Conjugated linoleic acid (CLA) is a group of positional and geometric isomers of conjugated dienoic derivatives of linoleic acid. The cis-9, trans-11 isomer of CLA, also known as rumenic acid, accounts for >90 % of the total CLA intake and is mostly found in beef and dairy products (Lin et al. Reference Lin, Boylston, Chang, Luedecke and Schultz1995). Other minor isomers are also detected in the human diet in smaller proportions. Recent studies investigating the health benefits of CLA isomers focused on their anticarcinogenic and antiatherogenic properties, as well as on their ability to reduce body fat and enhance lean body mass in several animal models (Dugan et al. Reference Dugan, Aalhus, Schaefer and Kramer1997; Park et al. Reference Park, Storkson, Albright, Liu and Pariza1999; Blankson et al. Reference Blankson, Stakkestad, Fagertun, Thom, Wadstein and Gudmundsen2000; DeLany & West, Reference DeLany and West2000; Kritchevsky et al. Reference Kritchevsky, Tepper, Wright, Tso and Czarnecki2000; Pariza et al. Reference Pariza, Park and Cook2000; Stangl, Reference Stangl2000; Zambell et al. Reference Zambell, Keim, Van Loan, Gale, Benito, Kelley and Nelson2000; Terpstra et al. Reference Terpstra, Beynen, Everts, Kocsis, Katan and Zock2002; Wang & Jones, Reference Wang and Jones2004). These latter properties may suggest that CLA supplementation could have beneficial effects on the management of body weight in man and therefore on the treatment and prevention of obesity. Postulated anti-obesity mechanisms of CLA are decreased energy and food intakes, decreased lipogenesis, and increased energy expenditure, lipolysis and fat oxidation (West et al. Reference West, Delany, Camet, Blohm, Truett and Scimeca1998; Takahashi et al. Reference Takahashi, Kushiro, Shinohara and Ide2002; Wang & Jones, Reference Wang and Jones2004).
All these mechanisms generally rely on the regulation of the expression of the key proteins of energy metabolism. Accordingly, CLA has been shown to modulate adipocyte gene expression in several animals and in vitro models (Ryder et al. Reference Ryder, Portocarrero and Song2001; Takahashi et al. Reference Takahashi, Kushiro, Shinohara and Ide2002; Brown & McIntosh, Reference Brown and McIntosh2003; Brown et al. Reference Brown, Boysen, Jensen, Morrison, Storkson, Renee, Pariza, Mandrup and McIntosh2003; Grandlund et al. Reference Grandlund, Juvet, Pedersen and Nebb2003; Kang et al. Reference Kang, Liu, Albright, Park and Pariza2003; Park et al. Reference Park, Storkson, Liu, Albright, Cook and Pariza2004; Wargent et al. Reference Wargent, Sennit, Stocker, Mayes, Brown, O'dowd, Wang, Einerhand, Mohede, Arch and Cawthorne2005). Among the genes that appeared to be relevant for the effects of CLA, the PPAR are ligand-activated nuclear hormone receptors that control the expression of sets of genes involved in cellular metabolism and differentiation. It has been reported that CLA isomer trans-10, cis-12 could inhibit PPAR γ expression and some of its downstream target genes (LPL, GLUT4, aP2) in rodent adipocytes (Brown & McIntosh, Reference Brown and McIntosh2003). Furthermore, Kang et al. (Reference Kang, Liu, Albright, Park and Pariza2003) have also shown that CLA decreases body fat gain and significantly inhibits the expression of PPAR γ and the differentiation into mature adipocytes. However, their results were not consistently found and other investigators have observed an increase in PPAR γ gene expression by CLA cis-9, trans-11 (Brown et al. Reference Brown, Boysen, Jensen, Morrison, Storkson, Renee, Pariza, Mandrup and McIntosh2003). In another study, it was shown that a mixture of the two CLA isomers induces an up-regulation of uncoupling protein 2 (UCP-2) gene expression, a target gene of the PPAR, in rat skeletal muscle and adipose tissue (Ryder et al. Reference Ryder, Portocarrero and Song2001).
Up to now, several human studies have been published but the effects of CLA consumption were rather inconsistent and appeared less important than what was expected from animal studies (Blankson et al. Reference Blankson, Stakkestad, Fagertun, Thom, Wadstein and Gudmundsen2000; Zambell et al. Reference Zambell, Keim, Van Loan, Gale, Benito, Kelley and Nelson2000; Smedman & Vessby, Reference Smedman and Vessby2001; Kamphuis et al. Reference Kamphuis, Lejeune, Saris and Westerterp-Plantenga2003; Riserus et al. Reference Riserus, Vessby, Arnlov and Basu2004; Terpstra et al. 2004; Desroches et al. Reference Desroches, Chouinard, Galibois, Corneau, Delisle, Lamarche, Couture and Bergeron2005; Tricon et al. Reference Tricon, Burdge, Williams, Calder and Yaqoob2005). For example, Blankson et al. (Reference Blankson, Stakkestad, Fagertun, Thom, Wadstein and Gudmundsen2000) reported a reduction in the fat mass of overweight and obese subjects after 12 weeks of supplementation with CLA (3·4 g/d) divided into three doses per day. Desroches et al. (Reference Desroches, Chouinard, Galibois, Corneau, Delisle, Lamarche, Couture and Bergeron2005) failed to show any beneficial metabolic effects and no differences were observed in the accumulation of adipose tissue compared with placebo after 4 weeks of supplementation with CLA incorporated into butter (4·22 g CLA/100 g butter) in overweight and obese subjects. In lean women, Zambell et al. (Reference Zambell, Keim, Van Loan, Gale, Benito, Kelley and Nelson2000) observed that supplementation of the diet with 3 g/d of CLA for 9 weeks had no effect on body composition and energy expenditure. The potential action of CLA on adipose tissue gene expression in man during dietary supplementation has not yet been investigated.
The aim of the present study was to determine whether 14-week CLA supplementation as triacylglycerols (3·76 g) with a 50 : 50 combination of the two main isomers (cis-9, trans-11 and trans-10, cis-12) added to flavoured yoghurt-like products was able to alter body composition in healthy human adults. And in addition, subcutaneous abdominal adipose tissue biopsies were performed to investigate in vivo the changes in the expression of several key adipose tissue genes, including PPAR γ, lipoprotein lipase (LPL), UCP-2 and hormone-sensitive lipase (HSL).
Material and methods
Material
The yoghurt-like products were made using a standard dairy process for fermented milk. They were made of skimmed milk inoculated with 107 colony-forming units of yoghurt bacillus (Streptococcus thermophilus, Lactobacillus bulgaricus), 8 % sugar, cherry flavour and 3·76 g triacylglycerols TONALIN 75 TG® for the CLA group and cream for the control group. To maintain a similar isoenergetic composition, the same amount of fat was used. The CLA mixture contained 35 % cis-9, trans-11 isomer and 35 % trans-10, cis-12 isomer. The major remaining fatty acids were 8·3 % C16-0, 2·9 % C18-0, 14·8 % 18: 1n-9 and 1·4 % 18: 2n-6. Less than 1 % of trans–trans CLA isomers were found. This composition has been verified by GC–MS as described later. The yoghurt-like products were produced and delivered by Danone Company, Palaiseau, France. TONALIN 75 TG® was supplied by Natural Lipids Ltd (Hovdebygda, Norway).
Study subjects
Forty-four healthy volunteers, twenty-two women and twenty-two men with a mean age of 28·9 (sem 1·14) years, moderately overweight mean weight of 72·5 (sem 1·17) kg, with a mean BMI of 25·2 (sem 0·21) kg/m2 were recruited from the local community by advertisement.
All subjects gave written informed consent to participate and complied with the following inclusion criteria: BMI 23–27·5 kg/m2 with a stable body weight for at least 3 months; having at least 135 min of moderate exercise per week; fasting plasma cholesterol < 6·5 mmol/l; fasting plasma triacylglycerol < 2 mmol/l; fasting plasma glucose < 7 mmol/l; not regular consumers of any fatty acid nutritional supplements or drugs known to affect body weight, lipid or glucose metabolism except for oral contraceptive for women. Pregnant women were not allowed to be part of this protocol.
The present study was approved by the Ethics Committee of the Hospices Civils de Lyon.
Study design
This was a randomised double-blind placebo-controlled study. Subjects were randomly assigned to receive either CLA-supplemented yoghurt-like products (CLA group, n 21) or placebo yoghurts (placebo group, n 23). Subjects were instructed to consume one yoghurt daily, at the end of their usual breakfast, for 14 weeks (98 d). Subjects maintained their usual dietary habits during the study and were asked to have regular physical activity (45 min, three times per week) and to hold a physical activity diary that was checked every 2 weeks. Subjects came to the Centre de Recherche en Nutrition Humaine de Lyon, at Hôpital Edouard Herriot, for blood sampling at 07.30 hours following a 12 h overnight fast, on days 0, 42 and 98.
Body composition and laboratory methods
At each visit, evaluation criteria were measured. After urine collection, weight was measured on a carefully calibrated scale (SECA, Les Mureaux, France). Height was measured using a calibrated height gauge. Total lean body mass and total fat mass were measured using a dual-energy X-ray absorptiometer (Hologic QDR 4500 A; Hologic Inc., Waltham, MA, USA) on the first (day 0) and on the last (day 98) visits. Blood samples were drawn for measurement of glycaemia, insulinaemia, triacylglycerols, NEFA, total and HDL cholesterol, liver enzymes, blood cell count, bleeding time, prothrombin, activated cephalin time, thiobarbituric acid reactive substances, CLA and fatty acid profile.
On days 0 and 98, subcutaneous abdominal adipose tissue biopsies were performed under local anaesthesia by aspiration from the per-umbilical area through a 15-gauge needle, as previously described (Vidal et al. Reference Vidal, Auboeuf, De Vos, Staels, Riou, Auwerx and Laville1996). Tissue samples were immediately frozen in liquid nitrogen and stored at − 80°C for later analysis. Respiratory exchange measurements were performed during a 1 h period by indirect calorimetry (Deltatrac Metabolic Monitor; Datex, Helsinki, Finland). Urine was collected during the calorimetry measurements to determine the nitrogen excretion rate. Total lipid oxidation rate and RMR were calculated as previously described (Binnert et al. Reference Binnert, Pachiaudi, Beylot, Hans, Vandermander, Chantre and Laville1998).
Plasma glucose, triacylglycerols and NEFA concentrations were measured by enzymatic methods. Plasma insulin was measured by RIA as previously described (Binnert et al. Reference Binnert, Pachiaudi, Beylot, Hans, Vandermander, Chantre and Laville1998). Blood cell counts, liver enzymes and activated cephaline time were determined using standard methods. Urinary nitrogen was determined by chemioluminescence (Antek 703C; Sopares, Paris, France). Lipid peroxidation was quantified through fluorometric measurements of thiobarbituric acid reactive substances (Richard et al. Reference Richard, Portal, Meo, Coudray, Hadjian and Favier1992).
Plasma fatty acids and CLA isomer concentrations (cis-9,trans-11 and trans-10, cis-12) were measured by GC–MS after extraction and esterification by the method of Bligh & Dyer (Reference Bligh and Dyer1995) and purification by TLC (hexane–diethyl ether–acetic acid, 80 : 20 : 1, by vol.). Precautions were taken for preventing the possible isomerisation of CLA into trans, trans isomers (Fay & Richli, Reference Fay and Richli1991), the absence of such isomers was checked at each analysis. Fatty acid methyl esters were separated by GC (capillary column, HP Innowax 60 m × 0·25 mm × 0·25 μm) and analysed by MS using a HP-5973 and Specific Ion Monitoring for CLA measurements (Lavillonnière et al. Reference Lavillonnière, Martin, Bougnoux and Sebedio1998).
Total RNA preparation
For practical purposes, RNA preparations were only made from the biopsies of twenty-two subjects from a random sub-selection: eleven subjects in the placebo group (six men and five women) and eleven subjects in the CLA group (five men and six women). One biopsy from one subject from the CLA group was not usable. Analysis was always double-blindly performed. Adipose tissue samples were pulverized in liquid nitrogen and total RNA was prepared using the Qiagen (Courtaboeuf, France) RNeasy total RNA kit. Average yields of total RNA were 1·2 (sem 0·2) μg/100 mg adipose tissue (wet weight). Total RNA solutions were stored at − 80°C until quantification of the target mRNA.
Determination of mRNA concentrations
We measured the steady-state expression level of mRNA encoding HSL, LPL, UCP-2 and PPAR γ. The concentrations of the target mRNA were measured by RT–competitive PCR. The method relies on the addition of known amounts of a competitor DNA molecule in the PCR to standardize the amplification process (Auboeuf & Vidal, Reference Auboeuf and Vidal1997). The construction of the competitors, the sequences of the primers, the validation of the assays and the conditions of the RT–competitive PCR assays have previously been described in full detail (Laville et al. Reference Laville, Auboeuf, Khadfallah, Vega, Riou and Vidal1996; Vidal et al. Reference Vidal, Auboeuf, De Vos, Staels, Riou, Auwerx and Laville1996; Auboeuf & Vidal, Reference Auboeuf and Vidal1997; Lefebvre et al. Reference Lefebvre, Laville, Vega, Riou, Van Gaal, Auwerx and Vidal1998). For each mRNA, the specific first strand cDNA was synthetized from 0·1 μg total RNA in the presence of the specific antisense primer and a thermostable RT enzyme to guarantee optimal synthesis of first strand cDNA (Auboeuf & Vidal, Reference Auboeuf and Vidal1997). Cy-5-5′-end labelled sense primers that were used during the competitive PCR products were analysed using an automated laser fluorescence DNA sequencer (ALFexpress, Pharmacia, Uppsala, Sweden) in 4 % denaturing polyacrylamide gels.
Statistical analysis
All results are given as means and their standard errors. Differences between groups from baseline to day 98 were assessed using overall tests. In the case of a significant overall test result, a Wilcoxon non-parametric test was used for differences between groups.
An interaction test was used to determine the effects of CLA, sex, type of subject (fat or lean) and the interactions CLA × sex and CLA × type of subject on body composition.
According to prior work (Blankson et al. Reference Blankson, Stakkestad, Fagertun, Thom, Wadstein and Gudmundsen2000), it was estimated that twenty subjects per group would be needed to detect a 5 % change in body fat mass levels at a significant level of 0·05 using a power of 0·85 and a standard deviation of 6.
All analyses were performed using Statview version 5.0 software (SAS Institute, Cary, NC, USA).
Results
Baseline status, compliance and tolerance
Baseline characteristics were similar in both groups (Table 1) and also in the subgroup chosen for biopsy (data not shown). All of the forty-four subjects completed the study. CLA-enriched products were well tolerated as no adverse events occurred. Compliance was excellent (99·9 %), using a product count every 2 weeks on the occasion of each yoghurt-like product delivery.
Table 1 Effect of dietary supplementation with conjugated linoleic acid (CLA) versus placebo on body composition and metabolic parameters from baseline (day 0) to day 98 (there were no differences between groups at baseline)† (Mean values with their standard errors)

Mean values were significantly different from those of day 0: *P = 0·07; **P = 0·03.
† For details of procedures, see Materials and methods section.
Blood parameters
Regarding blood parameters, no changes were observed in blood cell count, liver enzyme levels, bleeding time and prothrombin concentration (data not shown). However, activated cephalin time was significantly increased in men from the CLA group (33·6 (sem 1·1) and 34·4 (sem 1·4) s, on day 0 and day 98, respectively, P = 0·01) but not in women (31·1 (sem 0·8) and 31·0 (sem 1·2) s, on day 0 and day 98, respectively, NS). Thiobarbituric acid reactive substance formation was unaltered by CLA consumption, in men (3·37 (sem 0·08) and 3·03 (sem 0·10) mol/l, at day 0 and day 98, respectively) and in women (3·65 (sem 0·16) and 3·34 (sem 0·17) mol/l, at day 0 and day 98) from the CLA group.
Body composition, plasma lipids, glucose, insulin and leptin
The consumption of CLA-enriched yoghurt-like products for 14 weeks (98 d) affected neither the body weight nor the BMI of the subjects (Table 1), whether in male or in female (data not shown). Similarly, fat mass and free fat mass, determined by a dual-energy X-ray absorptiometer, were not modified by the CLA supplementation diet (Table 1) and there was also no effect of sex or type of subject (fat or lean) using an interaction test (data not shown). In addition, no changes occurred in any of the anthropometric parameters that were measured on day 42 of the protocol (data not shown). The consumption of CLA-enriched yoghurt-like products over 14 weeks did not alter any of the following metabolic parameters: glycemia, insulinemia, leptinemia, triacylglycerols, total and HDL-cholesterol concentrations (Table 1). Plasma NEFA concentrations were also left unchanged (Table 1).
Fatty acid composition of total lipids in plasma and adipose tissue
The evolution of CLA cis-9, trans-11 and CLA trans-10, cis-12 concentrations is presented in Fig. 1. Plasma CLA cis-9, trans-11 levels increased with CLA consumption (29·9 (sem 3·1) and 57·3 (sem 7·2) μmol/l, on day 0 and day 98, respectively, P < 0·02) whereas concentrations remained stable in the placebo group (37·2 (sem 3·6) and 40·8 (sem 3·4) μmol/l, on day 0 and day 98, respectively).
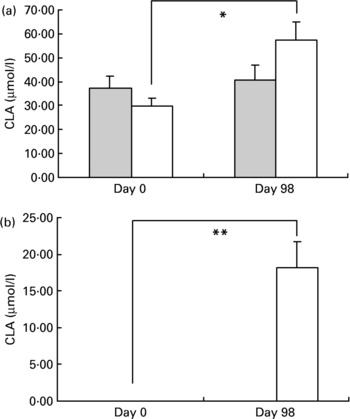
Fig. 1 Evolution of conjugated linoleic acid (CLA) isomers cis-9, trans-11 (a) and trans-10, cis-12 (b) in plasma before (day 0) and after (day 98) supplementation in the placebo () and CLA groups (). For details of procedures, see Materials and methods section. Values are means with their standard errors depicted by vertical bars. There were no differences between groups at baseline. Mean values indicated were significantly different: *P < 0·02; **P < 0·01.
CLA trans-10, cis-12 isomer was undetectable in plasma during the whole study in the placebo group while it increased significantly in the CLA-treated group (undetectable on day 0 and 18·2 (sem 3·6) on day 98, P < 0·01).
The CLA supplementation diet did not alter the relative concentration of n-3 or n-6 PUFA in plasma NEFA. Values in the CLA group were similar to those in the placebo group (data not shown).
In adipose tissue, CLA trans-12, cis-10 isomer was undetectable in both groups on day 0 and remained undetectable in the placebo group on day 98 but increased significantly in the CLA group from 0 to 1·96 (sem 0·4) nmol/mg. CLA cis-9, trans-11 isomer increased significantly from 8·26 (sem 0·5) to 10·47 (sem 0·9) nmol/mg (P = 0·01) in the CLA group between day 0 and day 98 but remained stable in the placebo group.
Effects of conjugated linoleic acid supplementation on energy expenditure
Total lipid oxidation rates were affected by CLA consumption (0·68 (sem 0·06) mg/kg per min v. 0·58 (sem 0·07) mg/kg per min on day 98 and day 0, respectively; Table 1). When referred to total body weight, basal energy expenditure tended to increase more in the CLA group (93·2 (sem 1·9) kJ/kg per d on day 98 v. 89·5 (sem 1·8) kJ/kg per d on day 0, P = 0·07), whereas no changes were observed in the placebo group (88·6 (sem 1·7) kJ/kg per d at day 98 v. 88·6 (sem 1·3) kJ/kg per d at day 0, NS). However, when basal energy expenditure was expressed by reference to the kg free fat mass, a significant increase was found in the CLA group (123·3 (sem 2·5) kJ/kg free fat mass per d on day 98 v. 118·7 (sem 2·3) kJ/kg free fat mass per d on day 0, P = 0·03). On the contrary there was no change in the placebo group (120·4 (sem 2·2) kJ/kg free fat mass per d on day 98 v. 121·6 (sem 2·3) kJ/kg FFM per d on day 0, NS).
Effects of conjugated linoleic acid supplementation on gene expression in adipose tissue
The mRNA expression of candidate genes was determined in adipose tissue samples from twenty-one subjects: ten subjects from the CLA group and eleven subjects from the placebo group. Baselines characteristics were similar in these sub-groups and consistent with those found in the entire CLA and placebo groups (data not shown). Figure 2 illustrates the variations in mRNA levels of LPL, HSL, PPAR and UCP-2 between day 0 and day 98 of the experimental protocol.
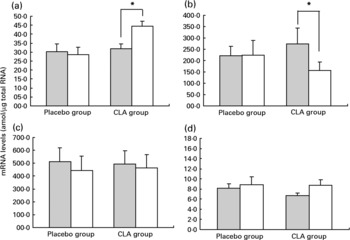
Fig. 2 Variations in mRNA levels of PPAR γ (a), hormone-sensitive lipase (b), lipoprotein lipase (c) and uncoupling protein 2 (d) in adipose tissue between day 0 () and day 98 (□). For details of procedures, see Materials and methods section. Values are means with their standard errors depicted by vertical bars. There were no difference between groups at baseline. Mean values indicated were significantly different: *P < 0·01.
PPAR γ mRNA expression increased significantly in the CLA group (31·8 amol/μg total RNA at day 0 v. 44·4 amol/μg total RNA at day 98, P < 0·01) while it remained unchanged in the placebo group (30·1 amol/μg total RNA at day 0 v. 28·5 amol/μg total RNA at day 98, NS). Moreover, the consumption of CLA-enriched yoghurt-like products induced a significant reduction in HSL mRNA levels in adipose tissue ( − 42 (sem 7) %, P = 0·01), while the consumption of placebo yoghurt-like products did not significantly affect HSL mRNA level (49 (sem 24) %, NS). The levels of UCP-2 mRNA were not altered during the trial, either in the CLA group (29 (sem 13) %, NS) or in the placebo group (3 (sem 11) %, NS). LPL mRNA expression was also not affected over the study period, either in the CLA group (25 (sem 27) %, NS) or in the placebo group ( − 3 (sem 16) %, NS).
Discussion
Effects of conjugated linoleic acid on gene expression in adipose tissue
The present study presents the changes induced by the consumption of CLA-enriched dairy products in subcutaneous abdominal adipose tissue gene expression in healthy subjects. The in vivo changes in the expression of several key adipose tissue genes, including PPAR γ, LPL, a key enzyme in lipid metabolism that hydrolyses fatty acids from circulating triacylglycerols, UCP-2, a mitochondrial uncoupling protein that decreases ATP synthesis coupled to energetic substrates oxidation and HSL, the enzyme controlling lipolysis in white adipose tissue, were investigated. The 98 d CLA supplementation diet increased PPAR γ gene expression and decreased HSL mRNA levels without any changes in LPL mRNA levels or in UCP-2 mRNA levels.
PPAR γ is mainly expressed in adipose tissue and is a central regulator of adipocyte gene expression and differentiation (Lowell, Reference Lowell1999). Besides these effects, PPAR γ plays a determinant role in body fat distribution (Lefebvre et al. Reference Lefebvre, Laville, Vega, Riou, Van Gaal, Auwerx and Vidal1998). Heterozygotous PPAR γ-deficient mice have a significant reduction in total body fat mass (Kubota et al. Reference Kubota, Terauchi and Miki1999). Recently, many studies have been designed to demonstrate the effects of CLA on PPAR γ gene expression and on its downstream target genes. All were performed in animal models or using cultured human or murine adipocytes and, to our knowledge, no study on the regulation of gene expression by dietary intake of CLA has been performed in man. Tsuboyama-Kasaoka et al. (Reference Tsuboyama-Kasaoka, Takahashi, Tanemura, Kim, Tange, Okuyama, Kasai, Ijemoto and Ezaki2000) reported important changes in gene expression in the adipose tissue of CLA-treated mice, including an increase in TNFα and UCP-2 mRNA expression and a decrease in PPAR γ, sterol regulatory element binding protein and fatty acid synthetase expression while LPL was unaffected. The changes were associated with a large decrease in adipose tissue weight and with an apoptosis state (Tsuboyama-Kasaoka et al. Reference Tsuboyama-Kasaoka, Takahashi, Tanemura, Kim, Tange, Okuyama, Kasai, Ijemoto and Ezaki2000). Such dramatic metabolic changes were not observed in the present experiment in man.
Some studies have shown a reduction in LPL activity caused by CLA supplementation and this effect was mainly allotted to the trans-10, cis-12 CLA isomer (Wang & Jones, Reference Wang and Jones2004). Kang et al. (Reference Kang, Liu, Albright, Park and Pariza2003) also showed that trans-10, cis-12 CLA inhibited the differentiation of preadipocytes and significantly inhibited the expression of PPAR γ and its target LPL. In another study performed in mice (Takahashi et al. Reference Takahashi, Kushiro, Shinohara and Ide2002), CLA decreased PPAR γ mRNA levels in adipose tissue and more specifically in brown adipose tissue. It is interesting to note that the greatest effects were obtained in the mouse strain that was the most sensitive to diet-induced obesity. The present results were obtained using a 50 : 50 mixture of the two main isomers, trans-10, cis-12 and cis-9, trans-11. Again, this was associated with an important decrease in fat mass. Brown et al. (Reference Brown, Boysen, Jensen, Morrison, Storkson, Renee, Pariza, Mandrup and McIntosh2003) observed the isomer-specific effects of CLA on gene expression in cultured adipocytes. Results showed that trans-10, cis-12 CLA decreased the expression of genes influencing differentiation: PPAR γ and genes regulated by PPAR γ; whereas cis-9, trans-11 increased the expression of PPAR γ-dependent genes. Therefore, Brown et al. (Reference Brown, Boysen, Jensen, Morrison, Storkson, Renee, Pariza, Mandrup and McIntosh2003) suggested that these mixed isomers might negate one another and so induce no change in adiposity or PPAR γ expression. CLA isomers are also known to activate PPAR γ with very low efficacy and another hypothesis proposed by Brown et al. (Reference Brown, Boysen, Jensen, Morrison, Storkson, Renee, Pariza, Mandrup and McIntosh2003) was that CLA directly affects PPAR γ activity by competing with other ligands with higher efficacy, and so the two isomers might mutually antagonize each other's activity. Therefore, discrepancies with other studies in regard to PPAR γ gene expression could be related to the use of a mixture of the two isomers whose effects may have cancelled each other out.
In the present study, the determination of mRNA expression was performed in crude adipose tissue biopsies that have been taken before and at the end of the dietary supplementation of a CLA mixture containing 35 % cis-9, trans-11 isomer and 35 % trans-10, cis-12 isomer. Under such conditions, we observed significant changes in the expression of PPAR γ and of HSL. These effects, however, were not associated with major changes in lipid metabolism during supplementation as illustrated by the lack of modification of plasma NEFA or triacylglycerol concentrations.
Effects of conjugated linoleic acid on body composition and metabolism
We found that the consumption of CLA-enriched dairy products providing 3·76 g CLA in triacylglycerols for 98 d did not alter body composition. The present result is different from those previously found in animals (Dugan et al. Reference Dugan, Aalhus, Schaefer and Kramer1997; Park et al. Reference Park, Storkson, Albright, Liu and Pariza1999; Blankson et al. Reference Blankson, Stakkestad, Fagertun, Thom, Wadstein and Gudmundsen2000; DeLany & West, Reference DeLany and West2000; Kritchevsky et al. Reference Kritchevsky, Tepper, Wright, Tso and Czarnecki2000; Stangl, Reference Stangl2000) and in man (Gaullier et al. Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen2004). However, it is consistent with the results obtained by Zambell et al. (Reference Zambell, Keim, Van Loan, Gale, Benito, Kelley and Nelson2000), Medina et al. (Reference Medina, Horn, Keimn, Havel, Benito, Kelley, Nelson and Erickson2000), Malpuech-Brugère et al. (Reference Malpuech-Brugère, Verboeket-van de Venne and Mensink2004) and Pariza (Reference Pariza2004). The differences between man and animals could be due to a dose effect since most studies in animals used higher doses per kg body weight. Another explanation can rely on a specie difference in metabolic rate since rodents generally have a higher metabolic rate than man. Also, as in many studies performed in man, dietary intake and activity in the present study were controlled but not standardized, which may interfere with the effects of CLA. Discrepancy in results between the human studies can be due to several differences in methodology and trial design. In fact, the duration of the trial (14 weeks, 1 year, etc.), the frequency (once a day, three times a day, etc.) and quantity of CLA intake, the way of administration and the food matrix containing CLA (associated diet, solution, capsules, triacylglycerols, enriched butter, enriched yogurt-like product, etc.), and the use of mixed or separate isomers could explained the variability of results found in human studies.
Importantly, the 14-week supplementation diet produced a significant increase in RMR in subjects. However, this observed increase in RMR was of low magnitude (about 3·76 kJ/kg per d) and suggests that an effect on body weight might be obtained after a longer dietary intervention. In fact, 3·76 kJ/kg per d represents for a 70 kg subject 263 kJ per d and 95 995 kJ per year, if we assume the same increase in energy expenditure would be maintained for an entire year with CLA supplementation. Thus, 95 995 kJ of energy expenditure represents 2·4 kg fat, that means a 70 kg subject from the CLA group would, in theory, lose 2·4 kg fat/year. In the study of Gaullier et al. (Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen2004), the subjects lost 2·4 kg fat after 1 year of supplementation of 2·4 g CLA (triacylglycerols)/d. This confirms the calculated results; it might be therefore assumed that the duration of the trial was not long enough to produce a measurable reduction in body weight or fat mass, as in other studies in which no effects on body fat were observed. Maybe the body fat-lowering effect of CLA needs a long period of time to start and be effective. Another possible explanation for this change in energy expenditure not associated with a significant modification in body weight, fat mass or body composition could be a parallel increase in energy intake with CLA consumption. However, none of the subjects reported any modification in their daily food intake. Besides, such an explanation is not supported by literature data according to which CLA does not affect food intake or appetite in man (Medina et al. Reference Medina, Horn, Keimn, Havel, Benito, Kelley, Nelson and Erickson2000; Terpstra, Reference Terpstra2004). Therefore, we assume that a 98 d supplementation is not enough to obtain significant results in reducing body weight.
Regarding the mechanisms potentially involved in the observed change in the RMR, we did not find modification in the mRNA UCP-2 levels in adipose tissue. UCP participate in energy expenditure as possible candidates to explain mitochondrial proton leaks in skeletal muscle and adipose tissue (Ricquier & Bouillaud, Reference Ricquier and Bouillaud2000). A positive correlation has previously been found between adipose tissue UCP-2 mRNA levels and RMR adjusted for lean body mass in obese women after diet standardization (Barbe et al. Reference Barbe, Millet, Larrouy, Galitzky, Berlan, Louvet and Langin1998). In a study by Takahashi et al. (Reference Takahashi, Kushiro, Shinohara and Ide2002), CLA supplementation greatly increased UCP-2 mRNA expression in brown adipose tissue in mice. However, in their study, the energy expenditure of the animals was not measured. So, further studies are required to understand the roles of UCP and energy expenditure with regard to CLA supplementation.
In most human studies, subjects were administered CLA orally in capsules, in the form of triacylglycerols or NEFA. In a recent study performed in overweight and obese men, CLA was incorporated into butter and the 4-week supplementation diet induced no beneficial effects either on total or HDL cholesterol, or on adipose tissue accumulation (Desroches et al. Reference Desroches, Chouinard, Galibois, Corneau, Delisle, Lamarche, Couture and Bergeron2005). In the present study, measurements of both CLA isomer concentrations in blood after consumption of CLA-enriched yoghurt-like products demonstrate that concentrations increased following yoghurt-like product intake. So, administration of CLA once a day as triacylglycerols in a yoghurt seems to be a relevant way of administration, well tolerated and accepted by subjects. However, further pharmacokinetic studies should help to understand the specific effects of both CLA isomers on body composition as well as their bio-availability to optimise the way of administration.
In conclusion, a 98 d supplementation diet with a 50 : 50 mixture of the two CLA isomers cis-9, trans-11 and trans-10, cis-12 in a flavoured yoghurt-like product was unable to alter body composition, although a significant increase in the RMR could be recorded in the subjects of the CLA group. It seems therefore that the 14 weeks of the trial were not long enough to produce significant changes in body composition. We also found an effect of CLA supplementation on gene expression in adipose tissue, more specifically on PPAR γ. The observed increase in PPAR γ gene expression is different from what could be expected from the results generally obtained in rodents or in vitro. This could be could be due to the use of mixed isomers. However, it is important to note that, to our knowledge, this is the first observation of CLA supplementation-induced gene expression in human tissues. Recent studies wondered about the potential detrimental effects of CLA, such as altered blood lipid composition or impaired insulin sensitivity. So, these points, as well as the specific effects of both CLA isomers, will require further investigation and longer nutritional interventions in order to understand fully the effects of CLA on body composition.





