Diabetes mellitus (DM) represents one of the greatest threats to modern global health and its incidence is rapidly increasing. The WHO estimated that nearly 300 million of individuals will develop DM in 2025( Reference Agbaje, Rogers and McVicar 1 ), and these numbers may tend to aggravate due to some risk factors related to lifestyle, such as being overweight, having an unhealthy diet or smoking. Type 2 diabetes mellitus is the most common type of DM( Reference Tuei, Maiyoh and Ha 2 ), and, nowadays, is affecting children, adolescents and young adults who will face the burden of the disease for a very long period. The complexity of DM diagnosis has led to the establishment of an intermediate state known as ‘prediabetes’. In this state, some, but not all, of the diagnostic criteria for DM are met. Impaired fasting glucose, glucose intolerance and/or insulin resistance, as well as mild hyperglycaemia are associated with prediabetes( Reference Tabak, Herder and Rathmann 3 ), and, as a result, body glucose metabolism becomes compromised. The brain uses glucose as the primary fuel source, and thus it is expected that dysfunctional glucose metabolism will lead to severe brain damage (for a review, see Tabak et al. ( Reference McCall 4 ) and McCall( Reference Alves, Oliveira and Socorro 5 )). In fact, hyperglycaemia, even if transient, may produce several deleterious effects on cerebral function( Reference Diaz-Parejo, Stahl and Xu 6 ). Noteworthy, hyperglycaemia is known to differently affect different brain regions, particularly the cortex, which is highly sensitive to glucose fluctuations( Reference Cardoso, Santos and Seica 7 ). Indeed, cortical cells are more vulnerable to glucose metabolism deregulation than cells from the striatum or hippocampus( Reference Xu, Sapolsky and Giffard 8 ). Moreover, DM increases the vulnerability of specific brain areas to neuronal damage, with the cortex being particularly sensitive( Reference Bree, Puente and Daphna-Iken 9 ). Increased oxidative stress (OS) has been proposed to be one of the major causes of hyperglycaemia-induced complications (for a review, see Valko et al. ( Reference Valko, Leibfritz and Moncol 10 )). The abnormal enhancement of free radical production and the decline in antioxidant defence mechanisms lead to oxidative damage and insulin resistance (for a review, see Maritim et al. ( Reference Maritim, Sanders and Watkins 11 )). Persistent hyperglycaemia has been reported to play a major role in the onset of cognitive and affective disorders through OS-mediated mechanisms( Reference de Morais, de Souza and da Silva 12 ). Interestingly, different brain regions present different susceptibilities to OS, and it has been reported that the cortex possesses a lower basal level of antioxidant defences and, thus, is more prone to OS( Reference Srivastava and Shivanandappa 13 ).
Medicinal plants have been used in traditional medicine to prevent and treat a wide range of diseases. Tea (Camellia sinensis (L.)) is one of the most widely consumed beverages in the world, surpassed only by water( Reference Cheng 14 ). It is composed of several bioactive compounds and has been reported to promote numerous health benefits( Reference Carvalho, Jerónimo and Valentão 15 – Reference Dias, Alves and Tomás 19 ). Among those, antidiabetic( Reference Dieren, Uiterwaal and Shouw 20 ) and neuroprotective( Reference López and Calvo 21 ) properties of tea have been attributed to the high content of phenolic compounds, particularly catechins, and to their antioxidant activity( Reference Almajano, Carbó and Jiménez 22 ). Moreover, catechins are known to interact with large biomolecules such as proteins and enzymes( Reference Higdon and Frei 23 ). Indeed, tea supplementation has been reported to alter several metabolic pathways, and it is known to suppress insulin resistance and improve insulin sensitivity( Reference Hininger-Favier, Benaraba and Coves 24 ). Green tea supplementation also induces different metabolic effects during rest and exercise( Reference Hodgson, Randell and Boon 25 ). Plasma metabolite profile of men consuming green tea extract revealed that it enhances fat oxidation, activates the Krebs cycle, reduces amino acid catabolism and enhances glycolysis( Reference Hodgson, Randell and Boon 25 ). Interestingly, green tea stimulation of lactate concentration during exercise has been suggested to inhibit lipolysis and limit fat oxidation rates( Reference Hodgson, Randell and Boon 25 ). White tea, the least processed tea after green tea, is one of the less studied types of tea. It is prepared from very young tea leaves or buds covered with tiny, silvery hair, which are harvested only once a year in the early spring( Reference Rusak, Komes and Likić 26 ). It has been reported that white tea contains the highest levels of antioxidants among the various types of teas( Reference Dias, Tomás and Teixeira 17 , Reference Dias, Alves and Tomás 19 ). Tea polyphenols (TP) seem to be the most important components of white tea leaves, due to their relative abundance, with good bioactive properties (for a review, see Dias et al. ( Reference Dias, Tomás and Teixeira 17 )). Due to its formation process, white tea contains relatively high concentrations of catechins. In fact, the concentration of polyphenols, caffeine, gallic acid and others is significantly higher in white tea than in green tea( Reference Hilal and Engelhardt 27 ). In a recent study, our research team has determined the phytochemical profile of white tea and demonstrated that ( − )-epigallocatechin-3-gallate (EGCG) is the most abundant phytocomponent in white tea, followed by caffeine( Reference Dias, Tomás and Teixeira 17 , Reference Martins, Alves and Bernardino 18 ). Thus, the possible increased antioxidant activity of white tea could be related to higher concentrations of several of the major constituents, explaining its beneficial effects on health. Several studies have reported that tea phenolic compounds, mainly catechins, are potent antioxidant agents, scavenging reactive oxygen species (ROS)( Reference Nakagawa and Yokozawa 28 ) and metal chelators( Reference Atoui, Mansouri and Boskou 29 ). Continuous administration of EGCG for 30 d significantly improved rat brain antioxidant defences, ameliorating age-induced OS( Reference Srividhya, Jyothilakshmi and Arulmathi 30 ). In general, herbal medicinal preparations are complex mixtures that contain different compounds, and the synergistic action of these phytochemicals, as total extracts, is expected to be responsible for the full beneficial effects of herbal preparations( Reference Loew and Kaszkin 31 ).
The potential effects of tea phytochemicals on health depend on the amount ingested and on their bioavailability. EGCG and other catechins are absorbed intestinally( Reference Okushio, Matsumoto and Kohri 32 ), and it was reported that saliva possesses catechin esterase activity, which illustrates that EGCG may be degalloylated in the mouth and the oesophagus( Reference Yang, Lee and Chen 33 ). Moreover, EGCG can easily cross the blood–brain barrier and reach the brain parenchyma( Reference Suganuma, Okabe and Oniyama 34 ). Tea phytochemicals, especially catechins, are potent antioxidant agents, with positive effects on human health. In the present study, we report the effect of daily consumption of white tea on cerebral cortex glucose metabolism and OS in streptozotocin (STZ)-induced prediabetic rats.
Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich, unless otherwise stated.
White tea infusion
White tea samples were purchased on the Portuguese market, which originated from China and were produced in European Union. Tea leaves were subjected to infusion (1 g/100 ml of distilled water at 100°C), during 3 min, according to the manufacturer's instructions. The resulting infusion was filtered through a 0·2 μm cellulose acetate filter (VWR). The phytochemical profile of white tea was determined in previous work from our team( Reference Martins, Alves and Bernardino 18 ).
Rats
A total of eighteen male Wistar rats (Rattus norvegicus), aged 3 months old, were housed under a 12 h light–12 h dark cycle and constant room temperature (20 ± 2°C) in our accredited animal colony vivarium (Health Science Research Center, University of Beira Interior). Rats were maintained with ad libitum food and water. All animal experiments were performed according to the ‘Guide for the Care and Use of Laboratory Animals’ published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996) and the European rules for the care and handling of laboratory animals (Directive 86/609/EEC).
Rat model and experimental design
Prediabetes is a prodromal stage of DM characterised by elevated blood glucose levels, although not sufficient to meet the criteria for established diabetes( Reference Association 35 ). Prediabetic individuals present impaired fasting glucose levels and/or impaired glucose tolerance, and its prevalence is now increasing among young people( Reference Association 35 ). Prediabetes was induced by intraperitoneal (IP) administration of a low dose of STZ, in accordance with the method described by Iwase et al. ( Reference Iwase, Kikuchi and Nunoi 36 ), with slight modifications. In brief, 2-d-old male Wistar rats from the prediabetic group were injected with STZ (40 mg/kg, IP) freshly diluted in citrate buffer (0·1 m-sodium citrate, pH 4·5). The control group received only the vehicle solution in an equivalent volume. Rats were fed ad libitum with a standard chow diet (4RF21 certificate; Mucedola). At 1 month of age, STZ-treated rats were randomly divided into two groups, and one group consumed white tea during 2 months (PrDM+WTea group). The other group of STZ-treated rats (PrDM group) and control rats (control group) consumed water. Each group consisted of six rats, which were caged in groups. Weight and blood glucose levels of the rats were monitored every 6 d. Non-fasting glycaemic levels were determined using a glucometer (One Touch Ultra Lifescan-Johnson). At the end of the treatment, rats were killed by decapitation. The brain was removed, and cortex tissue samples were collected, weighed, snap frozen and stored at − 80°C.
Insulin and glucose tolerance tests
At 3 months of age, rats were subjected to a glucose tolerance test (GTT), as described by Rato et al. ( Reference Rato, Alves and Dias 37 ). In brief, at 14.00–18.00 hours before the test, rats were deprived of food. An IP injection with 6 ml glucose (30 % (w/v) per kg body weight) was given to each rat. Blood samples for glucose measurement were obtained from the tail vein immediately before and 30, 60, 90 and 120 min after glucose administration. Rats were also subjected to an insulin tolerance test (ITT), as described by Rato et al. ( Reference Rato, Duarte and Tomas 38 ). In brief, at 16.00–18.00 hours before the test, rats were deprived of food. An IP injection with 0·75 units of insulin/kg body weight was administered to each rat. Blood samples for glucose measurement were collected, as described above. The AUC for glucose tolerance (AUCGTT) and insulin tolerance (AUCITT) tests were calculated using the trapezoidal rule, as described previously( Reference Rato, Duarte and Tomas 38 ).
Ferric reducing–antioxidant power assay
The ferric reducing–antioxidant power assay in cerebral cortex tissue samples was performed according to the colorimetric method described by Benzie & Strain( Reference Benzie and Strain 39 ). In brief, cortex tissue samples were homogenised in PBS (pH 7·4) and protein concentration determined by the Bradford micro-assay using bovine serum albumin as the standard. The antioxidant potential of the samples was determined against the standards of l-ascorbic acid, by monitoring the changes in absorbance at 595 nm due to the reduction of the Fe3+–2,4,6-tripyridyl-s-triazine (TPTZ) complex to a coloured Fe2+–TPTZ complex induced by the samples. Absorbance was corrected using a blank of H2O instead of the sample. The changes in absorbance values of the tested reaction mixtures were used to calculate the ferric reducing–antioxidant power value of the samples (μmol of antioxidant potential/mg protein).
Thiobarbituric acid-reactive species assay
Thiobarbituric acid-reactive species are formed as a by-product of lipid peroxidation, and can be detected by the thiobarbituric acid-reactive species assay using thiobarbituric acid as a reagent. The thiobarbituric acid-reactive species assay was carried out by the method described by Iqbal et al. ( Reference Iqbal, Sharma and Rezazadeh 40 ), with slight modifications. In brief, 20 μg of tissue homogenate were mixed with Tris–HCl buffer (150 mm, pH 7·1), ferrous sulphate (1·0 mm), l-ascorbic acid (1·5 mm) and H2O. This mixture was then incubated at 37°C for 15 min. The reaction was stopped by the addition of TCA (10 %, w/v). Subsequently, thiobarbituric acid (0·375 %, w/v) was added, and all samples were incubated for 15 min at 100°C. Finally, the samples were centrifuged at 1000 g for 10 min. The amount of malondialdehyde formed was estimated by measuring optical density at 532 nm using an Anthos 2010 microplate reader (Biochrom) against a blank. Results are expressed as nmol of thiobarbituric acid reactive species/mg tissue.
Analysis of carbonyl groups
Protein carbonyl content is commonly used as a marker for protein oxidation. Evaluation of protein carbonyl group contents was performed using the slot–blot technique. First, the samples were derivatised using 2,4-dinitrophenylhydrazine according to the method developed by Levine et al. ( Reference Levine, Garland and Oliver 41 ). The derivatised samples were then diluted in PBS and transferred to activated polyvinylidenedifluoride membranes using the slot–blot technique, which was performed using a Hybri-slot manifold system (Biometra). The membranes were then blocked by incubation for 90 min with 5 % non-fat milk TBS solution with 0·05 % Tween-20. Afterwards, the membranes were incubated overnight with rabbit anti-2,4-dinitrophenol antibody (1:5000, D9656; Sigma-Aldrich) and then incubated with an anti-rabbit alkaline phosphatase-linked IgG (IgG-AP, 1:5000, SC-2007; Santa Cruz Biotechnology). The membranes were then reacted with an enhanced chemifluorescence substrate (GE Healthcare) and read using a Bio-Rad FX-Pro-Plus (Bio-Rad). Densities from each band were quantified using the BIO-PROFIL Bio-1D Software from Quantity One (VilberLourmat).
Glutathione assay
Total glutathione and reduced glutathione (GSH) levels in cerebral cortex tissues were evaluated using a method developed by Baker et al. ( Reference Baker, Cerniglia and Zaman 42 ), with slight modifications. In brief, 50 mg of cortex tissues were homogenised in 5 % 5-sulfosalicylic acid and centrifuged at 10 000 g for 10 min. The supernatant was then collected, diluted 5-fold and used as samples for the total glutathione assay. To evaluate GSH levels, samples were first derivatised with 2-vinylpyridine. This was done by incubating each sample in a 2-vinylpyridine solution (1 m) for 60 min. Glutathione levels were measured using a kinetic assay, in which glutathione causes a continuous reduction of 5,5′-dithiobis-(2-nitrobenzoic acid) to 2-nitro-5-mercaptobenzoic acid, which can be spectrophotometrically measured at 412 nm. Standards with known concentrations (50, 25, 12·5, 6·25 and 3·125 μm) were prepared, and a standard curve was plotted. The levels of oxidised glutathione were calculated by subtracting the values obtained for total glutathione and GSH. Results are expressed as nmol of total glutathione/mg tissue.
Western blot
Total proteins were isolated from the cerebral cortex tissues using radio-immunoprecipitation assay (RIPA) lysis buffer (1 × PBS, 1 % NP-40, 0·5 % sodium deoxycholate, 0·1 % SDS, 1 mm-phenylmethylsulfonyl fluoride, supplemented with 1 % protease inhibitor cocktail and 100? mm-sodium orthovanadate). Western blot was performed as described previously( Reference Alves, Machado and Sardão 43 ). Membranes were incubated with rabbit anti-GLUT 1 (GLUT1, 1:200, CBL242; Millipore), rabbit anti-GLUT 3 (GLUT3, 1:250, SC-31 838; Santa Cruz Biotechnology), rabbit anti-phosphofructokinase-1 (PFK-1, 1:1000, SC-67 028; Santa Cruz Biotechnology), rabbit anti-monocarboxylate transporter 4 (1:1000, SC-50 329; Santa Cruz Biotechnology), rabbit anti-lactate dehydrogenase (LDH, 1:5000, ab52488; Abcam) or mouse anti-catalase (1:4000, C0979; Sigma Aldrich). Mouse anti-α-tubulin was used as the protein loading control (1:2500, T9026; Sigma Aldrich). The immunoreactive proteins were detected separately with goat anti-rabbit IgG-AP (1:5000, SC-2007; Santa Cruz Biotechnology) or goat anti-mouse IgG-AP (1:5000, SC-2008; Santa Cruz Biotechnology). Membranes were exposed to an enhanced chemifluorescence detection system (GE Healthcare) and read with the Bio-Rad FX-Pro-Plus (Bio-Rad). The Quantity One Software (Bio-Rad) was used to obtain band densities following standard procedures. The densities of each band were divided by the corresponding α-tubulin, and are expressed as a fold change relative to the control group.
Lactate dehydrogenase activity assay
The activity of LDH was assessed spectrophotometrically by determining the cleavage of a colorimetric substrate as described previously( Reference Alves, Martins and Rato 44 ) using a commercial kit (Promega), according to the manufacturer's instructions. In brief, LDH activity was calculated by measuring the shift in absorbance (492 nm) that resulted from the conversion of a tetrazolium salt (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride; INT) into a red formazan product. The amount of formazan formed is directly proportional to the activity of LDH. The method was calibrated with LDH positive control included in the assay kit. The attained activities were calculated using the molar absorptivity of formazan, and are expressed as a fold change relative to the control condition.
1H NMR
Intracellular metabolites of cerebral cortex samples were extracted using a methanol–chloroform–water strategy as described previously( Reference Martineau, Tea and Loaec 45 ). The upper methanol–water phase containing the water-soluble cellular metabolites was carefully separated and lyophilised. For 1H-NMR analysis, the lyophilised samples were dissolved in 2H2O. 1H NMR spectra of the samples were acquired at 14·1 T and 25°C, using a Bruker Avance 600 MHz spectrometer equipped with a 5 mm QXI probe with a z-gradient (Bruker Biospin) using standard methods( Reference Alves, Neuhaus-Oliveira and Moreira 46 , Reference Alves, Oliveira and Carvalho 47 ). Sodium fumarate was used as an internal reference (singlet, 6·50 parts per million) to quantify the metabolites in the solution (multiplet, parts per million): valine (doublet, 1·02); aspartate (double doublet, 1·8); γ-aminobutyric acid (triplet, 2·28); glutamate (multiplet, 2·0); lactate (doublet, 1·33); alanine (doublet, 1·47); taurine (triplet, 3·4); N-acetylaspartate (doublet, 7·9); fumarate (singlet, 6·5). The relative areas of 1H NMR resonances were quantified using the curve-fitting routine supplied with the NUTSpro NMR spectral analysis program (Acorn NMR, Inc.).
Statistical analysis
Statistical differences between the experimental groups were assessed by one-way ANOVA, followed by the Bonferroni post-test. All data are presented as means with their standard errors (n 6 for each condition). Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software). P< 0·05 was considered significant.
Results
Daily consumption of white tea ameliorates glucose and insulin tolerance in prediabetic rats
At the end of the treatment, rats from the three experimental groups presented similar body weights (Table 1). STZ-treated rats developed typical characteristics of prediabetes. Mean glycaemic values increased from 5·00 (sem 0·06) mmol/l in the control group to 6·60 (sem 0·11) mmol/l in the prediabetic group (Table 1). These mild hyperglycaemic values fit the diagnostic for the prodromal stage of type 2 diabetes mellitus, prediabetes. To evaluate glucose tolerance and insulin sensitivity of all rats, the AUC was calculated. STZ-treated rats developed glucose intolerance, as shown by the higher AUCGTT value (23 364 (sem 1095) arbitrary units), when compared with the control group (17 661 (sem 670) arbitrary units) (Table 1). The insulin resistance test further confirmed that STZ-treated rats developed the characteristics of prediabetes because when compared with the control group (6870 (sem 597) arbitrary units), these rats exhibited a lower shift in blood glycaemia when subjected to the insulin resistance test, illustrating increased insulin resistance (Table 1). The PrDM+WTea group exposed to an IP injection of glucose exhibited a smaller AUCGTT value (17 760 (sem 1446) arbitrary units) compared with the PrDM group (Table 1). Furthermore, the PrDM+WTea group demonstrated lower insulin resistance (AUCITT 4907 (sem 871) arbitrary units) compared with the PrDM group (Table 1).
Table 1 Weight, blood glycaemia and AUC for glucose tolerance (AUCGTT) and insulin tolerance (AUCITT) tests in the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group) after 60 d of treatment (Mean values with their standard errors)
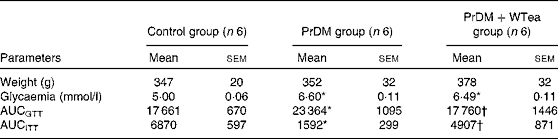
* Mean value was significantly different from that of the control group (P< 0·05).
† Mean value was significantly different from that of the PrDM group (P< 0·05).
Daily consumption of white tea decreases GLUT expression and lactate accumulation in the cerebral cortex of prediabetic rats
The expression of the two principal GLUT (GLUT1 and GLUT3) in the cerebral cortex was evaluated. The prediabetic state did not change GLUT1 protein levels in the cortex (Fig. 1(a)). Nevertheless, white tea consumption decreased the protein expression levels of GLUT1 in the cortex of prediabetic rats to 0·89 (sem 0·06)-fold change relative to the control group (Fig. 1(a)). Moreover, white tea consumption also decreased the protein expression levels of GLUT3 in the cortex of prediabetic rats (1·03 (sem 0·05)- to 0·82 (sem 0·06)-fold change relative to the control group; Fig. 1(b)). After glucose enters the brain, it follows the glycolytic pathway. The levels of PFK-1, which catalyses a major rate-limiting step in the glycolytic pathway, remained unchanged in the cortex of prediabetic rats (Fig. 1(c)). Also, white tea consumption did not alter the protein expression levels of PFK-1 in the cortex of prediabetic rats (Fig. 1(c)).

Fig. 1 Effect of daily consumption of white tea on the protein expression levels of GLUT and phosphofructokinase-1 (PFK-1) in the cerebral cortex of prediabetic rats. Protein expression levels of GLUT1 (a), GLUT3 (b) and PFK-1 (c) in the cerebral cortex of the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group). Values are means (n 6 per condition), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the PrDM group (P< 0·05).
Pyruvate from glycolysis is converted to lactate by LDH, and lactate overproduction is one of the major contributors for neuronal lesions related to hyperglycaemia. The present study shows that LDH protein expression level is maintained in the cortex of prediabetic rats even after daily consumption of white tea (Fig. 2(a)). However, LDH activity was increased (1·9 (sem 0·3)-fold change relative to the control group) in the cortex of the PrDM and PrDM+WTea groups (2·3 (sem 0·4)-fold change relative to the control group) (Fig. 2(b)).

Fig. 2 Effect of daily consumption of white tea on protein expression levels of lactate dehydrogenase (LDH) and monocarboxylate transporter 4 (MCT4), LDH activity and lactate content in the cerebral cortex of prediabetic rats. LDH protein expression levels (a) and activity (b), MCT4 protein expression levels (c) and lactate content (d) in the cerebral cortex of the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group). Values are means (n 6 per condition), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the PrDM group (P< 0·05).
After lactate is produced, it can be either exported through monocarboxylate transporter 4 or accumulated in the cells. Nevertheless, the protein expression levels of monocarboxylate transporter 4 in the cortex remained unaltered in prediabetic rats even after daily consumption of white tea (Fig. 2(c)). Interestingly, lactate content was decreased in the cortex of prediabetic rats to 13·8 (sem 0·9) nmol/mg tissue (Fig. 2(d)), and the daily consumption of white tea further decreased the cortical lactate content in prediabetic rats to 9·4 (sem 0·8) nmol/mg tissue (Fig. 2(d)).
Daily consumption of white tea decreases alanine content but does not restore the lactate:alanine ratio in the cerebral cortex of prediabetic rats
Pyruvate derived from glycolysis can also be converted to alanine. Daily consumption of white tea by prediabetic rats reduced alanine content in the cortex (Fig. 3(a)). Interestingly, the lactate:alanine ratio, which reflects the NADH/NAD+ equilibrium and thus the cellular redox state, was decreased to 7·8 (sem 0·4) in the cerebral cortex of prediabetic rats, compared with the control group (12·5 (sem 0·5)) (Fig. 3(b)). The daily consumption of white tea did not restore the lactate:alanine ratio in the cortex of prediabetic rats to 8·20 (sem 0·19; Fig. 3(b)).

Fig. 3 Effect of daily consumption of white tea on alanine content and lactate:alanine ratio in the cerebral cortex of prediabetic rats. Alanine content (a) and lactate:alanine ratio (b) in the cerebral cortex of the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group). Values are means (n 6 per condition), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
Daily consumption of white tea increases antioxidant capacity and catalase expression, preventing lipid peroxidation and protein oxidation in the cerebral cortex of prediabetic rats
Prediabetes decreased antioxidant capacity in the cerebral cortex from 20 (sem 2) μmol antioxidant potential/mg tissue under the control conditions to 16 (sem 1) μmol antioxidant potential/mg tissue (Fig. 4(a)). Interestingly, daily consumption of white tea increased the cortex antioxidant potential of prediabetic rats to 25 (sem 2) μmol antioxidant potential/mg tissue (Fig. 4(a)). Prediabetes also increased lipid peroxidation levels in the cortex from 0·29 (sem 0·03) nmol/mg tissue under the control condition to 0·46 (sem 0·03) nmol/mg tissue (Fig. 4(b)). Importantly, daily consumption of white tea prevented the increase in cortical lipid peroxidation levels of prediabetic rats to 0·26 (sem 0·03) nmol/mg tissue (Fig. 4(b)). ROS can induce protein oxidation, which leads to the formation of carbonyl groups. We found that prediabetes increased protein oxidation levels to 1·09 (sem 0·07)-fold change relative to the control group (Fig. 4(c)). The daily consumption of white tea decreased the carbonyl group content in the cortex compared with the PrDM and control groups (0·89 (sem 0·04)-fold change relative to the control group; Fig. 4(c)).

Fig. 4 Effect of daily consumption of white tea on antioxidant capacity, lipid peroxidation and protein oxidation levels in the cerebral cortex of prediabetic rats. Ferric reducing–antioxidant power (FRAP) (a), thiobarbituric acid-reactive substances (TBARS) (b) and carbonyl group levels (c) in the cerebral cortex of the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group). Values are means (n 6 per condition), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the PrDM group (P< 0·05).
Catalase is one of the major antioxidant defences present in the brain. The cortex of prediabetic rats exhibited a decreased expression level of catalase of 0·69 (sem 0·08)-fold change relative to the control group (Fig. 5(a)). The daily consumption of white tea restored catalase expression levels in the cortex of prediabetic rats to 0·96 (sem 0·10)-fold change relative to the control group (Fig. 5(a)). Total glutathione levels were reduced in the cortex of prediabetic rats to 0·11 (sem 0·01) nmol/mg tissue, and daily consumption of white tea did not restore total glutathione content in the cortex of prediabetic rats (Fig. 5(b)). No alterations in oxidised glutathione content were detected in the cortex of the PrDM and PrDM+WTea groups compared with the control group (Fig. 5(b)). However, GSH content was decreased to 0·03 (sem 0·01) nmol/mg tissue in the cortex of the PrDM group, and 0·04 (sem 0·01) nmol/mg tissue in the cortex of the PrDM+WTea group compared with the control group (0·08 (sem 0·02) nmol/mg tissue) (Fig. 5(b)).

Fig. 5 Effect of daily consumption of white tea on catalase protein expression levels and glutathione content in the cerebral cortex of prediabetic rats. Catalase protein expression (a) and glutathione content (b) in the cerebral cortex of the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group). Values are means (n 6 per condition), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). † Mean value was significantly different from that of the PrDM group (P< 0·05). ![]() , Oxidised glutathione;
, Oxidised glutathione; ![]() , reduced glutathione.
, reduced glutathione.
Daily consumption of white tea restores valine content in the cerebral cortex of prediabetic rats
No differences in the levels of glutamate, aspartate and taurine were detected in the brain of the control, PrDM and PrDM+WTea groups (Table 2). Concerning N-acetylaspartate levels in the cerebral cortex of the control, PrDM and PrDM+WTea groups, the present results show that prediabetes decreased the levels of N-acetylaspartate in the cortex to 3·4 (sem 0·1) nmol/mg tissue compared with 4·6 (sem 0·5) nmol/mg tissue in the control group. Choline was increased in the cortex of the PrDM and PrDM+WTea groups (3·5 (sem 0·1) and 3·5 (sem 0·3) nmol/mg tissue, respectively), when compared with the control group (1·6 (sem 0·2) nmol/mg tissue) (Table 2). Concomitantly, in the cortex of the PrDM and PrDM+WTea groups, the levels of γ-aminobutyric acid, which is a primary inhibitor of neurotransmission, remained unaltered when compared with the control group (Table 2). Interestingly, daily consumption of white tea restored valine levels, a crucial amino acid involved in protein synthesis, in the cerebral cortex of prediabetic rats compared with the control rats. The valine levels in the cortex of the control rats was 0·26 (sem 0·02) nmol/mg tissue, which increased to 0·36 (sem 0·03) nmol/mg tissue in the cortex of prediabetic rats and was restored after daily consumption of white tea (0·26 (sem 0·02) nmol/mg tissue) (Table 2).
Table 2 Metabolite levels in the cortex of the control rats (control group), prediabetic rats drinking water (PrDM group) and prediabetic rats drinking white tea (PrDM+WTea group) (Mean values with their standard errors)
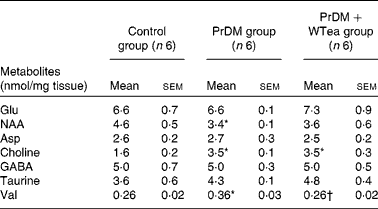
NAA, N-acetylaspartate; GABA, γ-aminobutyric acid.
* Mean value was significantly different from that of the control group (P< 0·05).
† Mean value was significantly different from that of the PrDM group (P< 0·05).
Discussion
Tea (C. sinensis) is a medicinal plant that deserves special merit since it is the second most consumed beverage in the world, and has been reported to possess several health benefits, mainly due to its antioxidant potential (for a review, see Dias et al. ( Reference Dias, Tomás and Teixeira 17 )). However, the number of studies on white tea consumption is, so far, negligible and the underlying mechanisms of action remain largely unknown. In contrast, mild hyperglycaemia is a characteristic feature of prediabetes, and is associated with complications in several organs, including the brain (for a review, see Roriz-Filho et al. ( Reference Roriz-Filho, Sa-Roriz and Rosset 48 )). Within the brain, the cortex has been reported to be very sensitive to hyperglycaemia( Reference Cardoso, Santos and Seica 7 , Reference Bree, Puente and Daphna-Iken 9 ). Thus, herein we studied the effect of the daily consumption of white tea in the cerebral cortex of STZ-induced prediabetic rats.
The results reveal that STZ-treated newborn rats developed typical prediabetic features. Blood glucose levels were mildly elevated, not enough to meet the criteria for the establishment of type 2 diabetes mellitus, and they developed glucose intolerance and insulin insensitivity. Although daily consumption of white tea did not decrease blood glycaemia to normal levels, it improved glucose tolerance and insulin sensitivity. TP have been suggested to be responsible for these actions. In fact, the capacity of TP to improve glucose and insulin sensitivity in hyperglycaemic conditions has been reported in in vitro studies( Reference Snoussi, Ducroc and Hamdaoui 49 ), animal experiments( Reference Sabu, Smitha and Kuttan 50 ) and even in clinical observations( Reference Hosoda, Wang and Liao 51 ). There are several mechanisms that may mediate these effects; however, they are suggested as being primarily mediated by the strong antioxidant action of TP( Reference Sabu, Smitha and Kuttan 50 ). It has been suggested that TP could be responsible for the improvement of glucose homeostasis probably through the modulation of intestinal absorption of nutrients( Reference Snoussi, Ducroc and Hamdaoui 49 ), and inhibition of intestinal glucose uptake by sodium-dependent GLUT( Reference Kobayashi, Suzuki and Satsu 52 ). Green tea extracts and green tea catechins, such as EGCG, have also been reported to decrease blood glucose levels( Reference Roghani and Baluchnejadmojarad 53 ), although the underlying mechanisms remain largely unknown.
Extracellular glucose enters the cerebral cortex through the action of GLUT. Daily consumption of white tea decreased GLUT levels in the cortex of prediabetic rats. The inhibition of glucose transport through the modulation of GLUT expression levels has been attributed to TP. It has been reported that intestinal glucose uptake is inhibited by TP through competitive inhibition of GLUT( Reference Kobayashi, Suzuki and Satsu 52 , Reference Shimizu, Kobayashi and Suzuki 54 ). Moreover, in vitro studies in Sertoli cells, which are metabolically active cells, have also shown that white tea extract modulates GLUT expression levels( Reference Martins, Alves and Bernardino 18 ). Prediabetic rats drinking white tea presented reduced GLUT1 expression levels when compared with prediabetic rats drinking water as well as decreased GLUT3 expression levels compared with prediabetic rats drinking water and control rats. Thus, reduced brain GLUT expression levels in prediabetic rats drinking white tea may explain persistent hyperglycaemia though glucose tolerance is improved. The decrease in GLUT expression levels may lead to a lower amount of glucose entering the brain that increases its concentration in the periphery, which may explain persistent hyperglycaemia after daily consumption of white tea. After glucose reaches the cortex, it is metabolised via glycolysis. Following the decrease in GLUT expression levels, a decrease in PFK-1 protein expression levels could be expected, illustrating an inhibition of glucose metabolism in the cerebral cortex induced by the consumption of white tea. However, no changes were detected. The expression of PFK-1 was studied but its activity may be altered thus, the evaluation of PFK-1 activity in the future may elicit a mechanism by which the consumption of white tea changes the glycolytic profile of the cerebral cortex. Interestingly, lactate content was decreased in the cortex of prediabetic rats and further decreased after daily consumption of white tea. Lactate is an important energy substrate for neurons during activation( Reference Alessandri, Schwandt and Kamada 55 ). Moreover, lactate may be a crucial metabolic fuel for the cerebral cortex under stressful conditions (for a review, see Schurr( Reference Schurr 56 )). Thus, as expected, moderate hyperglycaemia in prediabetic rats leads to less lactate accumulation in the cortex due to an increase in its metabolism. This was in accordance with the increase of LDH activity that operates in both ways (promoting lactate production or lactate degradation). Moreover, this is also concomitant with the maintenance of monocarboxylate transporter 4 levels, which indicates that although glycolysis is stimulated, the lactate produced is metabolised instead of being exported. Pyruvate derived from glycolysis can also be converted to alanine. Daily consumption of white tea decreased alanine content in the cerebral cortex of prediabetic rats, illustrating a metabolic adaptation to the decrease in lactate content. The lactate:alanine ratio reflects the intracellular redox state since the conversion of pyruvate to lactate or its conversion to alanine is coupled with re-oxidation of NADH into NAD+( Reference O'Donnell, Kudej and LaNoue 57 ). The results suggest that consumption of white tea by prediabetic rats partly restores the intracellular redox state, as can be observed by the increased lactate:alanine ratio, although not reaching the control condition values.
Glycaemic fluctuations, particularly hyperglycaemia, can amplify brain OS inducing several alterations. Within the brain, the cerebral cortex is particularly vulnerable to ROS production since it has a limited antioxidant capacity (for a review, see Wang & Michaelis( Reference Wang and Michaelis 58 )). As expected, prediabetes reduced antioxidant capacity and increased cortical oxidative damage, as evaluated by lipid peroxidation and protein oxidation levels. Moreover, the results show that catalase expression, which is one of the most important enzymatic defences in the cortex, is reduced in prediabetic rats. In addition, total glutathione and GSH contents, usually used as a measure of a balance between ROS production and antioxidant defences, were also decreased in the cerebral cortex of prediabetic rats. Much attention has been paid to natural products that can control hyperglycaemia and OS. The antioxidant potential of white tea, attributed to its polyphenol content, make it a very effective ROS scavenger. Daily consumption of white tea restored the cerebral cortex antioxidant capacity and lipid peroxidation levels of prediabetic rats to normal levels. Importantly, in prediabetic rats, protein oxidation levels were decreased when compared with the control group. Interestingly, recent studies have reported a neuroprotective effect of tea extracts with low EGCG content( Reference Rodrigues, Assunção and Lukoyanov 59 ), particularly against lipid and protein oxidation, illustrating that other components may also play a crucial neuroprotective role.
In the present study, the metabolic and oxidative status of the cerebral cortex was altered in prediabetic rats; thus, as expected, the levels of several intracellular metabolites were also altered. N-Acetylaspartate and choline are two metabolites that have been reported to be diminished( Reference Sinha, Ekka and Sharma 60 ) and increased( Reference Geissler, Fründ and Schölmerich 61 ), respectively, in the brain of diabetic patients. In fact, the results obtained herein are in accordance with those of previous studies. Nevertheless, white tea consumption did not revert that fact. In contrast, the daily consumption of white tea restored valine levels in the cerebral cortex of prediabetic rats to normal levels. Valine is a crucial metabolite involved in protein synthesis, and the results demonstrated that daily consumption of white tea decreases protein oxidation and restores protein synthesis in the cerebral cortex of prediabetic rats.
Undoubtedly, impaired glucose metabolism induces OS that contributes to brain damage. Although daily consumption of white tea did not decrease blood glycaemic levels, it improved glucose tolerance and insulin sensitivity in prediabetic rats. Moreover, daily consumption of white tea altered the cerebral cortical glycolytic profile of prediabetic rats. The effect was particularly evident in the decrease of GLUT expression levels and the content of lactate and alanine. These changes were followed by a clear improvement in the oxidative status of the cerebral cortex. Overall, the cortex of prediabetic rats drinking white tea exhibited greater antioxidant capacity and normalised expression levels of catalase. The protective effect of white tea consumption is mediated not only by the sum of the action of isolated compounds but by the synergistic action of a mixture of compounds. For instance, studies that associate coffee consumption with a reduced risk of some types of cancer have shown that these effects may be due to the synergistic effect of all the compounds present in coffee, and not to a single compound in particular, such as caffeine (for a review, see Nkondjock( Reference Nkondjock 62 )). In addition, many studies have shown that long-term consumption of several tea phytochemicals together can result in better antimicrobial benefits than the action of single components (for a review, see Friedman( Reference Friedman 63 )). However, more studies are needed to unveil the mechanisms responsible for the synergistic action of the compounds present in tea. Moreover, in usual dietary practice, tea is generally prepared by using 1·5 g of green tea leaves per 100 ml of boiling water, but the bioavailability of TP is poor (for a review, see Khan & Mukhtar( Reference Khan and Mukhtar 64 )). In contrast, the amounts of tea consumed by humans are much lower than those given to animals. The differences between animal models and human subjects may also hamper the correct interpretation, extrapolation and practical application of the results and conclusion. Thus, more studies are needed. Nowadays, the interest for traditional medicine, which relies on the use of natural products, including teas, is increasing. Herein, we report for the first time the protective effects of the daily consumption of white tea in the cerebral cortex of a prediabetic model, supporting the idea that white tea ingestion can represent an alternative co-adjuvant in the treatment of DM and/or prevention of DM-related dysfunction in the brain cortex.
Acknowledgements
The present study was supported by the ‘Fundação para a Ciência e a Tecnologia’ – FCT (PEst-OE/SAU/UI0709/2014) co-funded by the Fundo Europeu de Desenvolvimento Regional – FEDER via Programa Operacional Factores de Competitividade – COMPETE/QREN. M. G. A. (SFRH/BPD/80451/2011) and A. C. C. (SFRH/BPD/69643/2010) were funded by the Fundação para a Ciência e a Tecnologia (FCT). P. F. O. was funded by the FCT through Fundo Social Europeu (FSE) and Potencial Operacional Potencial Humano (POPH) funds (Programa Ciência 2008).
The authors' contributions are as follows: A. R. N., M. G. A., G. D. T., V. R. C. and A. C. C. performed the experiments; A. R. N., M. G. A. and P. F. O. contributed to the data interpretation and statistical analysis; P. I. M., P. F. O. and B. M. S. contributed to the overall study design and data interpretation. All authors contributed to the discussion of the content, and writing and reviewing/editing of the manuscript.
There are no conflicts of interest.










