Binge eating (BE) is a behaviour characterised by eating in a discrete period of time an amount of food that is definitely larger than what most people would eat in a similar period of time under similar circumstances, accompanied by a sense of lack of control over eating. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition( 1 ), when such behaviour occurs at least once weekly for 3 months without compensatory behaviours, it is defined as binge eating disorder (BED). BE is also common in other eating disorders such as bulimia nervosa (BN), some subtypes of anorexia nervosa (AN) and eating disorders not otherwise specified (EDNOS)( 1 ). It is particularly prevalent in subjects seeking a weight loss or maintenance programme, among whom BE prevalence is 17 %, with increased prevalence in women as well as in younger and in obese subjects( Reference Bertoli, Leone and Ponissi 2 ). Beyond the higher risk of developing obesity, binge eaters have a worse dietary pattern( Reference Bertoli, Spadafranca and Bes-Rastrollo 3 ), a higher smoking status( Reference Solmi, Veronese and Sergi 4 ) and lower physical activity levels than subjects free of BE( Reference Bertoli, Leone and Ponissi 2 ).
Obesity and lifestyle are crucial factors associated with cardiometabolic diseases( Reference Sperling, Mechanick and Neeland 5 , Reference Gomez-Huelgas, Jansen-Chaparro and Baca-Osorio 6 ), whereas the contribution of BE behaviour is still a debated topic( Reference Mitchell 7 ). A 5-year longitudinal study of 134 individuals with BED compared with age- and sex-matched controls (the authors tried to match even for BMI, but failed in their purpose) showed an increased risk for dyslipidemia in subjects with BED, after adjusting for sex, age, baseline BMI and BMI changes. The authors concluded that BED might confer a risk of components of the metabolic syndrome (MS) over and above the risk attributable to obesity alone( Reference Hudson, Lalonde and Coit 8 ). Subsequently, a cross-sectional study carried out on 3551 participants of the Framingham Heart Study reported that subjects with objective BE, but not subjects with subclinical BE, had a higher risk of impaired fasting glucose compared with non-binge eaters, after adjusting for sex, age, BMI, smoking status, alcohol intake, education and depressive symptoms( Reference Abraham, Massaro and Hoffmann 9 ). A further cross-sectional study carried out on 2225 patients waiting for bariatric surgery reported a positive association between BED status and impaired fasting glucose, after controlling for age, sex, education, BMI and psychiatric disorders( Reference Mitchell, King and Pories 10 ). More recently, a 16-year longitudinal study observed a higher incidence of type 2 diabetes mellitus in subjects with BED and BN, but not AN, compared with age- and sex-matched controls( Reference Raevuori, Suokas and Haukka 11 ). All these evidences suggest that BE may increase the likelihood of developing components of MS( Reference Mitchell 7 ). Nevertheless, whether this increased risk is attributable to an independent contribution of BE or to a higher presence of obesity and unhealthy lifestyle is unclear( Reference Mitchell 7 ). Previous studies, indeed, have not taken, or only partially taken, into account the effects of nutritional status and lifestyle on cardiometabolic risk factors. Therefore, the relative contribution of BE to cardiometabolic risk is presently unknown.
In this context, the aim of this study was to evaluate the contribution of BE to cardiometabolic risk in a large sample of subjects starting a weight loss or maintenance programme, taking into account the potential effects of nutritional status, dietary habits, smoking status and physical activity.
Methods
Study design
We performed a cross-sectional study on 5466 consecutive adults who self-referred to the International Center for the Assessment of Nutritional Status (University of Milan) from September 2010 to January 2015, in order to participate in a structured weight loss or weight maintenance programme. On the same day, they underwent a clinical examination, an anthropometric assessment and a structured interview by a trained dietitian, in order to obtain information about marital status, educational level, smoking status and structured physical activity. Physical activity was investigated by asking the subjects the following questions: ‘Do you practice any structured physical activity?’ and ‘How many hours per week do you spend on this activity?’. Subjects who spent ≥2 h/week performing any structured physical activity were considered as active( Reference Bertoli, Leone and Ponissi 2 ). Moreover, all the patients filled in two questionnaires to evaluate adherence to the Mediterranean diet( Reference Martinez-Gonzalez, Fernandez-Jarne and Serrano-Martinez 12 , Reference Schröder, Fito and Estruch 13 ) and the presence of BE( Reference Gormally, Black and Daston 14 , Reference Di Bernardo, Barciulli and Ricca 15 ). From the initial number of subjects recruited for the study, thirty subjects were excluded because they were diagnosed as having acute infective, neurological, gastrointestinal, cardiac, renal and pulmonary disorders or were unable to understand and fill in the questionnaires. The present study was carried out according to the Declaration of Helsinki, and all participants gave their written informed consent to participate. The institutional review board approved the study procedures.
Anthropometric measurements
Anthropometric measurements were obtained according to conventional criteria and measuring procedures proposed by Lohmann et al.( Reference Lohmann, Roche and Martorell 16 ). Body weight (BW, kg) was measured using Column scale (Seca 700 balance; Seca Corporation) up to 100 g with subjects wearing only light underwear and after bladder emptying. Body height (BH, cm) was measured to the nearest 0·1 cm using a vertical stadiometer. BMI was calculated using the following formula: BMI (kg/m2)=BW (kg)/BH2 (m2). BMI was classified into four categories: underweight (BMI<18·5 kg/m2), normal weight (BMI 18·5–24·9 kg/m2), overweight (BMI 25·0–29·9 kg/m2) and obese (BMI>30·0 kg/m2). Waist circumference was measured midway between the lower rib margin and the superior anterior iliac spine to the nearest 0·5 cm with a non-stretch tape applied horizontally.
Laboratory measurements
Fasting total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG and glucose were measured using enzymatic methods (Cobas Integra 400 Plus; Roche Diagnostics)( Reference Spadafranca, Cappelletti and Leone 17 ). Blood pressure was measured by a physician using a random-zero mercury sphygmomanometer following the Joint National Committee 7 guidelines( Reference Chobanian, Bakris and Black 18 ).
The metabolic syndrome and clinical outcomes
The MS was diagnosed using the harmonised international definition( Reference Alberti, Eckel and Grundy 19 ). Large waist was defined as waist circumference ≥102 cm in men and ≥88 cm in women, low HDL-cholesterol as values <40 mg/dl in men and <50 mg/dl in women, high TAG as values ≥150 mg/dl or treatment with TAG-lowering drugs, high blood pressure as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or treatment with pressure-lowering drugs, and high glucose as values ≥100 mg/dl or treatment with glucose-lowering drugs. The MS was defined as three or more of the above components. In addition, high total cholesterol was defined as total cholesterol ≥200 mg/dl and high LDL as values ≥130 mg/dl.
Mediterranean dietary pattern
Adherence to the Mediterranean diet was evaluated using a validated fourteen-item questionnaire( Reference Schröder, Fito and Estruch 13 ), which is the extension of an original nine-item questionnaire( Reference Martinez-Gonzalez, Fernandez-Jarne and Serrano-Martinez 12 ). A fourteen-item Mediterranean score (Medscore) was obtained from this questionnaire following the guidelines of the PREvención con DIeta MEDiterránea (PREDIMED) study (www.predimed.es) and a specific validation study by Schröder et al.( Reference Schröder, Fito and Estruch 13 ) with some adaptations already used in previous studies( Reference Bertoli, Spadafranca and Bes-Rastrollo 3 , Reference Babio, Bulló and Basora 20 – Reference Soldati, Bertoli and Terranegra 22 ). In brief, 1 point was attributed for each of the following: (1) olive oil as the main cooking fat, (2) olive oil ≥4 tablespoons/d, (3) vegetables ≥2 servings/d (≥1 portion raw or on salad), (4) fruits ≥3 servings/d, (5) red or processed meat <1 serving/d, (6) butter or cream or margarine <1/d, (7) soda drinks <1/d, (8) wine ≥3 glasses/week, (9) legumes ≥3 servings/week, (10) fish/seafood ≥3 servings/week, (11) commercial sweets and confectionery <3/week, (12) nuts ≥1/week, (13) white more than red meats (yes) and (14) use of sofrito sauce ≥2/week. Subjects with a MEDscore ≥9 points were considered to have a high adherence to the Mediterranean diet( Reference Bertoli, Spadafranca and Bes-Rastrollo 3 , Reference Bertoli, Leone and Vignati 21 , Reference Soldati, Bertoli and Terranegra 22 ).
Psychological assessment
Eating behaviour was evaluated using the Italian version of the Binge Eating Scale (BES)( Reference Gormally, Black and Daston 14 , Reference Di Bernardo, Barciulli and Ricca 15 ). BES consists of sixteen forced-choice questions, each with a set of three to four answer choices. BES gives a score ranging from 0 to 46. BE was defined as a BES score ≥18( Reference Marcus, Wing and Hopkins 23 ). The questionnaire was considered invalid when more than 10 % of items was missing. Following such criteria, further 261 subjects were excluded.
Statistical analysis
Statistical analyses were performed on a final number of 5175 subjects. Most continuous variables had non-Gaussian distributions, and all are reported as 25th, 50th and 75th percentiles. Discrete variables are reported as counts and frequencies.
A Poisson working regression model (PWRM) with robust 95 % CI was used to estimate prevalence and its predictors( Reference Lumley, Kronmal and Ma 24 , Reference Barros and Hirakata 25 ). A PWRM was used because a binomial regression model failed to converge for some of the regressions of interest. Expectedly, the estimates made by the binomial regression model and by the PWRM were similar in all cases where both converged. Sex- and age-adjusted and multiple-adjusted PWRM were used to evaluate the associations of MS, its components, high cholesterol and high LDL with BES. The response variable of all PWRM (MS, MS components, high cholesterol, low LDL) was discrete (0=no; 1=yes). Besides BES (discrete, 0=non-binge eater; 1=binge eater, or continuous, score units), predictors of the multivariable PWRM were the following: (1) sex (discrete, 0=female; 1=male), (2) age (continuous, years/10), (3) BMI (continuous, kg/m2), (4) Medscore (continuous, score units), (5) smoking status (discrete, 0=no; 1=ex; 2=yes) and (6) physical activity (discrete, 0=no; 1=yes). Adjusted probabilities of the responses of interest were calculated from the PWRM( Reference Williams 26 ).
We also evaluated the association between the continuous MS components (TAG, HDL, glucose, systolic blood pressure and diastolic blood pressure), total cholesterol and LDL-cholesterol and the BES score using a multivariable median regression model (MRM) using the same covariates of the PWRM – that is, sex, age, BMI, Medscore, smoking status and physical activity. Adjusted estimates of the responses of interest were calculated from the MRM( Reference Williams 26 ).
Degree 2 multivariable fractional polynomials (MFP) were used to test whether the relationships of continuous predictors with the responses were non-linear( Reference Royston and Sauerbrei 27 ). MFP selected an inverse square root transformation of BMI for all PWRM models and no transformation, a log e transformation, an inverse transformation or an inverse square-root transformation for age depending on the model. There was only a modest gain in the linearity of continuous predictors when MFP were applied to the MRM; therefore, all continuous covariates were kept untransformed for the MRM analysis. Adjusted estimates of the responses of interest were calculated from the MRM( Reference Williams 26 ).
Statistical analyses were performed using Stata 14.2 (Stata Corporation LP).
Results
The continuous measurements of the 5175 study subjects are given in Table 1. Women made up 72 % of the study population.
Table 1 Measurements of the study subjects

P50, 50th percentile; P25, 25th percentile; P75, 75th percentile; BES, Binge Eating Scale score; Medscore, Mediterranean diet score.
The distribution of age, BMI status, BE, MS and lifestyle factors is given in Table 2.
Table 2 Distribution of age, BMI status, Binge Eating Scale (BES), lifestyle factors and the metabolic syndrome (MS) (Numbers and percentages)
The prevalence of BE in the pooled sample was 0·16 (95 % CI 0·15, 0·17) and was higher in women (0·20; 95 % CI 0·19, 0·21) than in men (0·07; 95 % CI 0·06, 0·08, P<0·001, PWRM).
The prevalence and prevalence rate ratios (PRR) of MS, its components, high total cholesterol and high LDL-cholesterol are given in Table 3.
Table 3 Prevalence and prevalence rate ratios (PRR) of the metabolic syndrome (MS), MS components, high cholesterol and high LDL in binge eaters and non-binge eaters† (Prevalence, PRR and robust 95 % confidence intervals obtained from Poisson working regression model using BES score as discrete variables (BES<18: non-binge eaters, BES≥18: binge eaters)

BES, Binge Eating Scale; Medscore, Mediterranean score.
** P<0·01.
† The multiple-adjusted model included sex, age, BMI, Medscore, smoking status and physical activity as covariates.
The prevalence of MS in the pooled sample was 0·28 (95 % CI 0·26, 0·29). This prevalence was higher in binge eaters (0·33; 95 % CI 0·28, 0·37) compared with non-binge eaters (0·27; 95 % CI 0·25, 0·28) in the sex- and age-adjusted model (P=0·011, PWRM). However, the statistical difference was lost after inclusion of nutritional status and lifestyle factors in the multiple-adjusted model. The prevalence of high blood pressure was marginally higher in binge eaters (0·52; 95 % CI 0·47, 0·57) compared with non-binge eaters (0·47; 95 % CI 0·45, 0·49) in the sex- and age-adjusted model (P=0·076, PWRM). However, the statistical difference was lost after inclusion of nutritional status and lifestyle factors in the multiple-adjusted model. No differences in the prevalence of high TAG, low HDL, high glucose, high total cholesterol and high LDL were observed between binge eaters and non-binge eaters in both models.
Table 4 reports the PRR of MS, its components, high total cholesterol and high LDL associated with an increase of 1 unit in the BES score in the sex- and age-adjusted model and in the multiple-adjusted model (sex, age, BMI, Medscore, smoking status and physical activity).
Table 4 Prevalence rate ratios (PRR) of the metabolic syndrome (MS), MS components, high cholesterol and high LDL associated with an increase of 1 unit in Binge Eating Scale (BES) before and after correction for confounders (sex, age, BMI, Medscore, smoking status and physical activity)Footnote † (PRR and robust 95 % confidence intervals obtained from Poisson working regression model using the BES score as continuous variable)

Medscore, Mediterranean score.
** P<0·01, *** P<0·001.
† The multiple-adjusted model included sex, age, BMI, Medscore, smoking status and physical activity as covariates.
We observed an increased risk in MS, high blood pressure and high glucose associated with an increase of 1 unit in the BES score in the sex- and age-adjusted model. However, such increments were no more significant in the multiple-adjusted model. Only a weak inverse association was observed between the BES score and the risk of low HDL.
Fig. 1 plots the prevalence of MS, its components, high cholesterol and high LDL as a function of continuous BES, taking into account the effects of sex, age, BMI, Medscore, smoking status and physical activity (PWRM, see the ‘Statistical analysis’ section for details). The values graphed on the x-axis represent the 5th, 25th, 50th, 75th and 95th percentiles of the BES score.

Fig. 1 Prevalence of the metabolic syndrome (MS), components of the MS, high cholesterol and high LDL as a function of continuous Binge Eating Scale (BES). Values are adjusted probabilities estimated from the Poisson working regression model described under the ‘Statistical analysis’ section. The values graphed on the x-axis represent the 5th, 25th, 50th, 75th and 95th percentiles of BES. Only the relationship with low HDL was statistically significant (P=0·007). BP, blood pressure; GLU, glucose; CH, total cholesterol.
Fig. 2 plots the median values of TAG, total cholesterol, HDL, LDL, glucose, systolic blood pressure and diastolic blood pressure as a function of continuous BES, taking into account the effects of sex, age, BMI, Medscore, smoking status and physical activity (MRM, see the ‘Statistical analysis’ section for details). The values graphed on the x-axis represent the 5th, 25th, 50th, 75th and 95th percentiles of the BES score. The association with BES was linear in all cases and was statistically significant only for HDL, which was higher in patients with BE (P<0·001).
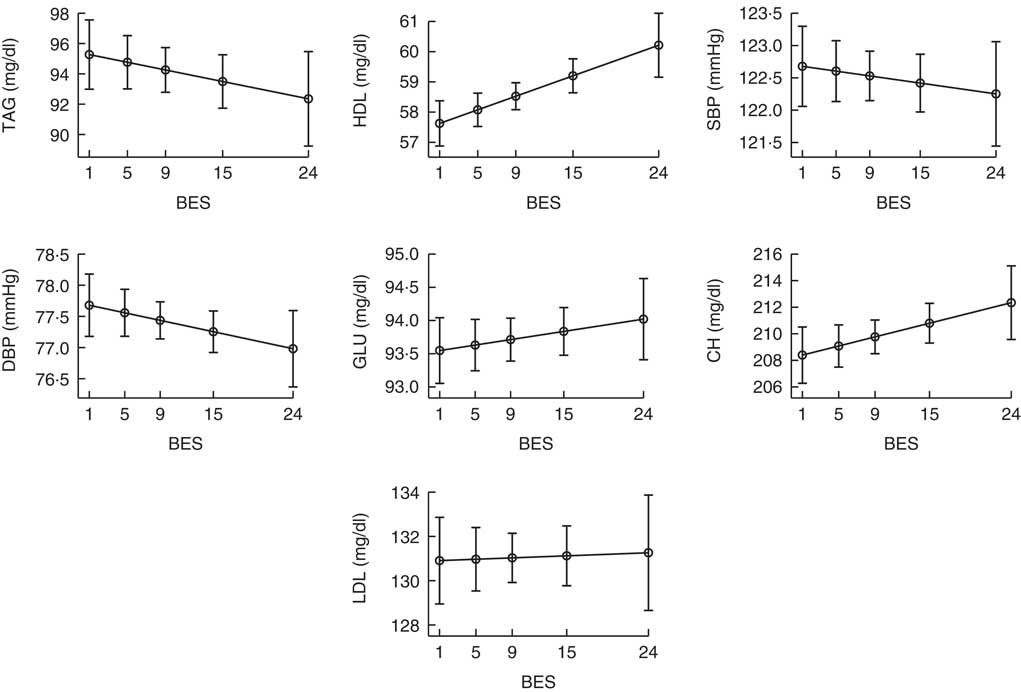
Fig. 2 Values of laboratory measurements as a function of continuous Binge Eating Scale (BES). Values are adjusted probabilities estimated from the median regression model described under the ‘Statistical analysis’ section. The values graphed on the x-axis represent the 5th, 25th, 50th, 75th and 95th percentiles of BES. Only the relationship with HDL level was statistically significant (P<0·001). MS, metabolic syndrome; SBP, systolic blood pressure; DBP, diastolic blood pressure; GLU, glucose; CH, total cholesterol.
Discussion
This is the first study to explore, in a large sample, the association between BE and cardiometabolic risk factors, taking into account the effects of nutritional status and lifestyle. Our study confirms that BE is common in patients seeking a weight loss or maintenance programme, with higher prevalence in women than in men( Reference Bertoli, Leone and Ponissi 2 ). Moreover, we found an increased risk of MS, high blood pressure and high glucose with increasing BES scores in the sex- and age-adjusted model. However, such associations were lost after inclusion of nutritional status and lifestyle factors in the model. Only an inverse, but clinically irrelevant, association was found between BES score and low HDL risk. These results are of great relevance in order to better understand the independent contribution of BE to cardiometabolic risk. The present study, indeed, adds to the information that BE is unlikely related to MS and other metabolic parameters. On the basis of such findings, and on previous evidences suggesting an association between BE and obesity, low adherence to the Mediterranean diet( Reference Bertoli, Spadafranca and Bes-Rastrollo 3 ), a higher smoking status( Reference Solmi, Veronese and Sergi 4 ) and low physical activity level( Reference Bertoli, Leone and Ponissi 2 ), we can speculate that BE leads to MS through the development of obesity and of an unhealthy lifestyle. However, only a longitudinal trial can confirm such a speculation.
Previous case–control studies, carried out on small samples of obese patients with BED, have not found any significant difference in binge eating episodes or severity of eating disorder psychopathology between subjects with and without MS( Reference Roehrig, Masheb and White 28 , Reference Blomquist, Milsom and Barnes 29 ). Nevertheless, Roehrig et al.( Reference Roehrig, Masheb and White 28 ) found that some lifestyle behaviours including fewer episodes of weight cycling and regular meal skipping were significant predictors of MS. However, Blomquist et al.( Reference Blomquist, Milsom and Barnes 29 ) failed to replicate these findings, adding that dietary restraint, depressive symptoms or self-esteem did not differ between subjects with and without MS. A non-significant association between BE and MS has also been reported in two cross-sectional studies( Reference Abraham, Massaro and Hoffmann 9 , Reference Mitchell, King and Pories 10 ) and in a longitudinal study( Reference Hudson, Lalonde and Coit 8 ). In agreement with these studies, we did not find any increased risk of MS with increasing BE severity after inclusion of nutritional status and lifestyle factors.
Even though the two cross-sectional studies involving subjects of the Framingham Heart Study( Reference Abraham, Massaro and Hoffmann 9 ) and patients waiting for bariatric surgery( Reference Mitchell, King and Pories 10 ) did not find any association between BE and MS, they observed a higher risk of impaired fasting glucose in subjects with BED compared with subjects without BED, after adjustment for nutritional status and other potential confounders. However, they did not adjust for important lifestyle factors such as dietary pattern and physical activity, and this could be the reason for the discrepancy between the results. In fact, we observed that the initial significant association between BES score and the risk of impaired fasting glucose was lost after adjusting for nutritional status and lifestyle factors. These considerations may also explain why our findings are inconsistent with results reported by Raevuori et al.( Reference Raevuori, Suokas and Haukka 11 ) and de Jonge et al.( Reference de Jonge, Alonso and Stein 30 ). These two studies, using, respectively, a longitudinal( Reference Raevuori, Suokas and Haukka 11 ) and cross-sectional design( Reference de Jonge, Alonso and Stein 30 ), observed an increased risk of type 2 diabetes mellitus in subjects with BED and BN. However, BMI and lifestyle data were not available, and therefore all analyses were not adjusted for nutritional status and other confounders. Moreover, as the authors stated, subjects with BED were more prone to develop overweight and obesity( Reference Raevuori, Suokas and Haukka 11 ). These strong limitations do not allow to establish whether the increased risk of diabetes is attributable to BE or to a worse nutritional status.
In contrast, a longitudinal study, carried out on a small sample of overweight and obese individuals, reported a higher 5-year incidence of dyslipidemia in subjects with BED compared with age- and sex-matched controls after adjustment for nutritional status( Reference Hudson, Lalonde and Coit 8 ). Even though this study is worthy for its longitudinal design, it has the strong limitation of using self-reported metabolic values, which may be less accurate, and thus the quantification of associations would have been less reliable. Moreover, they did not take into account any variable describing the lifestyle of individuals, and, for example, a lower physical activity level and a higher sedentary lifestyle, which are behaviours typical in subjects with BED( Reference Levine, Marcus and Moulton 31 ), may be the reasons for the higher risk of dyslipidemia. Our findings show an increment in serum HDL levels with increasing BES scores. However, the difference was small and clinically irrelevant. In fact, it should be noted that the difference in the serum level of HDL-cholesterol between BES scores corresponding to the 5th and 95th percentiles was only approximately 3·5 mg/dl.
Strengths of this study include its sample size and the fact that we are the first to adjust the analysis for nutritional status and lifestyle variables such as adherence to the Mediterranean diet, smoking status and physical activity. Moreover, we decided to include normal weight subjects as well, as we recently showed that this category of individuals, especially young women, is not BE-free( Reference Bertoli, Leone and Ponissi 2 ). However, it is not free of limitations. First, its cross-sectional nature does not allow us to establish a cause–effect relationship. Second, we enrolled subjects seeking a weight loss or maintenance programme, and therefore our results cannot be extrapolated to the general population. Third, we did not have any information concerning the onset of binging. Finally, we focused on BE, the primary diagnostic criterion for several disorders including BED, BN, AN and EDNOS, whose presence, however, does not necessarily involve the presence of an objective eating disorder.
In conclusion, BE does not seem to confer a risk of MS and other metabolic parameters over and above the risk attributable to obesity and lifestyle alone. However, considering the higher risk for developing obesity and having an unhealthy lifestyle among subjects suffering from BE, the screening and treatment of BE are issues of clinical relevance for the prevention of obesity and cardiometabolic diseases.
Acknowledgements
The authors thank the ICANS research staff and especially Chiara Caporali, Giulia De Carlo, Valentina Giustizieri, Chiara Lessa, Lidia Lewandowski, Diana Osio and Giovanna Croce for their help during this study.
This study was supported by ICANS internal grants.
A. L. and S. B. designed the research; A. L., V. P., A. B., V. B., P. M., M. R. and S. B. conducted the research; A. L. and G. B. performed statistical analysis; A. L. wrote the manuscript; A. L. had primary responsibility for the final content; and all authors read and approved the final manuscript.
The authors have no conflicts of interest to declare.









