The metabolic syndrome (MetS) is a common metabolic condition that is characterised by a clustering of biochemical and clinical characteristics, including central adiposity and insulin resistance. It is associated with an increased risk of both diabetes( Reference Grundy, Cleeman and Daniels 1 ) and CVD( Reference Isomaa, Almgren and Tuomi 2 – Reference Grundy, Hansen and Smith 6 ). The prevalence of the MetS has increased globally in the past few decades, and this may be related to the increasing prevalence of obesity and diabetes. Impaired glucose tolerance, dyslipidaemia and hypertension are features of the MetS, and more recently a low-grade systemic inflammation has been reported to be associated with it( Reference Sutherland, McKinley and Eckel 7 ). Obesity is also associated with a chronic inflammatory response that includes activation of inflammatory signalling pathways, abnormal cytokine production and an increased acute-phase response( Reference Berg and Scherer 8 ). Moreover, circulating levels of inflammatory markers, such as C-reactive protein (CRP)( Reference Frohlich, Imhof and Berg 9 ) fibrinogen( Reference Ford 10 ), and pro-inflammatory cytokines, such as IL-6( Reference Edalat, Sharifi and Badamchizadeh 11 ), IL-10( Reference Van Exel, Gussekloo and de Craen 12 ) and TNF-α( Reference Moon, Kim and Song 13 ), have been reported to be associated with the MetS( Reference Yudkin, Juhan-Vague and Hawe 14 ). IL-1α and IL-1β as members of IL-1 family are both responsible for a variety of inflammatory and metabolic effects. However, the data have not been consistent( Reference Choi, Lee and Lee 15 – Reference Matsushita, Yatsuya and Tamakoshi 17 ). Furthermore, a great number of experimental and epidemiological studies suggest that low-grade inflammation links variables, particularly attributed to the MetS, to the increased risk of CVD. It is known that a low-grade systemic inflammation precedes and predicts the progress of both diabetes and atherothrombotic diseases. The mild inflammatory state is associated with insulin resistance and obesity( Reference Kern, Ranganathan and Li 18 ). However, Matsushita et al. ( Reference Matsushita, Yatsuya and Tamakoshi 17 ) studied 624 middle-aged Japanese men without a history of CVD or cancer and reported no significant associations of TNF-α and IL-6 with the MetS or its components. These inconsistent findings may be explained by several reasons, including variations in lifestyle, including diet, severity of associated disease, small sample size, ethnic origin and concurrent medication. Since in previous studies the relationship between the MetS and CVD and a limited numbers of individual inflammatory markers were assessed, in the present study we have measured the serum concentrations of a profile of twelve biomarkers of various categories (pro-inflammatory, anti-inflammatory and growth factors: epidermal growth factor (EGF), interferon-γ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1 (MCP-1), TNF-α and vascular endothelial growth factor (VEGF)) in groups of individuals with or without the MetS.
Methods
Phenotypic definition of the metabolic syndrome
We used the International Diabetes Federation criteria to define the MetS, according to which the MetS is defined by the following characteristics: central obesity (defined as waist circumference of ≥ 94 cm for male or ≥ 80 cm for female) plus any two of the following four factors: raised TAG: ≥ 150 mg/dl (1·7 mmol/l); reduced HDL-cholesterol: < 40 mg/dl (1·03 mmol/l) in males < 50 mg/dl (1·29 mmol/l) in females; raised systolic blood pressure (SBP) ≥ 130 or diastolic blood pressure (DBP) ≥ 85 mmHg; raised fasting blood glucose ≥ 100 mg/dl (5·6 mmol/l).
Population
A total of 303 participants were recruited, including 155 patients with the MetS and 148 controls without the MetS from Mashhad (Iran) who had no known history of infectious diseases or major systemic inflammation and were without a family history of stroke, myocardial infarction and diabetes mellitus. The population samples were recruited from Mashhad city and the surrounding regions in northeast Iran.
Exclusion criteria were medical illnesses such as chronic liver and/or renal diseases, pregnant women and alcohol consumption based on a questionnaire, taking any drugs including dietary supplements and anti-inflammatory drugs including aspirin and non-steroidal anti-inflammatory drugs. All participants completed a standardised questionnaire including the following information: sex, ethnicity, age, dietary habits, family history of myocardial infarction, stroke, diabetes mellitus and past medical history. In addition, dietary intake was determined by means of a questionnaire that was designed on the basis of a 24-h dietary recall. Analysis of macronutrient intake was performed using Diet Plan 6 software (Forestfield Software Limited). A trained interviewer performed face-to-face interviews, and individuals were asked about all foods and beverages that were consumed. Informed consent was obtained from all participants using protocols approved by the Ethics Committee of the Mashhad University of Medical Sciences (MUMS).
Anthropometric measurements
Anthropometric parameters (height, body weight, waist and hip circumference) were measured using standardised procedures, as described( Reference Mirhafez, Mohebati and Feiz Disfani 19 ). BMI was calculated as body weight (kg) divided by squared height in meters (m2), and BMI of 20–24·9, ≥ 25–29·9 and ≥ 30 kg/m2 were considered as normal, over-weight or obese, respectively. SBP and DBP (SBP or DBP) were measured in duplicate by sphygmomanometers. Total cholesterol, HDL, LDL and TAG, and fasting blood glucose concentrations were evaluated by standard enzymatic techniques, while serum CRP levels were determined by polyethylene glycol-enhanced immunoturbidimetry, as described previously( Reference Mirhafez, Mohebati and Feiz Disfani 19 ).
Measurement of cytokines and growth factors
The concentrations of serum cytokines and growth factors, including IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, IFN-γ, MCP-1, EGF and VEGF, were measured using the EV 3513 cytokine biochip array (Randox Laboratories) and competitive chemiluminescence immunoassays (Randox Laboratories), according to the manufacturer's instructions( Reference Molloy, Mc Connell and Lamont 20 , Reference Fitzgerald, Lamont and McConnell 21 ), using the Randox Evidence Investigator. The Evidence Investigator is an automated analyser for the simultaneous detection of multiple cytokines from a single sample and uses sandwich chemiluminescent methods( Reference Molloy, Mc Connell and Lamont 20 , Reference Fitzgerald, Lamont and McConnell 21 ). Quantitative and qualitative results are also available. Intra- and inter-assay CV for most cytokine markers were ≤ 10 % (see online Supplementary Table S1). Randox cytokine controls are intended for use as an assayed quality control in the routine monitoring of accuracy and precision for the analyses listed in this study.
Network analysis
Functional and physical interactions between pro- and anti-inflammatory factors were retrieved using STRING( Reference Nepusz, Yu and Paccanaro 22 ). Data were analysed using Cytoscape version 2.8.3 (an open-source Java tool), and networks were merged with embedded analysis tools, while the Biological Networks Gene Ontology plug-in provided ontologies for the cluster analyses( Reference Nepusz, Yu and Paccanaro 22 ).
Statistical analysis
Data were analysed using SPSS-16 software (SPSS, Inc.). The normality of distribution was assessed using the Kolmogorov–Smirnov test. Descriptive statistics including mean, frequency and standard deviation were determined for all variables and was expressed as means with standard deviations for normally distributed variables (or as the median and interquartile range for non-normally distributed variables). For normally and non-normally distributed variables, Student's t test and Mann–Whitney U test were used to compare the clinical characteristics and baseline demographics between the groups, respectively. The χ2 test and/or Fisher's exact test was used for comparing categorical variables. The correlations between the cytokines and growth factors were assessed using Spearman's correlation analysis. Logistic regression analysis was used to calculate association of serum cytokines level and the MetS in present of confounders such as age, sex, smoking and dietary intake. Multivariate analysis was then undertaken for the variables that had a P< 0·05 in the univariate analysis. Residual regression was used for adjusting of total energy intake. All the analyses were two-sided and statistical significance was set at P< 0·05.
Results
Clinical characteristics of the population
The clinical and baseline characteristics of patients with and without the MetS are summarised in Tables 1 and 2. Individuals with the MetS had a significantly higher BMI, waist circumference, hip circumference, fasting blood glucose, high-sensitivity CRP, TAG, SBP and DBP (P< 0·05), while no differences were found for age, sex, height, serum total cholesterol and LDL cholesterol between the groups (Table 1). Moreover, we measured fat percentage and waist:hip ratio in our population. These data showed that the ratio of waist:hip measurements was significantly (P< 0·05) increased in the MetS group, compared with the control group. Moreover, the mean and standard deviations of macronutrients and their P-values are presented in Table 2. In the present study, the mean total energy intake in the MetS group was less than that of control groups (P= 0·027). The mean intakes of protein, total fat, SFA, MUFA and PUFA in the MetS group were less than those of control groups (P< 0·05); however, after adjusting for total energy intake, significant differences remained for total fat, MUFA and PUFA (P< 0·05).
Table 1 Characteristics data from all subjects in each group* (Mean values and standard deviations; number of participants and percentages; median values and interquartile ranges (IQR))

MetS, metabolic syndrome; WC, waist circumference; HC, hip circumference; LDL-C, LDL cholesterol; TC, total cholesterol; HDL-C, HDL cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; hs-CRP, high-sensitivity C-reactive protein.
* Student's t test and Mann–Whitney U test were used to compare the demographic and clinical characteristics in normally and non-normally distributed variables, respectively.
Table 2 Comparison of the dietary intake of macronutrients between subjects under study * (Mean values and standard deviations)

MetS, metabolic syndrome.
* The dietary intake was adjusted based on total energy intake, as described previously( Reference Nazeminezhad, Tajfard and Latiff 44 ).
Altered serum cytokines and growth factor profile in individuals with metablolic syndrome
As shown in Table 3, individuals with the MetS had significantly higher serum concentrations of IL-1α/-β/-2/-4/-6/-8/-10, TNF-α, IFN-γ, MCP-1 and EGF compared with the control group (P< 0·05). Conversely, the serum concentration of VEGF was significantly lower in individuals with the MetS (P< 0·05; Table 3). Based on these results, we built two separate networks and functional analyses; the first was composed of all possible interactions for cytokines, while the second included interactions retrieved for proteins that had a differential expression (Fig. 1). These analyses showed the physical interaction of cytokines and growth factors in patients with the MetS, suggesting that IL-6/8, VEGF, TNF and chemokine (C-C motif) ligand 2 (MCP-1) were activated, followed by induction of IL-1A/-1B (Fig. 1).
Table 3 Serum concentration of cytokines and growth factors levels in two groups with and without the metabolic syndrome (MetS)* (Median values and interquartile ranges (IQR))
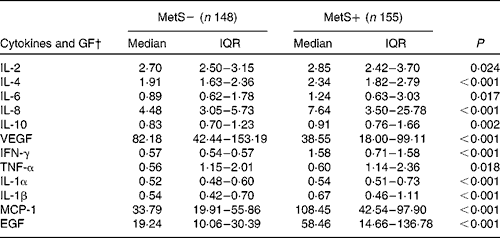
GF, growth factors; VEGF, vascular endothelial growth factor; IFN-γ, interferon-γ; MCP1, monocyte chemo attractant protein; EGF, epidermal growth factor.
* The Mann–Whitney U test was performed.
† Serum cytokines level is expressed as pg/ml.

Fig. 1 Network analysis. Functional and physical interactions between pro-inflammatory and anti-inflammatory cytokines and growth factors were retrieved using STRING and data were loaded in Cytoscape version 2.8.3. Arrows show the direction of interaction. CCL2 (monocyte chemo attractant protein-1), GIF (interferon-γ), EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; GIF, anti-interferon gamma antibody. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Association of serum cytokine and growth factor
We also evaluated the correlation between the cytokines and growth factors in each group (Tables 4 and 5). This additional analysis revealed a significant correlation between the expression levels of IL-8 and TNF-α (P< 0·001, r 0·287) or IFN-γ and IL-4 (P= 0·002, r 0·256) or IL-4 and VEGF (P= 0·001, r − 0·272) in individuals without the MetS. However, we observed a significant correlation between some of cytokines in the MetS group (e.g. correlation of IL-6 with IL-8, P= 0·001, r 0·274, Tables 4 and 5).
Table 4 Correlation matrix between growth factors, inflammatory and anti-inflammatory cytokines in the group without the metabolic syndrome (n 148) using Spearman's correlation analysis

VEGF, vascular endothelial growth factor; IFN-γ, interferon-γ; MCP-1, monocyte chemo attractant protein; EGF, epidermal growth factor.
* Statistically significant (P< 0·05).
Table 5 Correlation matrix between growth factors, inflammatory and anti-inflammatory cytokines in the group with the metabolic syndrome (n 155) using Spearman's correlation analysis

VEGF, vascular endothelial growth factor; IFN-γ, interferon-γ; MCP-1, monocyte chemo attractant protein; EGF, epidermal growth factor.
* Statistically significant (P< 0·05).
Association of serum cytokine and growth factor profiles with the metabolic syndrome
To evaluate the association of serum cytokine and growth factor concentrations with the MetS, univariate and multivariate analyses were employed (Table 6). This analysis showed an independent significant (P< 0·05) relationship between the presence of the MetS and serum concentrations of IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-α, VEGF and MCP-1, after removing the effects of age, sex and smoking. IFN-γ and IL-1α showed the greatest independent association with the MetS (Table 6). Then we created an inflammatory score to provide a summary statistic representative of the panel of markers using regression coefficient of each marker in multivariate model multiple-by variation range of cytokines and growth factors level. This analysis suggested that IFN-γ is the factor most related with the MetS (see online Supplementary Table S2).
Table 6 Association between cytokine and growth factor concentrations and the metabolic syndrome† (Odds ratios and 95 % confidence intervals)

VEGF, vascular endothelial growth factor; IFN-γ, interferon-γ, MCP-1, monocyte chemoattractant protein; EGF, epidermal growth factor; hs-CRP, high-sensitivity C-reactive protein.
* In present of age, sex, smoking and dietary intake (including energy intake, protein, adjusted carbohydrate, adjusted total fat SFA, adjusted MUFA and adjusted PUFA, which were significantly different between groups). Multivariate analysis was done when P value of univariate was < 0·05.
† Association of inflammatory markers level with the metabolic syndrome in calculated by binary logistic regression model.
Discussion
We have evaluated the association of twelve serum cytokines and growth factors (EGF, IFN-γ, IL-1α/-1β/-2/-4/-6/-8/-10, MCP-1, TNF-α and VEGF) in individuals with and without the MetS. We found that the serum concentrations of EGF, IFN-γ, IL-1α/-1β/-2/-4/-6/-8/-10, MCP-1 and TNF-α were significantly higher in the individuals with the MetS, while the circulating levels of VEGF were markedly lower in the group with the MetS; these findings are in agreement with previous studies( Reference Sutherland, McKinley and Eckel 7 ).
There is accumulating evidence of an association between the MetS and inflammation( Reference Sutherland, McKinley and Eckel 7 , Reference Eckel, Grundy and Zimmet 23 ). The increases in serum pro-inflammatory cytokines, such as IL-6 and TNF-α, are probably related to the enhanced production by the expanded adipose tissue mass( Reference Fernández Real and Ricart 24 ). It has been suggested that resident monocyte-derived macrophages in the adipose tissue are a major source of pro-inflammatory cytokines( Reference Trayhurn and Wood 25 ). However, little is known about the pro-/anti-inflammatory cytokine and the growth factor profile in this condition. We found significant differences in the concentrations of circulating cytokines and growth factors in the individuals with the MetS. The limited numbers of studies concerning the relationship between inflammation and the MetS as a particular disorder stems from the great overlap between inflammatory features of metabolic disorders such as obesity and diabetes as well as the range of variables involved in the pathogenesis of the MetS. Different components of the MetS have been implicated to be associated with low-grade inflammation mediators including pro-inflammatory, inflammatory and anti-inflammatory markers in numerous studies( Reference Berg and Scherer 8 , Reference Fernández Real and Ricart 24 ). Ridker et al. ( Reference Ridker, Buring and Cook 26 ) explored the relationship between CRP, the MetS and incidence of cardiovascular events among 14 719 apparently healthy women. They observed that the measurement of CRP could add clinically important prognostic information to the MetS( Reference Ridker, Buring and Cook 26 ). Furthermore, abdominal obesity as a major manifestation of the MetS leads to an increased expression and elaboration of various adipokines, which eventually results in persistent low-grade inflammation.
Here, we observed that individuals with the MetS had a significantly higher concentration of CRP and IL-6 than individuals without this syndrome. IL-6 and TNF-α are both expressed in adipose tissue( Reference Matsushita, Yatsuya and Tamakoshi 17 , Reference Miyazaki, Pipek and Mandarino 27 ), and have been reported as two of the most important inflammatory markers associated with the MetS( Reference Kern, Ranganathan and Li 18 ). There is increasing evidence that the elevated circulating levels of IL-6, IL-8, IL-1β and TNF-α predict the risk of type 2 diabetes( Reference Giulietti, van Etten and Overbergh 28 , Reference Odegaard and Chawla 29 ). In addition, in line with a previous finding( Reference Yaturu, Rains and Jain 30 ), we observed a significant increase in the concentrations of the aforementioned cytokines and IL-2 in the subjects with the MetS. In agreement with our findings, the correlation between elevated concentrations of IL-2 along with other pro-inflammatory cytokines and occurrence of metabolic disorders such as diabetes mellitus has been demonstrated in several studies( Reference Yaturu, Rains and Jain 30 ). The potential protective effects of some anti-inflammatory factors against metabolic disturbances associated with adiposity, including glucose intolerance( Reference Fleischman, Shoelson and Bernier 31 ). We found significantly higher levels of serum anti-inflammatory cytokines, IL-4 and IL-10 in the individuals with the MetS; the reason for this is uncertain, but may be a compensatory response to the overall pro-inflammatory state. Makki et al. ( Reference Makki, Thiel and Miller 32 ) have recently described that the activation of EGF receptor is implicated in blood pressure regulation, endothelial dysfunction, atherogenesis and cardiac remodelling, and increased circulating EGF-like ligands might mediate vascular disease associated with chronic inflammation. We found higher concentrations of serum EGF and lower concentration of VEGF in the group of individuals with the MetS than in the control subjects. We also found a correlation between VEGF with TNF-α in the control group. This is in accord with the work reported by Azimi-Nezhad et al. ( Reference Azimi-Nezhad, Stathopoulou and Bonnefond 33 ), who found that VEGF expression is modulated by TNF-α in peripheral blood mononuclear cells, which might be a plausible connection between VEGF and inflammatory markers; however, further studies are needed to explore their cross talk.
There is a growing body of evidence showing a relationship between chronic inflammation and the disruption of cellular metabolism( Reference Emanuela, Grazia and Marco de 34 , Reference Hotamisligil and Erbay 35 ). Zhang and collaborators( Reference Zhang and Shi 36 ) showed that mast cells contributed to the pathogenesis of type 2 diabetes by generating of IFN-γ. IFN-γ desensitises adipocyte to insulin and inhibits the maturation of pre-adipocyte in a Janus kinase 1/signal transducer and activator of transcription 1 (JAK1/STAT1) dependent manner. Moreover, IFN-γ disrupts energy expenditure and metabolic homeostasis( Reference Li, Zhao and Wu 37 ). In the present study, we observed that serum concentration of IFN-γ significantly increased from 0·57 pg/ml (interquartile range 0·54–0·57) to 1·58 pg/ml (interquartile range 0·71–1·58), P< 0·05 and was associated with higher OR (3·87) in the MetS group, compared to other markers, as determined by univariate and multivariate analysis. Additional cluster analysis using regression coefficient of each of the cytokines/growth factor in multivariate model multiple-by variation range of each marker suggested IFN-γ as the most related factor, with the MetS among the top thirteen markers. Similar results were observed for IL-1α as the most related factor with the MetS after IFN-γ, showing that patients with the MetS had an OR of 2·28 (P= 0·049) after adjustment for age, sex, smoking and dietary intake. In line with our observation, Um et al. ( Reference Um, Rim and Kim 38 ) have proposed that IL-1α, a pro-inflammatory cytokine secreted from adipose tissue, contributes to the morbidity associated with obesity and CVD( Reference Mehra, Ramgolam and Bender 39 , Reference Fearon and Fearon 40 ). In addition, network analysis illustrated potential interaction of cytokines and growth factors in MetS patients, suggesting the activation of IL-6/8 and TNF via induction of IL-1. In turn, IFN-γ induces nuclear translocation of NF-κB( Reference Li, Wang and Wu 41 ), which is the major transcription factor mediating the transcriptional activation of TNF-α and inflammatory genes in cellular response to the environmental factors( Reference Zhou, Scoggin and Gaynor 42 ). TNF is shown to activate the mammalian target of rapamycin signalling pathway, which is associated with insulin resistance and diabetic complications( Reference Blagosklonny 43 ).
A strength of the present study is that it was conducted in a well-characterised cohort of individuals, with or without the MetS, in whom a simultaneous profile of several potentially important cytokines was assessed. The main limitations were the cross-sectional study design and modest sample size. It has been shown that certain dietary components, found in fruits, berries, vegetables, nuts, whole grains, and foods of marine origin, might play a crucial role in reducing and mitigating chronic pro-inflammatory processes associated with chronic diseases. In the present study, we adjusted the effects of macronutrient intake( Reference Nazeminezhad, Tajfard and Latiff 44 ), which were significantly different between the groups in multivariate analysis to explore the association of cytokines and growth factor with the MetS (Table 6). It is possible that other lifestyle characteristics such as micronutrients may have an important influence on the cytokine profile. In particular, it has been suggested that culinary herbs and spices have anti-inflammatory activities and the extent to which they activate PPARα and PPARγ inhibit the activation of NF-κB and enhance expression of anti-inflammatory cytokines( Reference Jungbauer and Medjakovic 45 ), which might explain at least in part the inverse effect of some anti-inflammatory cytokines in our population. The impact of treatment with anti-inflammatory agents on the biochemical and clinical outcomes of individuals with the MetS would clearly be important to explore, as would the value of cytokine profiles in predicting MetS patients. Moreover, in the present study we did not evaluate the relation between complement component 3 (C3) and the MetS, supporting further investigation on the role of this factor in the MetS.
In conclusion, we have shown that individuals with the MetS have altered levels of circulating pro- and anti-inflammatory cytokines and growth factors that may be implicated in clinical outcomes. Further multi-centre and prospective trials are required to unravel the role and association of emerging inflammatory markers with the MetS and cardiovascular outcomes.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001038
Acknowledgements
We would like to thank all the patients for their help and participating in the study.
This work was supported by the grant from MUMS, Research Council (M. G.-M., S. R. M., A. A. and A. P.). The results obtained in the present study are part of Mr Seyed Reza Mirhafez's PhD thesis (ID no. 910823) in MUMS. MUMS had no role in the design, analysis or writing of this article.
The authors' contributions are as follows: M. G.-M., A. P. and Z. M. contributed to the study design; S. R. M., M. M. and A. R. H.-B. conducted the experimental part; S. R. M., M. G.-M., H. R. R., S. E., H. M.-M., H. E. and A. M. contributed to the data analysis; S. R. M., A. P., A. A., G. A. F., A. P. and M. G.-M. contributed to the preparation of the manuscript.
The authors have no conflict of interest.










