Non-alcoholic fatty liver disease (NAFLD) is a progressive disorder that can lead to liver disease (e.g. fibrosis or cirrhosis) and cancer(Reference Park, Lee and Chung1). NAFLD is strongly associated with insulin resistance and is estimated to occur in 70–100 % of the obese population(Reference Farrell and Larter2). Although the pathogenesis of NAFLD is not completely understood, excessive fat accumulation and oxidative stress in the liver appear to play a direct role in its initiation and development(Reference Farrell and Larter2). Furthermore, an excessive and inappropriate dietary fat intake causes insulin resistance, increases NEFA delivery to the liver and alters gene expression involved in lipid metabolism, which results in fat accumulation in the liver(Reference Assy, Nassar and Nasser3, Reference Park, Choi and Um4). Therefore, current research is investigating whether phytochemicals in food exert beneficial effects on metabolism, such as preventing hepatic fat accumulation and improving insulin resistance.
Coumarin (1,2-benzopyrone) is a natural phenolic compound found in plant-based foods such as citrus fruits, tomatoes, vegetables and green tea. Coumarin has been reported to possess antibacterial, antiviral, vasodilatory, antimutagenic, antioxidant and anticancer activities(Reference Murat Bilgin, Atmaca and Deniz Obay5). In addition, coumarin has been shown to exert a protective effect against CCl4-induced hepatic injury in rats(Reference Atmaca, Bilgin and Obay6). Hsu & Yen(Reference Hsu and Yen7) reported that coumaric acid inhibits the expression of PPARγ and CCAAT/enhancer binding protein-α (C/EBPα), and up-regulates adiponectin levels in 3T3-L1 adipocytes. In addition, coumaric acid exerts anti-obesity and lipid-lowering effects(Reference Yuce, Danis and Ogan8–Reference Hsu, Wu and Huang10). Although a number of studies have reported various biochemical actions of coumarin, the ability of coumarin to protect against high-fat diet (HFD)-induced hepatic steatosis in animals and the molecular mechanisms underlying the effects of coumarin have not yet been reported in detail.
Therefore, we evaluated the effect of coumarin on HFD-induced hepatic steatosis in mice. To determine the mechanisms responsible for its effect, we evaluated enzyme activities and gene expression involved in hepatic lipid metabolism.
Experimental methods
Animals and diets
Male C57BL/6J mice (4 weeks old) were purchased from Orient Bio. C57BL/6J mice are prone to obesity when fed a HFD, developing characteristics observed in human obesity(Reference Cho, Jung and Choi11). After a 1-week adaptation period, mice were randomly divided into three groups (n 10 per group) and fed a normal diet (ND), HFD or HFD containing 0·05 % coumarin (CD) for 8 weeks. The HFD was prepared by supplementing the American Institute of Nutrition (AIN) 76 rodent diet with 15 % fat (maize oil 5 %, coconut oil 3 % and cocoa butter 7 %) and 0·5 % cholesterol (g/100 g) and consisted of 31·2 % fat, 18·5 % protein and 50·3 % carbohydrate. Feeding a HFD is crucial in the development of hepatic steatosis of the diet-induced obesity mouse model(Reference Cho, Jung and Choi11–Reference Oosterveer, Van Dijk and Tietge13). Coumarin was obtained from Sigma-Aldrich. The dose of coumarin was chosen from the results of our preliminary experiment, showing maximal inhibitory effects against increase in body fat and hepatic lipid accumulation (data not shown), and the results of previous animal studies(Reference Pari and Rajarajeswari14, Reference Ogawa, Sasai and Kamisako15). The mice were housed individually and maintained at a controlled temperature (22 ± 1°C), humidity (65 ± 5 %) and 12 h light–12 h dark cycle. Body weight and food intake were recorded three times per week for 8 weeks. The mice were allowed ad libitum access to food and water.
At the end of experimental period, blood was obtained from the retro-orbital sinus of mice in the fasting state. Liver and adipose tissues (epididymal and retroperitoneal adipose tissue) were removed, weighed and immediately snap-frozen in liquid N2, and stored at − 80°C until analysed. The present study was approved by the Animal Care Committee of the Korean Food Research Institute and performed in compliance with the Guide for the Care and Use of Laboratory Animals.
Blood analysis
Serum levels of total cholesterol (TC), HDL-cholesterol, TAG, NEFA and glucose were determined using a commercial kit (Shinyang Chemical Company). Serum apoA1 and apoB levels were determined using a commercial kit (Nittobo). Insulin and leptin levels were quantified by ELISA (R&D Systems).
Analysis of hepatic lipids and thiobarbituric acid-reactive substances
Hepatic lipids were extracted from the liver tissues according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley16). TC and TAG levels in the liver were determined using a commercial kit (Sinyang Chemical Company).
Hepatic lipid peroxidation was determined by evaluating the production of thiobarbituric acid-reactive substances (TBARS), according to the method of Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi17), in which the reaction of malondialdehyde (a product of lipid peroxidation) with thiobarbituric acid yields a compound that is quantified by measuring absorbance at 532 nm.
Hepatic and adipose tissue histology
Liver and epididymal adipose tissue were removed and fixed with 10 % buffered formalin. The paraffin-embedded samples were cut into 3-μm-thick sections on a microtome and stained with haematoxylin and eosin. Stained sections were analysed by light microscopy (BX50; Olympus), and digital images were captured. Adipocyte size was determined using the image analysis program Image J (National Institutes of Health; http://rsb.info.nih.gov/ij/).
Lipogenic enzyme activities
Hepatic tissue was homogenised in ice-cold buffer (pH 7·4) containing 0·25 m-sucrose and 0·5 mm-EDTA, and then centrifuged at 10 000 g at 4°C for 30 min. The supernatant was used to determine malic enzyme (ME) and fatty acid synthase (FAS) activities. ME activity was determined spectrophotometrically by monitoring the production of NADPH at 340 nm(Reference Ochoa, Colowick and Kaplan18). FAS activity was determined according to the procedures described by Gibson & Hubbard(Reference Gibson and Hubbard19). Protein concentration was determined by the method of Lowry et al. (Reference Lowry, Rosebrough and Farr20) using serum bovine albumin as the standard.
Western blot analysis
Hepatic tissues were homogenised in lysis buffer and then centrifuged at 13 000 rpm at 4°C for 20 min. Total protein (20 μg) was separated by 10 % SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Millipore). The membranes were blocked overnight with 5 % non-fat dry milk in Tris-buffered saline solution containing 0·05 % Tween-20 and then incubated for 4 h using rabbit polyclonal antibodies against sterol regulatory element-binding protein (SREBP)-1, FAS, acetyl-CoA carboxylase 1 (ACC1), C/EBPα, PPARγ and β-actin (1:1000; Santa Cruz Biotechnology, Inc.). β-Actin was used as the loading control. Immunoreactive proteins were detected by using the enhanced chemiluminescence (ECL) Western blotting detection system.
RNA extraction and quantitative real-time RT-PCR analysis
Total RNA was extracted from the liver tissue using the RNeasy Mini kit (Qiagen), according to the manufacturer's protocol. RT was carried out in a 20 μg reaction mixture using 1 μg total RNA and the Maxime RT PreMix Kit (iNtRON Biotechnology). The following primer pairs were used for real-time RT-PCR: SREBP-1c, 5′-GCAGCCACCATCTAGCCTG-3′ and 5′-CAGCAGTGAGTCTGCCTTGAT-3′; FAS, 5′-GGAGGTGGTGATAGCCGGTAT-3′ and 5′-TGGGTAATCCATAGAGCCCAG-3′; ACC1, 5′-CTGCCGAAACATCTCTGGGA-3′ and 5′-CTGCCGAAACATCTCTGGGA-3′ and β-actin, 5′-GGCTGTATTCCCCTCCATCG-3′ and 5′-CCAGTTGGTAACAATGCCATGT-3′. Amplification was carried out using SYBR green PCR Master mix (Toyobo) and a Roche LightCycler 480 System (Roche Applied Science). Expression levels of SREBP-1c, FAS and ACC1 were normalised to β-actin.
Statistical analysis
Statistical analysis was performed using the SPSS 14.0 package (SPSS, Inc.). Results are expressed as mean values with their standard errors. Groups were compared by one-way ANOVA and Duncan's multiple range test; P< 0·05 was considered significant.
Results
Effects of coumarin on body weight, food intake and adiposity
Body weight was increased in mice fed the HFD, compared with mice fed the ND, but this increase was significantly reduced by coumarin supplementation in the CD group (Table 1 and Fig. 1(A)). However, food intake did not differ significantly among the experimental groups (Fig. 1(B)).
Table 1 Effect of coumarin on body weight and food intake (Mean values with their standard errors, n 10 per group)

ND, normal diet; HFD, high-fat diet; CD, HFD+coumarin 0·05 %.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).

Fig. 1 Effects of courmarin on (A) body weight, (B) food intake, (C) abdominal fat pad weight and (D) epididymal adipocyte size in C57BL/6J mice fed experimental diets. Body weight gain and food intake were measured during the experimental period. Epididymal adipose tissues were isolated and stained with haematoxylin and eosin (H&E). Adipocyte size was determined in H&E-stained tissue (original magnification × 200) by using Image J analysis software (National Institutes of Health). Values are means, with their standard errors represented by vertical bars (n 10 per group). a,b,cMean values with unlike letters were significantly different (P< 0·05, Duncan's multiple range test). (A, B) ![]() , Normal diet (ND);
, Normal diet (ND); ![]() , high-fat diet (HFD);
, high-fat diet (HFD); ![]() , HFD containing 0·05 % coumarin (CD). (C) □, ND; ■, HFD;
, HFD containing 0·05 % coumarin (CD). (C) □, ND; ■, HFD; ![]() , CD. (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
, CD. (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
To determine whether the body weight gain in the HFD group was due to decreased fat accumulation, adipose tissue weight and adipocyte size were measured. As shown in Fig. 1(C), the weights of epididymal and retroperitoneal adipose tissues were 165 and 153 % higher in the HFD group than in the ND group, but this increase was attenuated by coumarin supplementation (by 36·7 and 39·5 %, P< 0·05). Similarly, the size of epididymal adipocytes was larger in the HFD group than in the ND group, but this effect was also attenuated by coumarin supplementation (P< 0·05, Fig. 1(D)). These results demonstrate that coumarin supplementation significantly decreased body fat accumulation.
Effect of coumarin on serum lipid, insulin and leptin levels
Next, the effects of coumarin on serum levels of lipids were evaluated. As shown in Table 2, mice fed the HFD had significantly higher serum TC and apoB levels than mice fed the ND. However, coumarin supplementation reduced serum TC, and mice in the CD group had apoB levels similar to that of the ND group. The atherogenic index and apoB:apoA ratio, which are sensitive biomarkers of CHD, were also elevated in the HFD group; however, these values were decreased in the CD groups. Furthermore, NEFA levels were significantly lower in the CD group than in the HFD group, but did not differ significantly between the ND and HFD groups. In contrast, HDL-cholesterol and TAG levels did not differ significantly among the experimental groups.
Table 2 Effect of coumarin on fasting serum lipid and lipoprotein levels in C57BL/6J mice fed an experimental diet for 8 weeks (Mean values with their standard errors, n 10 per group)

ND, normal diet; HFD, high-fat diet; CD, HFD+coumarin 0·05 %
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Atherogenic index = (total cholesterol–HDL-cholesterol)/HDL-cholesterol.
Finally, serum levels of insulin and leptin were significantly increased in the HFD group, but these values were reduced by 43·7 and 51·5 %, respectively, by coumarin supplementation (Fig. 2(A) and (B)). These results indicate that coumarin markedly improved blood lipid, insulin and leptin levels in HFD-fed mice.

Fig. 2 Effect of coumarin on serum concentrations of (A) insulin and (B) leptin in C57BL/6J mice fed experimental diets for 8 weeks. Values are means, with their standard errors represented by vertical bars (n 10 per group). a,b,cMean values with unlike letters were significantly different (P< 0·05, Duncan's multiple range test). ND, normal diet; HFD, high-fat diet; CD, HFD containing 0·05 % coumarin.
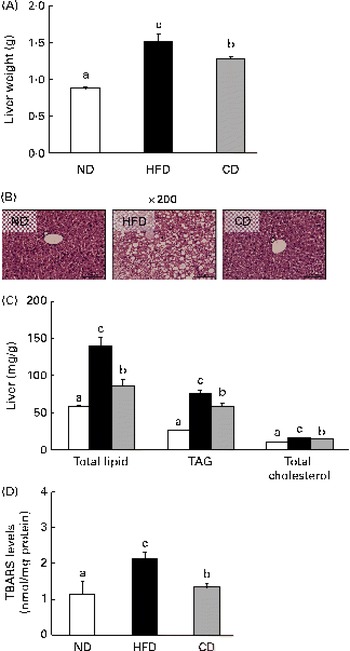
Effects of coumarin on hepatic lipid content and morphology
A HFD is associated with steatosis and metabolic disorders(Reference Park, Choi and Um4). Therefore, we investigated whether coumarin can protect against HFD-induced hepatic steatosis. We found that liver weight was significantly higher in HFD-fed mice than in ND-fed mice, but this increase was reduced by coumarin supplementation (Fig. 3(A)). Histological analysis showed that HFD-fed mice developed hepatocellular micro- and macrovesicular vacuolation as a result of fat accumulation. These changes were ameliorated by coumarin supplementation (Fig. 3(B)). HFD-induced increases in hepatic total lipid, TAG and TC levels were also significantly decreased by coumarin (Fig. 3(C)). These results demonstrate that coumarin suppressed HFD-induced hepatic fat accumulation in the mice.

Fig. 3 Coumarin alleviates high-fat diet (HFD)-induced fat accumulation in the liver. (A) Liver weight, (B) representative haematoxylin and eosin-stained liver section, (C) hepatic lipid concentrations and (D) hepatic thiobarbituric acid-reactive substance (TBARS) levels in mice fed experimental diets for 8 weeks. Values are means, with their standard errors represented by vertical bars (n 10 per group). a,b,cMean values with unlike letters were significantly different (P< 0·05, Duncan's multiple range test). □, Normal diet (ND); ■, HFD; ![]() , HFD containing 0·05 % coumarin (CD). (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
, HFD containing 0·05 % coumarin (CD). (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
To determine whether the HFD-induced hepatic fat accumulation was associated with increased oxidative stress, we assessed the effects of coumarin on TBARS levels, an indicator of lipid peroxidation. TBARS levels were 2-fold higher in the HFD group compared with the ND group; however, the HFD-induced increase was reduced by 38 % in the CD group (Fig. 3(D)). These findings suggest that coumarin can ameliorate hepatic oxidative stress associated with NAFLD.
Effects of coumarin on lipid-regulating enzyme activities in the liver
To determine the mechanism by which coumarin suppresses fat accumulation in the liver, we evaluated the activities of hepatic lipid-regulating enzymes. We found that FAS activity was elevated in the HFD group compared with the ND group, but that coumarin significantly decreased FAS activity (Table 3). In contrast, ME activity did not differ significantly between the ND and HFD groups, but was lower in the CD group compared with the other experimental groups.
Table 3 Effects of coumarin on the activities of lipid metabolism enzymes in C57BL/6J mice fed an experimental diet for 8 weeks (Mean values with their standard errors, n 10 per group)

ND, normal diet; HFD, high-fat diet; CD, HFD+coumarin 0·05 %.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Effects of coumarin on lipogenic gene expression in the liver
To further clarify the molecular mechanisms underlying the effects of coumarin on HFD-induced hepatic steatosis, we evaluated the expression of lipogenesis-related genes by Western blot analysis and quantitative RT-PCR. As shown in Fig. 4, SREBP-1c protein and mRNA levels were higher in the HFD group than in the ND group. Accordingly, expression of SREBP-1c target genes FAS and ACC1 were also up-regulated in the HFD group. However, protein levels of SREBP-1c, FAS and ACC1 were reduced in the CD group.

Fig. 4 Effect of coumarin on the expression of (A) protein and (B) mRNA involved in lipogenesis and adipogenesis in the liver of mice fed experimental diets. Protein levels were determined by Western blot analysis and normalised to β-actin. The mRNA levels of target genes were determined by real-time quantitative RT-PCR and normalised to β-actin. Values are means, with their standard errors represented by vertical bars (n 10 per group). a,b,cMean values with unlike letters were significantly different (P< 0·05, Duncan's multiple range test). □, Normal diet (ND); ■, high-fat diet (HFD); ![]() , HFD containing 0·05 % coumarin (CD). SREBP-1, sterol regulatory element-binding protein-1; C/EBPα, CCAAT/enhancer binding protein-α; ACC1, acetyl-CoA carboxylase 1; FAS, fatty acid synthase.
, HFD containing 0·05 % coumarin (CD). SREBP-1, sterol regulatory element-binding protein-1; C/EBPα, CCAAT/enhancer binding protein-α; ACC1, acetyl-CoA carboxylase 1; FAS, fatty acid synthase.
Protein levels of PPARγ and C/EBPα, regulators of lipogenesis and insulin signalling, were also increased in the HFD group, but were reduced by coumarin supplementation. Taken together, these findings suggest that coumarin regulates hepatic lipid metabolism by modulating the expression of lipogenic genes.
Discussion
In the present study, we examined the effect of coumarin on HFD-induced fat accumulation in C57BL/6J mice. Hepatic steatosis is usually observed in obese subjects. Several studies demonstrated that hepatic steatosis is closely related to body adiposity, indicating that reducing fat mass could be beneficial in obesity-related hepatic steatosis(Reference Samuel, Liu and Qu12, Reference Oosterveer, Van Dijk and Tietge13). In the present study, the present results show that increased body weight gain and adipose fat mass in HFD-fed mice were significantly lowered by coumarin supplementation. Fat reduction is typically accomplished by decreasing food intake, but we found that food intake was similar among the experimental groups in the present study. Consistent with these results, the weight of epididymal fat was lower in mice that received coumarin supplementation compared with the HFD group. Leptin is an adipocyte-derived hormone that plays a key role in appetite regulation and energy expenditure, and leptin levels correlate strongly with body fat in HFD-fed mice(Reference Fried, Ricci and Russell21); therefore, serum leptin levels are used as a marker of body fat accumulation. We found that coumarin significantly reduced serum leptin levels that were increased by the HFD. These results suggest that coumarin supplementation can inhibit HFD-induced body weight gain and fat accumulation.
Recent studies have documented a close relationship between NAFLD and hyperlipidaemia(Reference Min, Kapoor and Fuchs22, Reference Chatrath, Vuppalanchi and Chalasani23), which results in lipid and NEFA accumulation in hepatocytes, causing liver damage. Although TAG levels were similar among the experimental groups in the present study, coumarin supplementation significantly reduced serum levels of TC, NEFA and apoB. These results are consistent with the findings of Hsu et al. (Reference Hsu, Wu and Huang10), who reported that 8 weeks of coumaric acid supplementation suppressed HFD-induced increases in serum TAG and TC in obese rats. Similarly, Yuce et al. (Reference Yuce, Danis and Ogan8) reported that serum TC was lowered by 7,8-dihydroxy-3-(4-methylphenyl) coumarin in rats. It is plausible that the normalisation of serum lipid levels in the CD group prevented the accumulation of hepatic lipids.
The liver plays a central role in lipid metabolism. Results of human and animal studies indicate that hepatic steatosis is associated with accumulation of the end products of de novo fatty acid synthesis(Reference Araya, Rodrigo and Videla24, Reference Li, Huang and Li25). In the present study, histological analysis showed that the HFD caused a significant increase in the number of lipid droplets in the liver, which was ameliorated by coumarin supplementation. We also found that coumarin significantly reduced total lipids, TAG and TC levels in the liver and decreased the activity of enzymes involved in hepatic fatty acid synthesis (i.e. FAS and ME). FAS catalyses the synthesis of long-chain SFA from acetyl-CoA and malonyl-CoA in the presence of NADPH(Reference Cho, Jung and Choi11), and ME is a lipogenic enzyme involved in supplying NADPH for fatty acid biosynthesis(Reference Mong, Chao and Yin26). Reducing hepatic FAS and ME activity limits the availability of the long-chain fatty acids required for TAG synthesis. Thus, our finding that coumarin reduced hepatic FAS and ME activity in HFD-fed mice probably accounts for the decreased hepatic lipid accumulation.
The present study showed that coumarin altered hepatic lipid metabolism and the expression of genes involved in these processes. SREBP-1c is a transcription factor that regulates genes related to lipid synthesis. Its deficiency can prevent hepatic steatosis in ob/ob mice, and its overexpression leads to the up-regulation of ATP citrate lyase, ACC, FAS and stearoyl-CoA desaturase-1(Reference Tessari, Coracina and Cosma27). We found that the HFD-induced up-regulation of SREBP-1c was markedly suppressed by coumarin, along with expression of its target genes, FAS and ACC1. These results suggest that coumarin reduced hepatic lipid accumulation by regulating SREBP-1c, thereby reducing the expression of fatty acid synthesis-related genes.
PPARγ and C/EBPα are involved in fat accumulation in hepatocytes. Specifically, PPARγ is a ligand-activated transcriptional factor required for the differentiation of pre-adipocytes into mature adipocytes. Yu et al. (Reference Yu, Matsusue and Kashireddy28) reported that liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver and steatosis. In addition, PPARγ expression in the liver is increased by HFD(Reference Inoue, Ohtake and Motomura29). C/EBPα also plays an important role in hepatic lipid metabolism. Matsusue et al. (Reference Matsusue, Gavrilova and Lambert30) found that C/EBP-knockout mice had significantly reduced TAG levels and down-regulated expression of lipogenic genes, despite obesity. Finally, PPARγ and C/EBPα are up-regulated and subsequently activate the lipogenic target genes (cluster of differentiation 36 (CD36), lipoprotein lipase and acyl-CoA synthetase, etc.) in the liver of obese mice, thus promoting hepatic steatosis(Reference Park, Choi and Um4). In the present study, we examined the effects of coumarin on PPARγ and C/EBPα protein levels in the liver. We found that HFD induced the up-regulation of PPARγ and C/EBPα in the liver, and courmarin clearly reduced those changes. Consistent with these results, hepatic lipid levels were also significantly decreased by coumarin. These data indicate that the protective effect of courmarin against hepatic steatosis can be mediated, at least in part, by PPARγ- and C/EBPα-mediated lipogenic signalling.
On the other hand, another study reported that a HFD increases serum NEFA concentrations, which inhibits insulin signalling(Reference Boden and Shumlaman31). Insulin activates the transcription of SREBP-1c and PPARγ, inducing lipogenesis(Reference Kahn, Hull and Utzschneider32). We showed that reduced serum insulin levels coincided with lower SREBP-1c and PPARγ expression in mice treated with coumarin. Adiponectin is an adipose-derived hormone, and it could confer protective effects against hepatic steatosis via reduction of SREBP-1c and activation of AMP-activated protein kinase and PPARα(Reference You and Rogers33). In addition, adiponectin enhances insulin sensitivity that results in the increase of glucose uptake and fatty acid oxidation in the liver(Reference Lihn, Pedersen and Richelsen34). Although we did not determine adiponectin in the present study, our findings suggest that the effect of courmarin was mediated, in part, by regulating insulin and adiponectin levels. As summarised in Fig. 5, coumarin represses the lipogenic pathway by down-regulating SREBP-1c, PPARγ and C/EBPα, which reduces the rate of hepatic fat accumulation.
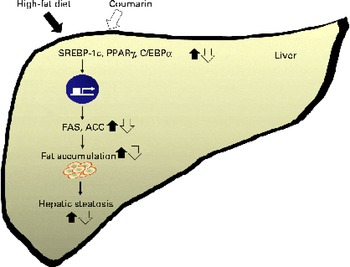
Fig. 5 Schematic diagram illustrating the effects of coumarin on high-fat diet-induced hepatic steatosis. Coumarin suppresses hepatic fat accumulation by regulating the expression of lipogenic genes. SREBP-1c, sterol regulatory element-binding protein-1c; C/EBPα, CCAAT/enhancer binding protein-α; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
In conclusion, we have demonstrated that coumarin protects against HFD-induced hepatic steatosis, which appears to be mediated through the regulation of genes involved in lipogenesis. These results suggest that coumarin may be useful in the alleviation of hepatic steatosis.
Acknowledgements
The present work was supported by the National Platform Technology Project from Ministry of Knowledge Economy and the Korea Food Research Institute. M. Y. U. performed the experimental work, acquisition of the data, analysis and wrote the original manuscript. M. K. M. and J. A. carried out the experiments. T. Y. H. designed the research and performed substantial editing. All authors reviewed the literature and contributed to the final report. The authors declare that there are no conflicts of interests.