporator at 30°C by progressively applying a vacuum up to − 70 mbars. The volume of ethanol was replaced by acidulated distilled water as reported previously(Reference Benito, Buxaderas and Mitjavila19). A mass spectrometer (Fisons MD800; Thermo Finnigan, Ringoes, NJ, USA) connected to a headspace gas chromatograph (GC8000-Top, Carlo Erba, Milan, Italy) was used to evaluate the residual ethanol in dealcoholised beers, with 2-methyl-1-propanol as the standard.
Beer analysis
Total phenols in 1:10 diluted beers were measured by the Folin–Ciocalteau colorimetric method(Reference Singleton and Rossi20), with gallic acid as the standard, and the results are expressed as mm-gallic acid equivalents. The reducing power in 1:20 diluted beers was evaluated using the method described by Oyaizu(Reference Oyaizu21). Briefly, beers were mixed with PBS (200 mmol/l, pH 6·6) and 1 % potassium ferricyanide and incubated for 20 min at 50°C, and 10 % TCA was then added. After centrifugation, 0·1 % ferric chloride was added and the absorbance was measured at 690 nm. Quercetin was used as the standard, and values are expressed as mm-quercetin equivalents.
The determination and quantification of individual phenolic compounds(Reference Gerhauser22) in dealcoholised beers (DLB and DDB) were analysed directly by LC tandem MS (LC-MS/MS)(Reference Urpi-Sarda, Monagas and Khan23) adapted to the matrix of beer. The concentrations of phenolic compounds in beers are expressed as mg/l.
LC tandem MS
LC analyses were performed using an Agilent 1200 system equipped with a quaternary pump and a refrigerated autosampler plate (Waldbronn, Germany). An Applied Biosystems API 3000 Triple Quadrupole mass spectrometer (PE Sciex, Concord, ON, Canada), equipped with a Turboionspray ionisation source in negative mode, was used. A Phenomenex Luna C18 analytical column (50 × 2·0 mm internal diameter, 5 μm; Luna, Torrance, CA, USA) with mobile phase A (0·1 % formic acid in water) and B (0·1 % formic acid in acetonitrile) was used. The linear gradient at a flow rate of 400 μl/min was (% mobile phase B, time (min)) (8, 0), (50, 4), (100, 5·2) and (100, 7)(Reference Urpi-Sarda, Monagas and Khan23). In each case, the column was re-equilibrated for 6 min, and the sample volume injected was 10 μl. MS/MS parameters used were as follows: capillary voltage, − 3700 V; focusing potential, − 200 V; entrance potential, − 10 V; declustering potential, − 50 V; nebuliser gas, 10 (arbitrary units); curtain gas, 12 (arbitrary units); collision gas, 5 (arbitrary units); auxiliary gas temperature, 400°C; auxiliary gas flow rate, 6000 cm3/min. The collision energy for phenolic acids was optimised for each compound as described elsewhere(Reference Urpi-Sarda, Monagas and Khan23). For quantification purposes, data were collected in the multiple reaction monitoring mode, in which the transition of parent and product ions specific for each compound is tracked.
Plasma analyses
At the end of the treatment period (20 weeks) and after a 14–16 h fasting, mice were anaesthetised with 150 mg/kg ketamine–10 mg/kg xilacin and exsanguinated by left ventricle puncture. Plasma was obtained by blood centrifugation at 1770 g for 15 min at 4°C and was stored at − 80°C until analysis.
Plasma concentrations of TAG and total cholesterol were evaluated enzymatically using commercial kits (Randox, Crumlin, UK).
Bioavailability of beer phenols in the plasma
The bioavailability of beer phenols was assessed by the analysis of phenolic microbial metabolites because the plasma samples were from fasted mice(Reference Rios, Gonthier and Rémésy24–Reference Urpi-Sarda, Llorach and Khan26). They were evaluated following a published and validated methodology as described previously(Reference Urpi-Sarda, Monagas and Khan23). Briefly, 400 μl plasma were spiked with ethyl gallate as an internal standard and subjected to enzymatic hydrolysis. Solid-phase extraction was performed using Oasis MCX ninety-six-well plates (Waters, Milford, MA, USA). The plates were conditioned with methanol and 2 % formic acid in water. The hydrolysed samples were then loaded onto a plate and washed in this acidified water. The analytes were then eluted with methanol and evaporated to dryness. Residues were reconstituted with taxifolin dissolved in mobile phase A (0·1 % formic acid in water) and analysed by LC-MS/MS.
To evaluate plasma samples from mice fed for 20 weeks with dealcoholised beers, we prepared calibration curves in the blank plasma in the range of concentrations expected, by supplementation with known concentrations of phenolic acids and flavonoids.
Atherosclerotic lesions
The thoracic aorta (including the ascending aorta, the aortic arch and the descending aorta) was isolated after perfusion with 4 % paraformaldehyde, cleaned of attached connective tissue, opened lengthwise and fixed in 4 % paraformaldehyde overnight. The atherosclerotic lesions were visible and clearly distinguishable from the lesion-free areas on the luminal surface of the vessels without staining. Images were taken on a digital camera (Olympus BX-40, Hamburg, Germany) and recorded in 24-bit true image format, and the lesions were quantified with the Analysis-Soft Imaging System software (Olympus Soft Imaging Solutions GmbH, Münster, Germany). The lesion area from each mouse is expressed as the percentage of the total luminal surface.
P-selectin, vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 mRNA expression by RT-PCR
RNA extraction from the total aorta (thoracic and abdominal aorta) was carried out using the NucleoSpin Macherey-Nagel kit. The quality of the extraction was checked in a 0·8 % agarose gel, and it was quantified by spectrophotometry (Genesis UV, Thermo Spectronic, Waltham, MA, USA).
RT was performed with 1 μg total RNA using the Roche Diagnostics kit, and the PCR was done with a Thermocycler Tpersonal 48 230 V (Biometra, Goettingen, Germany). We designed the oligonucleotide sequences for P-selectin, VCAM-1 and ICAM-1 (Table 2). The hypoxanthine–guanine phosphoribosyl transferase gene was used as an internal standard in the reaction for normalisation using specific primer sequences. Primers were tested for linearity over cycle number, and the analyses were performed in the linear portion of the curve, allowing semi-quantitative analysis of mRNA amount. The annealing temperature was set at 53°C for all gene sequences. PCR products were run on a 2 % agarose gel, and ethidium bromide bands were visualised and quantified with ImageJ 1.40 g (National Institutes of Health, Bethesda, MD, USA). Each amplified product was normalised by dividing the average grey level of the signal by that of the corresponding hypoxanthine–guanine phosphoribosyl transferase PCR band. Data are expressed as the percentage of mRNA expression with respect to the control group.
Table 2 Sequences of primers for semi-quantitative RT-PCR

VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; hprt, hypoxanthine–guanine phosphoribosyl transferase.
P-selectin, vascular cell adhesion molecule-1, intercellular adhesion molecule-1 and NF-κB expression by immunofluorescence
The heart was perfused, fixed with 4 % paraformaldehyde and extracted from the mice. Each heart was cryoprotected overnight with 35 % sucrose at 4°C under mild shaking and was then frozen on a cryostat mount with optical cutting temperature. Serial cross-sections of 10 μm thickness of the aortic root were obtained from the top of the left ventricle, where the aortic valves were first visible, up to where the valve cusps disappeared.
Five cross-sections (one for a negative control) of the aortic root of each mouse were washed in 10 mm-PBS and 20 mm-glycine and blocked with 1 % bovine serum albumin in 10 mm-PBS. We used the following primary antibodies: rabbit anti-mouse P-selectin (5 mg/l); rat anti-mouse VCAM-1 (20 mg/l); goat anti-mouse ICAM-1 (2 mg/l); rabbit anti-mouse NF-κB-p65 (Ser 276) (20 mg/l). After incubation with the corresponding secondary antibodies (donkey anti-rabbit 647, donkey anti-rat 488 and donkey anti-goat 546) in a concentration of 4 mg/l, the images were captured with a confocal inverted fluorescence microscope (Olympus Fluoview 500, IX-70), and the fluorescence was quantified in lesion areas by Metamorph analyser software (Universal Imaging Corporation, San Diego, CA, USA). Data are expressed as the percentage of NF-κB expression with respect to the control group.
Statistical analysis
Values are means with their standard errors. In the phenolic composition of dealcoholised beers, values are means and standard deviations. The LB:DLB and the DB:DDB were compared by Hotelling's T 2 statistic analysis, and the samples were independently drawn from two independent multivariate normal distributions of two dimensions. Comparisons between alcoholised and dealcoholised beers were carried out by Student's t test for paired samples. The differences between groups of mice treated with control diets and diets supplemented with dealcoholised beers were analysed by one-way ANOVA and least significant difference multiple comparison test for post hoc analysis. Differences were considered significant at P < 0·05. All statistical analyses were performed using SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Beer analyses
The peaks of residual ethanol and the standard (2-methyl-1-propanol) in beers after rotary evaporation were detected at 5·58 and 7·64 min, respectively, and the beers were considered non-alcoholic.
Dealcoholisation did not alter the total phenol content in either type of beer (5·68 (se 0·29) and 6·15 (se 0·54) mm-gallic acid equivalents for LB and DLB, respectively, and 5·96 (se 0·31) and 6·38 (se 0·91) mm-gallic acid equivalents for DB and DDB, respectively). No significant differences were observed in beers in terms of the reducing power before and after dealcoholisation (1·58 (se 0·04) and 1·54 (se 0·02) mm-quercetin equivalents for LB and DLB, respectively, and 1·69 (se 0·04) and 1·74 (se 0·06) mm-quercetin equivalents for DB and DDB, respectively), but DB had a significant greater reducing power than LB.
Individual phenolic composition of dealcoholised beers is shown in Table 3.
Table 3 Phenolic composition of dealcoholised lager beer (DLB) and dealcoholised dark beer (DDB)*
(Mean values and standard deviations)

MRM, multiple reaction monitoring; ND, not detected.
* Data were collected in the MRM mode.
Body weight and plasma analyses
All mice thrived and gained similar amounts of weight throughout the study (Table 4).
Table 4 Effect of dietary supplementation with dealcoholised lager beer (DLB) and dealcoholised dark beer (DDB) for 20 weeks on body-weight gain and plasma TAG and total cholesterol concentrations in apoE−/− mice*
(Mean values with their standard errors, n 8)
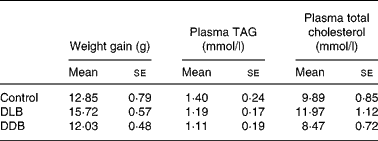
* There were no differences between groups (one-way ANOVA).
The concentrations of TAG and total cholesterol in the plasma after 20 weeks of treatment with dealcoholised beers were similar in the three groups (Table 4).
Plasma samples from mice treated with dealcoholised beers for 20 weeks were analysed by LC-MS/MS after sample hydrolysis and solid-phase extraction. Among the thirty-seven compounds studied, including phenols from beers and phenolic microbial metabolites, 4-hydroxyhippuric acid increased by 80 % (P = 0·005) and 68 % (P = 0·01) after the DLB and DDB diets, respectively, and 3,4-dihydroxyphenylacetic acid increased by 84 % (P = 0·019) and 82 % (P = 0·021) after the DLB and DDB diets, respectively. There were no other significant differences in the metabolites evaluated.
Dealcoholised beer-rich diets decreased the atherosclerotic lesions in the thoracic aorta of apoE−/− mice
Aortic lesions progressed to mature and complex fatty plaques resembling advanced human lesions. The mean percentage of the surface covered by the atherosclerotic lesions was 21 % in the control group. However, the lesions were less extensive in the DLB (12 %, P = 0·003) and DDB (11 %, P < 0·001)-supplemented apoE − / − mice (Fig. 1).

Fig. 1 Dietary supplementation with dealcoholised lager beer (DLB) and dealcoholised dark beer (DDB) for 20 weeks reduces the atheroma plaques in longitudinal sections of the thoracic aorta from apoE − / − mice. Percentage of atherosclerotic lesions relative to the total aortic area. Values are means, with standard errors represented by vertical bars (n 8). a,b Mean values with unlike letters were significantly different (P < 0·05).
Dealcoholised beer-rich diets decreased the mRNA expression of the endothelial adhesion molecules in the aorta of apoE−/− mice
The mRNA expression of P-selectin was significantly reduced by 17 % (P = 0·004) in the group supplemented with DDB (Fig. 2(A)) with respect to the control group. Beer supplementation had the greatest effect on the mRNA expression of VCAM-1 (20 %, P = 0·012 and 32 %, P = 0·001, of reduction for the DLB- and DDB-treated mice, respectively) (Fig. 2(B)). The mRNA expression of ICAM-1 was reduced in the two treated groups by about 14 % (Fig. 2(C)), but a significant difference (P = 0·014) was only detected for the DLB-treated mice.

Fig. 2 Dietary supplementation with dealcoholised lager beer (DLB) and dealcoholised dark beer (DDB) for 20 weeks reduces the mRNA and protein expression of adhesion molecules in the total aorta and the aortic root, respectively, from apoE − / − mice. Percentage of the mRNA or protein expression relative to the control group. (A) mRNA expression of P-selectin; (B) mRNA expression of vascular cell adhesion molecule-1 (VCAM-1); (C) mRNA expression of intercellular adhesion molecule-1 (ICAM-1); (D) protein expression of P-selectin; (E) protein expression of VCAM-1; (F) protein expression of ICAM-1. Values are means, with standard errors represented by vertical bars (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05). hprt, Hypoxanthine–guanine phosphoribosyl transferase.
Dealcoholised beer-rich diets decreased the protein expression of the endothelial adhesion molecules and NF-κB in the aortic root of apoE−/− mice
P-selectin, VCAM-1 and ICAM-1 were expressed in the aortic root of 24-week-old control apoE − / − mice. We observed a reduction in P-selectin in the DDB-treated group (37 %, P = 0·012). VCAM-1 was decreased by 48 % (P = 0·001) and 54 % (P < 0·001) in the DLB- and DDB-treated groups, respectively, and ICAM-1 was reduced by 25 % (P = 0·028) and 30 % (P = 0·018) in the DLB- and DDB-treated groups, respectively (Fig. 2(D)–(F)).
NF-κB expression in apoE − / − mice supplemented with DLB and DDB was reduced by 30 and 46 %, respectively (Fig. 3), but the reduction was only significant (P = 0·042) for the DDB-treated mice.
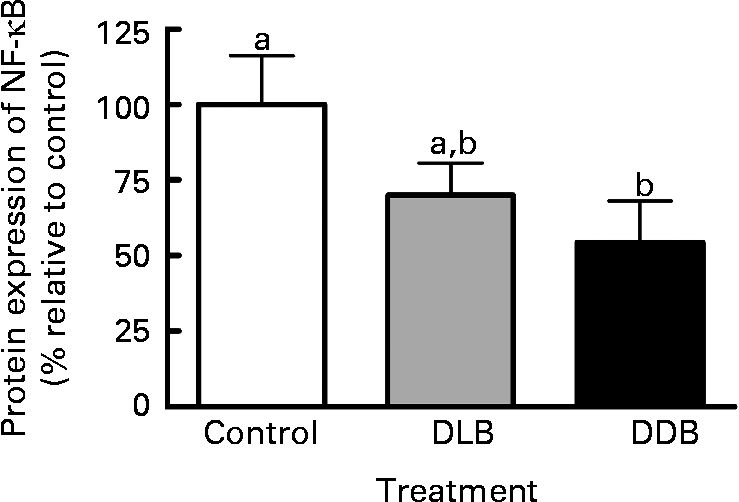
Fig. 3 Dietary supplementation with dealcoholised lager beer (DLB) and dealcoholised dark beer (DDB) for 20 weeks reduces the NF-κB expression in the aortic root from apoE − / − mice. Percentage of protein expression relative to the control group. Values are means, with standard errors represented by vertical bars (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05).
Discussion
Alcohol reduces the development of CHD(Reference Hashimoto, Futamura and Nakarai27) regardless of the type of drink(Reference Mukamal, Conigrave and Mittleman28). However, evidence also exists that components other than alcohol, such as polyphenols, might be involved in the protection afforded by beer(Reference Vinson, Mandarano and Hirst14). Most of the polyphenols in beer come from the barley and hops. Polyphenols have antioxidant activity(Reference Rice-Evans, Miller and Paganga11), and we show here that the dealcoholisation of beers does not affect their total phenolic content or reducing power, and that DB and DDB have a significantly greater reducing power than LB and DLB. Moreover, we demonstrate here that the phenolic compounds from beer are absorbed and metabolised. Significant amounts of 4-hydroxyhippuric and 3,4-dihydroxyphenylacetic acids were present in the plasma from mice fed with dealcoholised beers for 20 weeks. These metabolites have been reported as microbial metabolites after consumption of several polyphenols from cocoa(Reference Rios, Gonthier and Rémésy24, Reference Urpi-Sarda, Llorach and Khan26) or quercetin(Reference Selma, Espín and Tomás-Barberán25). Furthermore, both compounds inhibit the secretion of TNF-α in lipopolysaccharide-stimulated peripheral blood mononuclear cells in vitro (Reference Monagas, Khan and Andrés-Lacueva29). For all these reasons, we aimed to study the preventive effect of beer polyphenols on the development of atherosclerotic lesions and the possible underlying mechanisms.
The administration of alcohol-free beers did not change the plasma levels of TAG and total cholesterol in apoE − / − mice. A controversy exists regarding the efficacy of beer polyphenols in reducing circulating lipids(Reference Vinson, Mandarano and Hirst14, Reference Degrace, Moindrot and Mohamed30–Reference Tousoulis, Ntarladimas and Antoniades33), although most reliable data have been obtained from fasting animals. It is likely that many factors such as the amount of beer consumed, the age of initiation and the duration of the treatment, the presence or absence of alcohol or cholesterol in the diet and the species used would affect the results. In the present study, we used apoE − / − mice, which have defects in TAG and VLDL clearance(Reference Mahley and Rall2). We found that the aortic lesions were reduced in both groups of dealcoholised beer-treated mice, even without a reduction in plasma cholesterol and TAG concentrations.
The surface area occupied by the atheroma plaques is high in the aortic arch of apoE − / − mice, probably due to the turbulent flux generated by haemodynamic forces in the vascular endothelium(Reference Frangos, Gahtan and Sumpio34). Furthermore, Emeson et al. (Reference Emeson, Manaves and Singer35) postulated that the atheroma plaque deposition follows a caudal direction towards the abdominal aorta and secondary arteries. Controversial results also exist regarding the modulation of atherosclerotic lesions in alcohol-free beer-fed animals(Reference Vinson, Mandarano and Hirst14, Reference Escola-Gil, Calpe-Berdiel and Ribas36). In this sense, the present results can be compared to those of Vinson et al. (Reference Vinson, Mandarano and Hirst14), who administered diluted LB and DB to hamsters. It is evident that the protection afforded by beers in the present paper is due to their non-alcoholic compounds. The present results reinforce the proposal by Vinson et al. (Reference Vinson, Mandarano and Hirst14) that beer is equally as effective as red wine against atherosclerosis, despite the much greater content of polyphenols in wine. One reason for this could be the larger amount of beer consumed compared with wine in the US population(Reference Klatsky, Armstrong and Friedman15) and in Europe(Reference Pyorala16). Nevertheless, the mechanisms underlying the beneficial effect against atherosclerosis are unknown.
Atherosclerosis is associated with an inflammatory process, with accumulation of leucocytes in the subendothelial space and the involvement of the endothelial adhesion molecules in this step. There are studies based on the modulation of the expression of adhesion molecules and other inflammatory markers by phenolic compounds from alcoholic drinks in healthy subjects(Reference Martinez Alvarez, Belles and Lopez-Jaen32, Reference Tousoulis, Ntarladimas and Antoniades33, Reference Vazquez-Agell, Sacanella and Tobias37, Reference Imhof, Blagieva and Marx38) or in inflammatory processes such as coronary arterial disease or atherosclerosis(Reference Williams, Sutherland and Whelan39, Reference Appeldoorn, Bonnefoy and Lutters40). To the best of our knowledge, there are no reports focusing on the regulation of the inflammatory molecules by dealcoholised beers in atherosclerosis. Alcohol has been considered as responsible for the reduction of proinflammatory mediators(Reference Klatsky, Armstrong and Friedman15, Reference Imhof, Blagieva and Marx38). However, polyphenols from alcoholic drinks decrease the leucocyte interaction with the endothelium(Reference Appeldoorn, Bonnefoy and Lutters40) being also a clue for the protection in inflammation. A previous study by our group in which dealcoholised red wine was administered to healthy rats for 2 months showed reduced infiltration of polymorphonuclear leucocytes in an induced inflammatory process(Reference Lopez, Pavelkova and Gallova12), probably due to the antichemotactic effect of NO(Reference Hickey41) enhanced by wine polyphenols. This prompted us to perform the present study after administering dealcoholised beers to apoE − / − mice. The phenolic microbial metabolites in the plasma were similar in both groups treated with dealcoholised beers, indicating a similar bioavailability of phenols in beers, which is in accordance with the small differences in the reducing power of beers and the mRNA and protein expression of endothelial adhesion molecules.
The higher effect on the expression of VCAM-1 by dealcoholised beers can be related to the fact that it is an inducible molecule and its expression is restricted to lesions and lesion-predisposed areas, whereas ICAM-1 is a constitutive molecule that is expressed in all areas(Reference Iiyama, Hajra and Iiyama42). Moreover, P-selectin mediates the rolling of monocytes, and its decrease following the DDB-rich diet should be regarded as an additional factor in the reduction of VCAM-1. The decrease in the mRNA expression of P-selectin, VCAM-1 and ICAM-1 was corroborated by immunofluorescence. These findings suggest that dealcoholised beers attenuate the initiation of atherosclerosis by down-regulating the expression of endothelial adhesion molecules. Taken together, these results indicate that the protection against atherosclerosis afforded by the two types of beer is similar, which may be attributable to the fact that they have a similar phenolic content, and to the small difference in the reducing power in beers and to similar phenolic microbial metabolites in the plasma of mice treated with dealcoholised beers. Thus, the present study provides evidence for an in vivo anti-inflammatory mechanism that should be investigated in humans. However, since immoderate consumption has been implicated in processes such as cancer and heart disease, it would be unwise to consider beer as a healthy food without further qualification.
The regulation of the expression of endothelial adhesion molecules is complex and is controlled by numerous molecules implicated in inflammatory processes; among these, NF-κB plays an important role(Reference Barnes and Karin7, Reference Boyle, Kovacich and Canty43). We observed a decreased expression of NF-κB in mice fed the dealcoholised beer-rich diets, which may explain the reduction in the endothelial adhesion molecule expression. No other study have reported the implication of NF-κB in the beneficial signalling pathways of beer polyphenols. Nevertheless, other studies have supported the attenuation of NF-κB expression by cis-resveratrol, a polyphenol present in beer and other drinks such as wine, in previously activated peritoneal macrophages(Reference Leiro, Arranz and Fraiz44) or in the blood mononuclear cells of humans who had drunk red wine(Reference Blanco-Colio, Valderrama and Alvarez-Sala45). The procyanidins, another group of polyphenols, also inhibited the activation of this transcription factor in RAW 264.7 macrophages activated by lipopolysaccharide(Reference Terra, Valls and Vitrac46).
In summary, the present study indicates, for the first time, a reduction in the expression of the endothelial adhesion molecules caused by DLB and DDB. This reduction is supported by a slower progression of atheroma plaques in apoE − / − mice that spontaneously develop atherosclerosis when fed with dealcoholised beers, in the absence of a reduction in the high plasma levels of TAG and total cholesterol. Thus, we conclude that the protection afforded by DLB and DDB may be due to a decreased expression of NF-κB.
Acknowledgements
The authors thank the Serveis Científico-Tècnics from the Universitat de Barcelona for technical management and the Language Service at the Universitat de Barcelona for English language revision. The present study was financially supported by a grant from the Instituto de Cerveza y Salud, the Ministerio de Ciencia e Innovación (FIS project PI 05/2629; AGL2006-14228-C03-02/ALI) and the Instituto de Salud Carlos III (Alimentación saludable en la prevención primaria de enfermedades crónicas: Red PREDIMED, RD06/0045/0012). M. U.-S. thanks the Sara Borrell postdoctoral programme (CD09/00134) from the Ministry of Science and Innovation. N. M., M. U.-S., M. A. M.-G., C. A.-L. and M. T. M. designed the research and wrote the manuscript; N. M. and M. U.-S. conducted the research and analysed the data; C. A.-L. and M. T. M. provided essential materials and had primary responsibility for the final content. All the authors read and approved the final manuscript. None of the authors had a personal or financial conflict of interests.









