Compliance with nutritional guidelines is an important factor to consider when investigating the health effects associated with the consumption of a healthy, well-balanced diet. To that effect, the reliability and accuracy of dietary habits assessment are critical in establishing compliance, but this remains an enduring challenge for dietitians and health professionals. Indeed, several dietary assessment methods have been developed and used to measure nutritional habits, including food diaries, FFQ and 24-h recalls( Reference Subar 1 ). The attractiveness of these assessment tools resides in the easiness and affordability related to their administration. However, as these tools are based on self-reported data, their value has been challenged by some due to the perceived risk of biased data collection attributable, amongst others, to under- and/or over-estimating the consumption of certain foods( Reference Hebert, Hurley and Steck 2 ).
In this regard, there is increasing interest in the use of nutritional biomarkers (measured in blood, urine or saliva), as they have been suggested to offer a more reliable and accurate assessment of dietary exposure and nutritional status( Reference Subar, Kipnis and Troiano 3 ). For instance, urinary N is recognised as an excellent recovery biomarker of protein intake( Reference Bihuniak, Simpson and Sullivan 4 ). Doubly labelled water is the gold standard technique for measurement of energy metabolism( Reference Ainslie, Reilly and Westerterp 5 ) and estimation of energy requirements( Reference Redman, Kraus and Bhapkar 6 ) but remains a technique that is costly and requires specific technical skills and facilities. On the other hand, circulating carotenoids are recognised as good correlates of dietary carotenoid intakes( Reference Baldrick, Woodside and Elborn 7 ). Furthermore, as fruits and vegetables (FAV) provide more than 90 % of the daily carotenoid intake( Reference Maiani, Caston and Catasta 8 ), measuring plasma carotenoid concentrations has been suggested as a more precise evaluation of an individual’s compliance with current nutritional recommendations, focusing on increased consumption of FAV for a better health than self-reported data( Reference Crowe, Roddam and Key 9 – Reference Hastert, Beresford and Patterson 12 ). In line with this observation, we recently reported that plasma lutein and β-cryptoxanthin are better biomarkers of the consumption of FAV compared with plasma α-carotene, β-carotene or lycopene concentrations that are more likely to be affected by the consumption of foods other than FAV( Reference Couillard, Lemieux and Vohl 13 ).
Besides dietary carotenoid intake, circulating carotenoid concentrations are also affected by age, physical activity, smoking and alcohol consumption( Reference Gruber, Chappell and Millen 14 – Reference Woodside, Young and Gilchrist 17 ). In addition, obesity( Reference Moran, Nolan and Stack 18 ) and low total LDL- and HDL-cholesterol concentrations( Reference Moran, Nolan and Stack 18 , Reference Parker 19 ) have been associated with lower circulating carotenoid concentrations. A sex difference is also noted in circulating carotenoid concentrations in women displaying higher concentrations compared with men( Reference Al-Delaimy, van Kappel and Ferrari 15 , Reference Woodside, Young and Gilchrist 17 – Reference George, Thompson and Midthune 20 ). Therefore, the aim of the present study was to identify the correlates of plasma carotenoid concentrations among men and women and investigate whether variations in physical and metabolic parameters as well as dietary carotenoid intake contribute to the higher plasma carotenoid concentrations in women compared with men. We hypothesise that the difference noted in plasma carotenoid concentrations between men and women is explained at least in part by differences in adiposity and plasma lipoprotein–lipid profile.
Methods
Subjects
For the present analyses, we used data from a group of 155 men and 110 women who participated in a series of dietary interventions we previously conducted. The specific selection criteria of participants enrolled in each intervention have been previously published( Reference Lacroix, Charest and Cyr 21 – Reference Labonté, Couture and Paquin 25 ). Briefly, men and women had to be healthy and not under treatment for hypertension, CVD, type 2 diabetes or other endocrine disorders. Smoking, alcohol consumption (>1 drink/d) and dietary supplement use were also considered reasons to exclude participants. Each participant gave their informed written consent to take part in the different interventions that were all approved by the Human Research Ethics Committee of Université Laval.
Dietary interventions
Data from a series of fully controlled isoenergetic dietary interventions conducted by our research group since 2006 were used for the present analyses. Complete detailed descriptions of Diet 1 (NCT00930137)( Reference Lacroix, Charest and Cyr 21 ), Diet 2 (NCT01351012)( Reference Jones, Senanayake and Pu 22 ), Diet 3 (NCT01293344)( Reference Bédard, Riverin and Dodin 23 ), Diets 4 and 5 (registered at ClinicalTrials.gov as NCT00988650)( Reference Richard, Couture and Desroches 24 ) and Diet 6 (NCT01163175)( Reference Labonté, Couture and Paquin 25 ) have been previously published( Reference Couillard, Lemieux and Vohl 13 ) and are provided as online Supplementary Table S1 in the present article. These dietary interventions covered a wide range of daily carotenoid intakes (from 13·6 to 51·3 mg/d) almost exclusively coming from mixed FAV consumption. In brief, Diets 1 (17·6 (sd 2·1) mg/d of carotenoids) and 6 (37·3 (sd 6·1) mg/d of carotenoids) were four-week control diets low in trans-fatty acids (TFA) of ruminant origin from studies investigating the metabolic impact of TFA from dairy; Diet 2 (25·5 (sd 4·8) mg/d of carotenoids) was part of a study looking at the metabolic impacts of different vegetable oils and consisted of a four-week, weight-maintaining diet of fixed macronutrient composition for protein (15 % of energy), carbohydrates (50 % of energy) and fats (35 % of energy), with a blend of maize and safflower oils (25:75), contributing to dietary fat intake (18 %); Diets 3 (28·6 (sd 5·1) mg/d of carotenoids) and 5 (31·0 (sd 4·3) mg/d of carotenoids) corresponded to five-week Mediterranean diets – that is, they contained key foods of the Mediterranean pyramid (e.g. olive oil, whole-grain products, FAV, legumes, nuts, cheese and yogurt, fish, poultry and red wine)( Reference Willett, Sacks and Trichopoulou 26 ). Diet 4 (mean intake: 29·4 (sd 4·3) mg/d of carotenoids) was a five-week control diet of a study assessing the impact of the Mediterranean diet and was designed to reflect average macro-nutrient intake in North American men( Reference Gray-Donald, Jacobs-Starkey and Johnson-Down 27 ).
In all cases, subjects arrived at our Clinical Investigation Unit (CIU) on weekdays to have their body weight measured and consume their lunch meal (approximately 35 % of daily energy intake) under staff supervision. All foods and beverages were prepared and packaged at the CIU, and participants were provided with their evening meals and the next day’s breakfast to take home, giving us the opportunity to control for energy, macro-nutrient and food intakes. Interventions were conducted under isoenergetic conditions to assure body weight maintenance, and a seven-d cyclic menu was used in all dietary interventions. Participants were instructed to consume only the foods provided, and compliance was found to be excellent (>95 % in all cases). Dietary interventions were formulated using the Nutrition Data System software (version 4.03_31; Nutrition Coordinating Center). Participants were instructed to maintain their usual physical activity concentration throughout the experiments except for the three d that preceded blood sampling at the end of the feeding period, during which they were asked to refrain from intense physical exercise. Dietary carotenoid intake was calculated using the 2010 Canadian Nutrient File (http://webprod3.hc-sc.gc.ca/cnf-fce/index-eng.jsp), which was also used to obtain the energy, macronutrient and micronutrient contents of the different diets.
Anthropometry
Body weight, height as well as waist circumference of each participant were measured using standardised procedures( Reference van der Kooy and Seidell 28 ). BMI was calculated by dividing the body weight (kg) by the height (m) squared.
Plasma measurements
Lipid profile
Post-intervention blood samples were collected from an antecubital vein into vacutainer tubes containing EDTA after a 12-h overnight fast, and assessment of the basic lipid profile was performed according to previously described procedures( Reference Goulet, Lamarche and Nadeau 29 ).
Carotenoids
Samples and standards used for the measurement of carotenoid concentrations were prepared as reported previously( Reference Couillard, Lemieux and Vohl 13 ). Briefly, aliquots of 100 µl of post-intervention fasting plasma samples maintained at –80°C were thawed a day before analyses. After being vortexed and centrifuged (3500 rpm, 10 min, 4°C), plasma samples were transferred to Eppendorf tubes (1·5 ml) along with 20 µl of 2-propanol and 20 µl of carotenoid standard. Samples were then transferred to a 400-µl fixed well plate (ISOLUTE® SLE+; Biotage) and mixed with 900 µl of hexane:isopropanol (90:10, v/v). After evaporation under N, the dried samples were reconstituted with 300 µl of methanol:dichloromethane (65:35, v/v), shaken for 10 min and finally transferred into HPLC glass vials for analysis.
HPLC
HPLC–UV analysis( Reference Gleize, Steib and Andre 30 ) of the samples was performed using an Agilent 1260 liquid handling system (Agilent) as described previously( Reference Couillard, Lemieux and Vohl 13 ). Carotenoids of the different samples were separated with a mobile phase consisting of methanol:water (98:2, v/v; eluent A) and methyl-tert-butyl ether (eluent B). Flow rate was set at 1 ml/min, and the gradient elution was as follows: 2 % eluent B (initial), 2·0–80 % eluent B (0·0–27·0 min), isocratic 80 % eluent B (27·0–31·0 min), 80·0–2·0 % eluent B (31·0–31·1 min) and isocratic 2 % eluent B (31·1–34·0 min). Identification of each carotenoid was confirmed with a UV detector (450 nm) and using retention time and UV spectra (190–640 nm) of the pure compounds. Data acquisition was carried out using Chemstation software (Agilent).
Statistical analyses
Data are presented as means and standard deviations unless stated otherwise. Spearman’s correlation coefficients were calculated to test for associations among variables. General linear multiple regression analysis was used to examine the effects of predictors of plasma carotenoid concentrations. The LSMEANS option was used with the PROC MIXED procedure to compare plasma carotenoid concentrations of men and women adjusted for intervention, body weight as well as plasma LDL- and HDL-cholesterol concentrations. All analyses were performed using the SAS statistical package (9.3; SAS Institute Inc.). Throughout the analyses, a P value ≤0·05 was considered as statistically significant.
Results
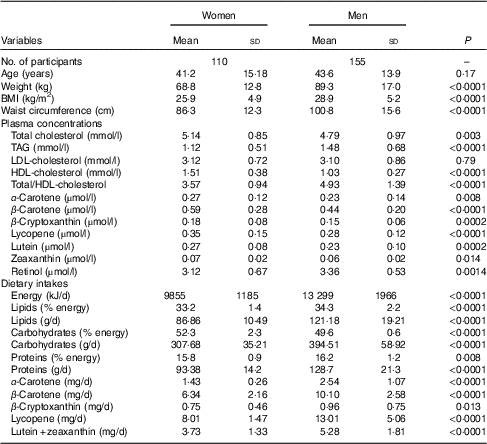
Table 1 presents physical characteristics of men and women who participated in the study. Men had a higher body weight, BMI and a larger waist circumference compared with women. Men were also characterised with higher plasma TAG and lower total and HDL-cholesterol concentrations compared with women, while no significant difference in plasma LDL-cholesterol concentrations (P=0·79) was noted between men and women. Energy as well as macronutrient intakes of the participants are also shown in Table 1. Daily energy intake was higher for men than for women (3443 (sd 1687) kJ/d; P<0·0001). Although dietary lipid, carbohydrate and protein intakes were higher in men compared with women, diets of both groups of participants were in line with recommended Canadian dietary guidelines( 31 ), that is the percentage of total energy content from lipids, carbohydrates and proteins were between 20–35, 45–65 and 10–35 %, respectively.
Table 1 Characteristics of study participants (Mean values and standard deviations)
As illustrated in Fig. 1, women had a lower total carotenoid dietary intake (–57 %, P<0·0001) as well as higher plasma total carotenoid concentrations (26 %, P<0·0001) compared with men. In addition, all individual carotenoid dietary intakes of women were lower than those of men (P<0·0001 for all with the exception of P<0·05 for β-cryptoxanthin, Table 1). In contrast, women showed higher plasma α-carotene (17 %, P<0·01), β-carotene (34 %, P<0·0001), β-cryptoxanthin (20 %, P<0·0005), lutein (17 %, P<0·0005), lycopene (25 %, P<0·0001) and zeaxanthin (17 %, P<0·05) concentrations compared with men (Table 1).
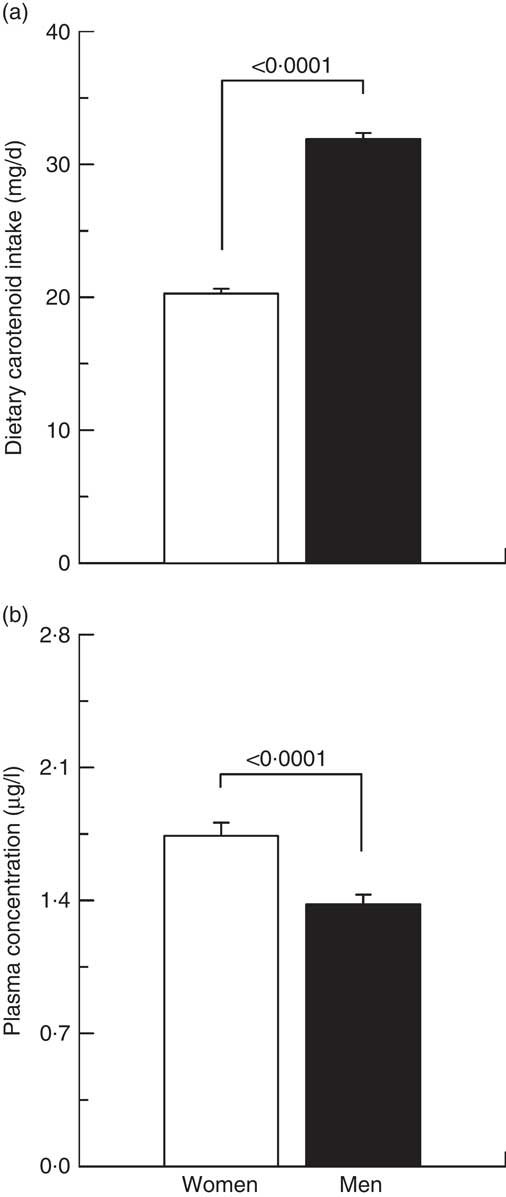
Fig. 1 Carotenoid (a) dietary intakes and (b) plasma concentrations in men (![]() ) and women (
) and women (![]() ).
).
Correlations of physical and metabolic characteristics with plasma carotenoid concentrations are shown in Table 2. We found that a higher body weight, BMI and waist circumference were associated with lower circulating total and individual carotenoid concentrations, with the exception of plasma lutein and zeaxanthin. On the other hand, elevated plasma total-, LDL- and HDL-cholesterol concentrations were positively correlated with plasma total as well as with individual carotenoids with the exception of the correlation between plasma zeaxanthin and HDL-cholesterol concentrations which only tended towards significance (P=0·0538; Table 2). The cholesterol:HDL-cholesterol ratio was negatively correlated with plasma total carotenoids as well as with α-carotene, β-carotene and lycopene. When men and women were examined separately (online Supplementary Table S2), similar patterns of associations were noted as in the entire group, with most correlations being stronger in men than in women. The positive correlations between individual plasma carotenoids and total- as well as LDL-cholesterol concentrations were all significant in both men and women, with the exception of the association between plasma LDL-cholesterol and lutein in women. On the other hand, plasma HDL-cholesterol was positively associated with all circulating carotenoids with the exception of lutein and zeaxanthin concentrations in men, while circulating HDL-cholesterol concentrations were positively correlated only with total carotenoids, α-carotene and lycopene concentrations in women (online Supplementary Table S2). Although total carotenoid dietary intake and plasma concentration were negatively associated, dietary intakes for β-cryptoxanthin, lutein and lycopene were positively correlated with their respective circulating concentrations in the entire cohort (Table 3). Positive correlations between dietary intakes and circulating concentrations for β-cryptoxanthin, lutein and lycopene were also noted in both men and women, while α-carotene and β-carotene dietary intakes and plasma concentrations were positively associated in men only (Table 3). There was no association between total carotenoid dietary intake and plasma concentration in men or in women. Also shown in Table 3 are the correlations between dietary and circulating carotenoids adjusted for HDL-cholesterol and body weight. We found that adjusting simultaneously for HDL-cholesterol and body weight strengthened the associations between dietary intakes and circulating concentrations for α-carotene, β-carotene, β-cryptoxanthin, lutein, lycopene and zeaxanthin but not for total carotenoids. Similar observations are made for β-cryptoxanthin, lutein and zeaxanthin when men and women are analysed separately (Table 3). On the other hand, adjusting correlations for body weight and HDL-cholesterol did not have a noticeable effect on the correlations for α-carotene, β-carotene and lycopene within men or women.
Table 2 Spearman correlations between physical and metabolic characteristics and plasma carotenoid concentrations in all participants
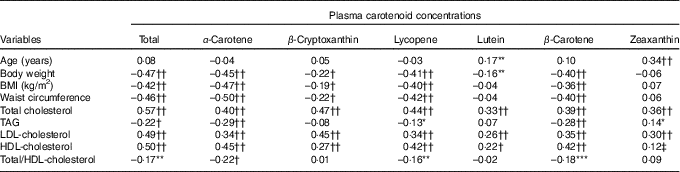
* P≤0·05; ** P≤0·01; *** P≤0·005.
† P≤0·0005; †† P≤0·0001.
‡ P=0·0538.
Table 3 Spearman correlations between carotenoid dietary intakes and plasma concentrations
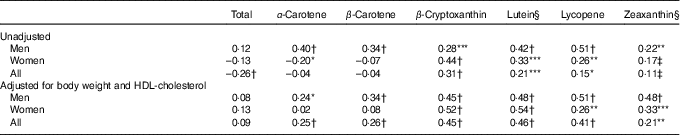
* P≤0·05; ** P≤0·01; *** P≤0·0005.
† P≤0·0001.
‡ P=0·07.
§ Dietary (lutein+zeaxanthin) intake was used for the analyses.
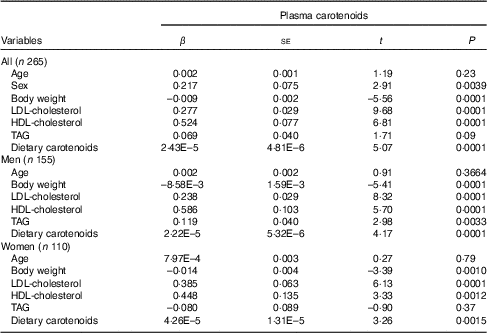
Multiple linear regression analysis (Table 4) revealed a significant portion of the variance in plasma total carotenoid concentration (adjusted R 2 0·547, P<0·0001). Age, female sex, LDL-cholesterol, HDL-cholesterol, TAG and dietary total carotenoid intake were positively associated with plasma total carotenoid concentrations. On the other hand, body weight was negatively associated with plasma total carotenoid concentrations. When men and women were analysed separately (Table 4), the same list of variables was found to be associated with circulating carotenoids in men (adjusted R 2 0·565, P<0·0001), whereas only LDL-cholesterol, HDL-cholesterol and dietary total carotenoid intake were positively associated and body weight negatively associated with plasma total carotenoid concentrations in women (adjusted R 2 0·440, P<0·0001).
Table 4 Multivariate linear regression analysis on predictors associated with plasma total carotenoids in men and women (β-Coefficients and standard errors)
Considering that physical and metabolic characteristics were found to be associated with circulating carotenoid concentrations, we investigated whether differences in body weight as well as in plasma LDL- or HDL-cholesterol concentrations between men and women could explain the sex difference observed in plasma carotenoid concentrations. As shown in Fig. 2, plasma total carotenoid concentrations remained higher in women compared with men after statistical adjustments for LDL-cholesterol (P<0·0001), whereas adjusting for body weight reduced the sex difference in circulating carotenoid concentrations (from 20 to 9 % in favour of women), but this difference remained statistically significant (P<0·05). On the other hand, after adjustment for plasma HDL-cholesterol concentrations, circulating total carotenoid concentrations were no longer different (P=0·66, Fig. 2), despite significantly lower dietary total carotenoid intake in women than in men (–36 %, P<0·0001). After the simultaneous statistical adjustment for plasma HDL-cholesterol concentrations and body weight, the differences in plasma β-carotene, β-cryptoxanthin, lutein, lycopene and zeaxanthin concentrations, but not α-carotene, were no longer significant. Similar results were obtained when statistical adjustments of specific plasma carotenoid concentrations were performed, that is, the adjustment for LDL-cholesterol had no impact on the sex difference in plasma α-carotene, β-carotene, β-cryptoxanthin, lutein, lycopene and zeaxanthin concentrations, with women still showing higher values after the adjustment when compared with men (online Supplementary Table S3). When adjustment for body weight was performed, the differences in plasma α-carotene, lycopene and zeaxanthin concentrations were no longer significant, while those of β-carotene, β-cryptoxanthin and lutein concentrations were still higher in women. Finally, β-carotene, β-cryptoxanthin, lutein, lycopene and zeaxanthin concentrations were no longer statistically different between men and women, but plasma α-carotene concentration was higher in men after adjustment for plasma HDL-cholesterol concentrations either alone or with body weight. Furthermore, statistical adjustment for the intervention each participant was enrolled in had no effect on the sex difference in circulating total carotenoids as well as in α-carotene, β-carotene, β-cryptoxanthin and lycopene concentrations that remained higher in women compared with men following the adjustment procedure (data not shown). On the other hand, plasma lutein and zeaxanthin concentrations were no longer significantly different between men and women after statistical adjustment for the intervention they were enrolled in.
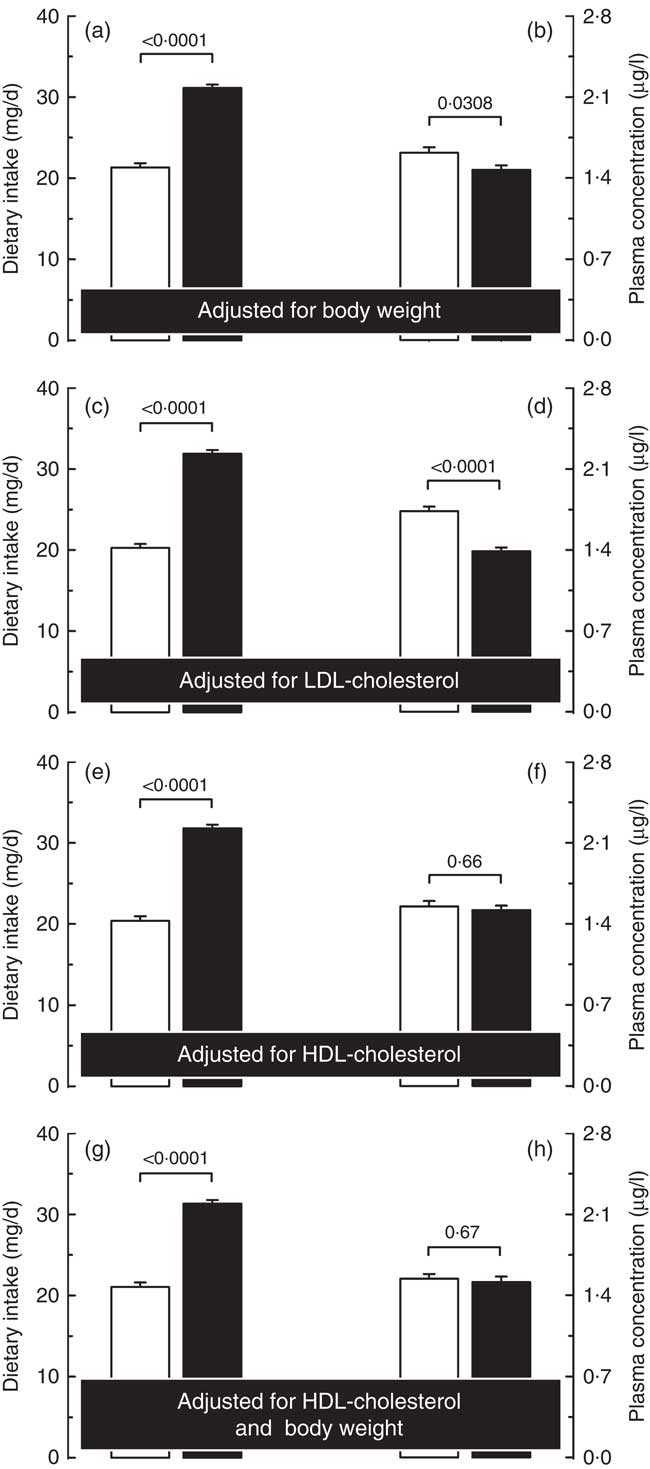
Fig. 2 Carotenoid dietary intakes (a, c, e and g) and plasma concentrations (b, d, f and h) in men (
![]() ) and women (
) and women (
![]() ) adjusted for body weight (a and b), plasma LDL-cholesterol (c and d) and HDL-cholesterol concentrations (e and f) as well as for HDL-cholesterol and body weight simultaneously (g and h).
) adjusted for body weight (a and b), plasma LDL-cholesterol (c and d) and HDL-cholesterol concentrations (e and f) as well as for HDL-cholesterol and body weight simultaneously (g and h).
Discussion
Results of the present study indicate that variations in circulating HDL-cholesterol concentrations are a significant correlate of the difference in plasma carotenoid concentrations observed between men and women. These results are in line with previous observations that identified lipoprotein cholesterol concentrations as determinants of circulating carotenoids( Reference Moran, Nolan and Stack 18 , Reference Goulinet and Chapman 32 , Reference Vioque, Weinbrenner and Asensio 33 ), and the present study extends these contributions to the sex difference in blood carotenoids.
Due to their hydrophobic nature, lipids are transported in the circulation by lipoproteins, namely, chylomicrons, VLDL, LDL and HDL( Reference Parker 19 ). Carotenoids are also hydrophobic molecules that are transported in varying proportions by lipoproteins. For instance, α-carotene, β-carotene and lycopene are present in the hydrophobic core of VLDL (10–16 %), LDL (58–73 %) and HDL (17–26 %)( Reference Parker 19 ). On the other hand, xanthophylls, which are more polar carotenoids, are also present on the surface of lipoproteins and distributed in VLDL (16 %), LDL (31 %) and HDL (53 %) for lutein and zeaxanthin, while β-cryptoxanthin is distributed in VLDL (16 %) and equally in LDL (40 %) and HDL (40 %)( Reference Parker 19 ). In line with these observations, and as previously reported( Reference Wang, Gaziano and Norkus 34 ), we found that plasma total carotenoid concentrations were positively correlated with circulating LDL- and HDL-cholesterol concentrations in men and women. On the other hand, there are known sex differences in lipid and lipoprotein metabolism, with women usually showing lower LDL-cholesterol and higher HDL-cholesterol concentrations compared with men( Reference Wang, Magkos and Mittendorfer 35 ). Although plasma LDL-cholesterol concentrations were similar between men and women of the present study, the latter had significantly higher circulating HDL-cholesterol concentrations suggesting a higher carotenoid transport capacity that may contribute to the higher circulating carotenoid concentrations in women compared with men. Our results support such a contribution, as the difference in plasma total carotenoid concentrations was eliminated when the latter was adjusted for plasma HDL-cholesterol concentrations. Interestingly, this lack of difference in plasma carotenoids was noted even if men had a significantly higher dietary carotenoid intake compared with women, suggesting lower incorporation of dietary carotenoids in HDL in men. A possible explanation for this observation could be related to differences in the physical characteristics of lipoprotein particles such as LDL and HDL between men and women. Indeed, besides differences in circulating concentrations, men have also been reported to have smaller, denser LDL and HDL particles compared with women( Reference Wang, Magkos and Mittendorfer 35 – Reference Magkos, Mohammed and Mittendorfer 38 ). In addition, small, dense LDL and HDL particles have been characterised with diminished carotenoid content( Reference Goulinet and Chapman 32 ). Thus, in the present study, although women have a lower dietary carotenoid intake than men, they may incorporate a greater proportion of their carotenoid intake into larger LDL and HDL particles, yielding higher plasma concentrations. Unfortunately, lipoprotein particle size and density measurements were not available in study participants, but we found that men had higher TAG and cholesterol:HDL-cholesterol ratio compared with women, which are, respectively, good correlates of the presence of small, dense LDL( Reference Lemieux, Pascot and Lamarche 36 , Reference St-Pierre, Cantin and Dagenais 39 ) and HDL( Reference Pascot, Lemieux and Bergeron 37 ) particles. Quantification of the carotenoid content of plasma LDL and HDL particles of study participants would also have been interesting to analyse.
Obesity is also associated, among others, with lower circulating carotenoid concentration( Reference Gruber, Chappell and Millen 14 ). Because of their lipophilic character, a greater uptake of carotenoids by adipose tissue may contribute to lowering plasma values in obese individuals( Reference Parker 19 ). The negative impact of body fat on circulating carotenoids has been recently underlined in women, as low serum α-carotene concentrations were found to be negatively correlated with BMI and body fat in women even after adjustment for age and dietary intake( Reference Nuss, Valentine and Zhang 40 ). Our results also support the negative influence of obesity and abdominal adipose tissue accumulation with regard to circulating carotenoids, as the sex difference in plasma carotenoid concentrations was reduced when values were adjusted for body weight or waist circumference (data not shown for the latter). When specific carotenoids were examined, we found that the sex difference in α-carotene, lycopene and zeaxanthin was eliminated by statistical adjustment for body weight (online Supplementary Table S3). Carotenoids are potent antioxidants( Reference Fiedor and Burda 41 ), and the higher oxidative stress in men( Reference Kaya, Uzunhasan and Baskurt 42 ) and in obese individuals( Reference Couillard, Ruel and Archer 43 , Reference Keaney, Larson and Vasan 44 ) could be responsible for the apparent lower ratio of circulating concentrations to dietary intake of carotenoids in men compared with women. However, we did not characterise the oxidative stress profile of our study participants and therefore could not test this hypothesis.
Dietary variables have also been shown to influence circulating carotenoid concentrations. First and foremost, dietary carotenoid intake is a significant contributor to circulating carotenoid. The significant correlations between carotenoid dietary intakes and circulating concentrations we report herein are supportive of this contribution. In the present study, men have a higher dietary carotenoid intake than women, an observation somewhat contradictory with their lower plasma carotenoid concentrations compared with women. However, as recently reviewed( Reference Burrows, Williams and Rollo 45 ), the correlations between both measures have been reported to be weak to moderate. In fact, β-cryptoxanthin shows the strongest associations between dietary intake and plasma concentration( Reference Burrows, Williams and Rollo 45 ), whereas such a significant correlation is not as strong and even absent with β-carotene( Reference Forman, Lanza and Yong 46 , Reference Hickenbottom, Follett and Lin 47 ). Furthermore, there is evidence that some individuals can be characterised as responders and non- or low responders to β-carotene intake( Reference Bowen, Garg and Stacewicz-Sapuntzakis 48 – Reference Johnson, Suter and Sahyoun 51 ), that is, the changes in circulating β-carotene concentration following the consumption of β-carotene are variable. Whether such processes can contribute to the difference in plasma carotenoids noted between men and women could not be investigated in the context of our study. In addition, fat intake has been shown to increase the bioavailability of carotenoids by creating a favourable environment for the absorption and transportation of carotenoids through the formation of micelles( Reference Parker 19 , Reference Reboul 52 ). However, a major influence of fat intake on carotenoid absorption in the present study seems unlikely, as men who had a significantly higher fat intake compared with women were also those showing lower plasma carotenoid concentrations. In contrast, dietary fibre intake has been shown to decrease the bioavailability of dietary carotenoids by limiting their absorption through a decrease in the formation of micelles( Reference Yeum and Russell 53 ) and by limiting the contact of circulating micelles with the intestinal wall( Reference van Het Hof, West and Weststrate 54 ). Again, results of the present study do not support a significant role of dietary fibre intake in the sex difference in plasma carotenoids, as women had higher fibre intake compared with men but were also those displaying higher plasma carotenoid concentrations.
Several proteins are implicated in dietary carotenoid absorption, including the scavenger receptor class B type 1, cluster determinant 36, Niemann–Pick C1-like 1 protein and ATP-binding cassette A1( Reference Reboul 52 ). Variations in the activity or expression of these proteins could therefore contribute to the inter-individual variability in carotenoid bioavailability. Accordingly, variants of genes involved in carotenoid absorption have been related to the bioavailability of β-carotene( Reference Borel, Desmarchelier and Nowicki 55 ), lutein( Reference Borel, Desmarchelier and Nowicki 56 ) and lycopene( Reference Borel, Desmarchelier and Nowicki 57 ). Our study did not explore the contribution of proteins (or their genetic variants) involved in absorption and transport of dietary carotenoids in the difference in plasma carotenoid concentrations. Future research should explore the relevance of genetic polymorphisms in the explanation of the sex-related variability in dietary carotenoid bioavailability.
The strengths of the present study include the analysis of data from a series of fully controlled nutritional intervention studies in which all foods were provided to the participants. This allowed a more precise assessment of dietary intakes than the usual self-reported data from dietary questionnaires. In addition, there was a large variability in carotenoid intakes (13·6–51·3 mg/d) between participants, allowing for greater representation of intakes. Our study also has limitations that must be acknowledged. First, we compiled data from intervention studies that were not primarily designed to study carotenoid bioavailability. For instance, although there were differences in the carotenoid content of the different diets, the intakes of different carotenoids were significantly higher than the median carotenoid intakes for women and men, that is, 5·47 and 6·88 mg/d, respectively( 58 ). Extending the dietary carotenoid range to lower intakes may have allowed us to investigate whether a potential plateau of absorption of dietary carotenoid contributed to the difference noted in plasma carotenoid concentrations between men and women or whether the latter is also present at lower dietary carotenoid intakes. Second, there are also variations in the proportion of individual carotenoids consumed within each intervention which may affect total carotenoids in circulation. Furthermore, the carotenoid contents of the fully controlled diets were calculated using the 2010 Canadian Nutrient File (http://webprod3.hc-sc.gc.ca/cnf-fce/index-eng.jsp), although direct measures of the carotenoid content of the different diets/meals consumed would have given an even more precise measure of the carotenoid intake of the participants. However, such measurements would have been extremely costly and time-consuming. Finally, our study does not take into account genetic characteristics of subjects or even seasonal variations in the carotenoid content of foods consumed, which are also factors that likely affect carotenoid metabolism in humans. The potential contributions of these factors in our results merit further investigations.
In conclusion, the present study demonstrates that HDL-cholesterol and to a lesser extent body weight are correlates of the difference in plasma carotenoid concentrations observed between men and women. Our results suggest that using crude circulating carotenoid concentrations without consideration for sex differences in plasma lipoprotein concentrations or adiposity could lead to under-/over-estimation of carotenoid intake in men and women. A better understanding of the factors affecting plasma carotenoid concentrations will lead to a more accurate use of nutritional biomarkers for the assessment of food intakes. In this regard, our results suggest that physical and metabolic characteristics of an individual should be taken into account when using plasma carotenoids to validate dietary questionnaires, including those measuring FAV intakes and therefore avoid mistakenly concluding to under- or over-reporting of dietary carotenoid and/or FAV intakes in men and women.
Acknowledgements
The authors would like to acknowledge the contribution of Pascal Dubé of the Institute of Nutrition and Functional Foods for the measurements of plasma carotenoid concentrations. The authors would also like to acknowledge the contributions of nurses and other research professionals as well as subjects who participated in the dietary interventions without whom, no clinical research would be possible.
Studies were made possible by grants from the Canadian Institutes of Health Research (MOP-68866, MOP-84568 and FHG-129921), the Natural Sciences and Engineering Research Council of Canada, the Dairy Farmers of Canada, the Canadian Dairy Commission, Novalait Inc., Dairy Australia, Agriculture and Agri-Food Canada, the Canola Council of Canada, the Flax Council of Canada, Dow Agrosciences, the Western Grains Research Foundation of Canada, the National Center for Research Resources (UL1 RR033184) and the National Center for Advancing Translational Sciences (UL1 TR000127). Funders of the studies had no role in the design, analysis or writing of the present article.
C. C. has received research funding from the Canadian Cranberry Growers Coalition and McCormick Science Institute and received honoraria or travel expense reimbursements from the Berry Health Benefits Symposium, Ocean Spray Cranberries Inc. and Mott’s Canada. C. C. and B. L. have received research funding from Atrium Innovations. B. L. has received research funding from the Danone Institute and honoraria from Unilever, Danone and the Dairy Farmers of Canada. B. L. is Chair in Nutrition and Cardiovascular Health, supported in part by Provigo/Loblaws. B. L. and P. C. have received research grants from the Dairy Farmers of Canada and Dairy Australia. The funders were not involved in the design, conduct, management, data collection and analysis, or preparation and review of the manuscript.
The authors’ responsibilities are as follows: T. A., C. C., S. L., M.-C. V., P. C. and B. L.: research design. P. C.: medical supervision of study participants. C. C., S. L., M.-C. V., P. C. and B. L.: data acquisition. T. A.: analysis and interpretation of the data and drafting of the manuscript. C. C., M.-C. V., P. C., S. L. and B. L.: critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
The authors were not aware of any conflicts of interest related to this article.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003045









