A vitamin-like substance, α-lipoic acid (ALA), functions as a cofactor in enzyme complexes related to oxidative decarboxylation of α-keto acid in animal tissues (Christensen, Reference Christensen and Riis1983; Bustamante et al. Reference Bustamante, Lodge, Marcocci, Tritschler, Packer and Rihn1998). Recently, a beneficial effect of ALA administration, stimulation of insulin-dependent glucose uptake in skeletal muscle, has been demonstrated, especially in insulin-resistant rats (Henriksen et al. Reference Henriksen, Jacob, Streeper, Fogt, Hokama and Tritschler1997; Khamaisi et al. Reference Khamaisi, Potashnik, Tirosh, Demshchak, Rudich, Tritschler, Wessel and Bashan1997; Streeper et al. Reference Streeper, Henriksen, Jacob, Hokama, Fogt and Tritschler1997; Bustamante et al. Reference Bustamante, Lodge, Marcocci, Tritschler, Packer and Rihn1998). In addition, hepatic gluconeogenesis due to ALA supplementation also decreases in fasted rats (Blumenthal, Reference Blumenthal1984; Khamaisi et al. Reference Khamaisi, Rudich, Potashnik, Tritschler, Gutman and Bashan1999). Thus, such findings indicate that a major effect of ALA supplementation on energy metabolism is in the carbohydrate metabolism responsible for hypoglycaemia (Khamaisi et al. Reference Khamaisi, Potashnik, Tirosh, Demshchak, Rudich, Tritschler, Wessel and Bashan1997; Bustamante et al. Reference Bustamante, Lodge, Marcocci, Tritschler, Packer and Rihn1998). In lipid metabolism, serum TAG was reported to decrease in rats that received an intraperitoneal injection of ALA (Segermann et al. Reference Segermann, Hotze, Ulrich and Rao1991). In addition, the response of lipids to ALA in mammalian species is suggested to relate to hepatic fatty acid metabolism as described later (Khamaisi et al. Reference Khamaisi, Rudich, Potashnik, Tritschler, Gutman and Bashan1999; Walgren et al. Reference Walgren, Amani, McMillan, Locher and Buse2004).
In avian species, there are few observations on the effects of ALA on energy metabolism (Shih, Reference Shih1983). The present author has focused on lipid metabolism in broiler chickens in response to dietary administration of ALA. An increase in plasma NEFA and a decrease in plasma TAG were observed when chickens received dietary ALA administration (Hamano et al. Reference Hamano, Sugawara, Kamota and Nagai1999). More recently, an interesting response of plasma lipids to dietary ALA administration has been observed in broiler chickens. When the dietary supplementation level was relatively high, concomitant increases in plasma NEFA and TAG were found, suggesting alteration of hepatic fatty acid utilisation (Hamano, Reference Hamano2002). In addition, ALA was also shown to increase plasma TAG, regardless of the negative metabolic action of corticosterone, which is a major glucocorticoid for avian species (Hamano, Reference Hamano2006). On the other hand, plasma NEFA level is an index not only of the lipolytic response of adipocytes but also of the lowered rate of fatty acid uptake in peripheral tissues, especially in the liver. Because the site of most fat (fatty acid) synthesis in avian species is known to be the liver (LeClercq, Reference LeClercq1984; Wellenreiter, Reference Wellenreiter1991; Hermier, Reference Hermier1997), the simultaneous increment of plasma TAG induced by ALA may result secondarily from re-esterification of excess fatty acids in the liver. Thus, the utilisation of acetyl-CoA, which will be derived from β-oxidation of fatty acids in chicken liver, may vary with different levels of ALA administration. Khamaisi et al. (Reference Khamaisi, Rudich, Potashnik, Tritschler, Gutman and Bashan1999) has also proposed that ALA has an inhibitory effect on hepatic fatty acid oxidation in rats. In a recent study by Walgren et al. (Reference Walgren, Amani, McMillan, Locher and Buse2004), R-(+)-ALA, a natural enantiomer, added to in vitro media inhibited oxidation rates of free palmitate in fasted rat hepatocytes. They also found that a marked response occurred as the ALA supplementation level rose in the medium containing a high level of NEFA.
In the liver, ketone bodies are produced by acetyl-CoA derived from the major precursors, NEFA, butyrate and amino acids (Madsen, Reference Madsen and Riis1983). In particular, this ketogenesis is activated due to fasting, resulting in acute elevation of plasma D-3-hydroxybutyrate (hydroxybutyrate), a ketone body (Emmanuel et al. Reference Emmanuel, Berzins and Robblee1982). Hence, if ALA diminishes hepatic fatty acid oxidation in chickens, ketone body production may also be reduced in vivo. There is the finding that ALA administration lowered in vitro ketogenesis in rat hepatocytes (Blumenthal, Reference Blumenthal1984). However, there are few observations of ALA inducing inhibition of ketogenesis with lowered fatty acid metabolism in chickens.
The aim of the present study, therefore, was to confirm the apparent ALA-induced increment in plasma NEFA in response to continuous intravenous infusion of ALA and to determine whether the response is closely associated with reduced ketogenesis in the liver.
Materials and methods
Animals and diets
The present study consisted of two independent experiments. Female broiler chicks (1 d old, Ross strain; Shosan Shoji Co., Tokyo, Japan) were housed in electrically heated battery brooders. At age 14 d, chicks were transferred to wire cages of four birds each and used for two experiments as described later. A commercial starter diet containing 230 g crude protein/kg and 12·5 MJ metabolisable energy/kg was provided until age 3 weeks, and thereafter, a grower diet containing 180 g crude protein/kg and 13·2 MJ metabolisable energy/kg was given. These diets and drinking water were provided ad libitum. The room was maintained under continuous lighting and temperature was kept at 25°C throughout the experiments. The present study was carried out in accordance with Guidelines for Animal Care and Experimentation of the Akita Prefectural College of Agriculture.
Experiment 1: dose effects of lipoic acid infusion on plasma lipids, free glycerol and hydroxybutyrate in chickens
Design
To determine the metabolic response of plasma glucose and lipids to ALA infusion, eighteen chickens, in the fed state, aged 28 to 41 d were randomly selected from each cage. The chickens were anaesthetised by injecting pentobarbital sodium (Nembutal® injection; Abbott Laboratories, North Chicago, IL, USA) into abdominal subcutaneous tissue. Two medical cannulas (inner diameter 0·48 mm, outer diameter 0·68 mm; Nipro, Osaka, Japan) were inserted into leg veins under general anaesthesia. In addition, the anaesthetised birds were laid on an electric warming sheet to prevent a reduction in body temperature during the experiment. One cannula was used for blood sampling, and the other was connected to a silicone tube with a syringe pump (Nipro, Osaka, Japan) for ALA infusion. The interior of the blood-sampling cannula was filled with saline containing heparin. ALA (Sigma-Aldrich, St Louis, MO, USA) dissolved in saline containing 200 mm-trishydroxyaminomethane (pH 7·4) was continuously infused at 0, 25 or 50 mg/kg per h (a solution volume of 3·5 ml/kg per h) for 90 min (n 6). Saline containing only trishydroxyaminomethane was continuously infused into control birds (n 6). Blood (1 ml) was sampled 30 min before infusion of ALA and again at 30 min intervals ( − 30, 0, 30, 60 and 90 min) using a heparinised syringe. To prevent confusion of age-related metabolic state, chickens received ALA, of which each infusion level was selected in random order during this trial. The blood samples were centrifuged to obtain plasma, and plasma samples were stored at − 20°C until chemical assay.
Analysis
Plasma metabolites were quantified using colorimetric and enzymic assay kits for glucose (Kyowa Medex, Tokyo, Japan), and NEFA (Kyowa Medex, Tokyo, Japan). Plasma free glycerol was assayed with a fluorometric method using glycerol dehydrogenase (Boobis & Maughan, Reference Boobis and Maughan1983). Plasma hydroxybutyrate was determined using an enzymic assay kit (Sigma-Aldrich). Data from plasma metabolite assays of the ALA-infused birds were calculated as the response area under the curve and above the baseline (concentration × min) to consider different initial concentrations between the treatment groups. The baseline that is an initial concentration before the ALA infusion (a mean of − 30 and 0 min) was fixed in individual chickens. Of the plasma metabolites, hydroxybutyrate during the ALA infusion was monitored only at 30 and 90 min. Such calculated areas to estimate metabolic responsiveness to ALA treatments were statistically analysed by the Scheffé test using a computer program (StatView; Abacus Concepts Inc., Berkeley, CA, USA).
Experiment 2: effects of intravenous infusion of lipoic acid on fatty acid mobilisation and plasma hydroxybutyrate response in fasted chickens
Design
This experiment was conducted to determine the effects of ALA on plasma hydroxybutyrate response stimulated by fasting-induced fatty acid mobilisation in chickens. Twenty chickens aged 36 to 40 d were randomly selected, and half the birds were fasted for at least 24 h before receiving an intravenous infusion of ALA. The bird was covered with a rubber suit made in the laboratory. Two medical cannulas were inserted into leg veins under local anaesthesia (5 g/l, lidocaine hydochloride; Astra Japan Ltd, Osaka, Japan). Thereafter, the bird was transferred to a two-shelf metal wire rack. The rubber suit containing the chicken was connected to rubber bands hanging from the upper shelf of the rack to hold it in a standing position on the bottom shelf during the experiment. One cannula was used for blood sampling, and the other was connected to a silicone tube with a syringe pump for infusion of ALA. The interior of the blood-sampling cannula was filled with saline containing heparin. Therefore, the chickens used in this experiment were infused with ALA without general anaesthesia. ALA dissolved in saline containing 200 mm-trishydroxyaminomethane (pH 7·4) was continuously infused at a rate of 100 mg/kg per h for 90 min (n 5). Control birds were infused with saline containing the solvent alone (n 5). Blood (1 ml) was drawn 30 min before infusion of ALA and at 30 min intervals ( − 30, 0, 30, 60 and 90 min) using a heparinised syringe. The blood samples were centrifuged to obtain plasma, and plasma samples were stored at − 20°C until chemical assay.
Analysis
Plasma metabolites in this experiment were determined using enzymic assay kits for plasma glucose (Kyowa Medex), TAG (Sanko Chemical, Tokyo, Japan), NEFA (Kyowa Medex) and hydroxybutyrate (Sigma-Aldrich). Of plasma metabolites, TAG during the ALA infusion was monitored only at 30 and 90 min. Plasma free glycerol was not analysed because this experiment focused on the involvement of ALA on plasma hydroxybutyrate with fasting-stimulated fatty acid mobilisation. Data that were calculated as response area (concentration × min) were statistically analysed by two-way ANOVA as a 2 × 2 factorial arrangement. If the interaction between ALA infusion and the feeding state was significant, the responses to ALA infusion in each feeding state were compared with those of controls using a Student's t test (StatView).
Results
Experiment 1: dose effects of lipoic acid infusion on plasma glucose, non-esterified fatty acids, free glycerol and hydroxybutyrate in chickens
Continuous infusion of ALA for 90 min did not change the plasma glucose level (Fig. 1) or its calculated response area (concentration × min; Table 1), but it gradually increased plasma NEFA (Fig. 2). Both infusion rates of ALA rapidly enhanced plasma NEFA, and its concentration remained high at 90 min, while plasma NEFA in controls gradually decreased below the basal level. The response area of the changes in plasma NEFA was statistically significant (P < 0·05) as shown by ANOVA. Regardless of the infusion level, the area under the curve of plasma NEFA was higher in the ALA-treated group than in the controls (P < 0·05). However, a dose-dependent difference was not observed in the response area. Changes in plasma free glycerol are shown in Fig. 3. The response areas of plasma free glycerol, overall, were not statistically significant by ANOVA. Changes in plasma hydroxybutyrate are shown in Fig. 4. The concentration was gradually enhanced for 90 min in controls but, in contrast, decreased following ALA infusion. A significant difference in plasma hydroxybutyrate as response area under the curve was observed from the ANOVA (P < 0·05; Table 1). Consequently, a significant decrease of plasma hydroxybutyrate in ALA-infused chickens was shown only at the low-dose level (P < 0·05) as compared with controls (Table 1).

Fig. 1 Changes in plasma glucose concentration with different continuous infusion levels of α-lipoic acid (ALA). Values are means for six birds with standard errors represented by vertical bars. (●), Controls; (■), 25 mg ALA/kg per h; (▲), 50 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significant difference are shown in Table 1.
Table 1 Areas under the curve and above the baseline of plasma metabolite responses in chickens during continuous infusion of α-lipoic acid† (Mean values with their standard errors for six birds)


Fig. 2 Changes in plasma NEFA concentration with different continuous infusion levels of α-lipoic acid (ALA). Values are means for six birds with standard errors represented by vertical bars. (●), Controls; (■), 25 mg ALA/kg per h; (▲), 50 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significant difference are shown in Table 1.

Fig. 3 Changes in plasma free glycerol concentration with different continuous infusion levels of α-lipoic acid (ALA). Values are means for six birds with standard errors represented by vertical bars. (●), Controls; (■), 25 mg ALA/kg per h; (▲), 50 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significant difference are shown in Table 1.

Fig. 4 Changes in plasma hydroxybutyrate concentration with different continuous infusion levels of α-lipoic acid (ALA). Values are means for six birds with standard errors represented by vertical bars. (●), Controls; (■), 25 mg ALA/kg per h; (▲), 50 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significant difference are shown in Table 1.
Experiment 2: effects of intravenous infusion of lipoic acid on plasma fatty acid mobilisation and ketogenesis in fasted chickens
Metabolic response to feeding condition
Metabolic alterations in response to different feeding conditions are shown in Table 2. Starvation for at least 24 h reduced plasma TAG (P < 0·001) and NEFA (P < 0·05) by 33 and 35 %, respectively, while plasma glucose was unchanged from the feeding condition. In contrast, plasma hydroxybutyrate was six times higher (P < 0·001) in fasted chickens than in those fed any food. Thus, the intensive increase in plasma hydroxybutyrate indicates that the plasma NEFA reduction resulted not from lowered fatty acid mobilisation but from an enhanced fatty acid requirement to supply energy.
Table 2 Effects of fasting on plasma metabolites in broiler chickens before infusion of lipoic acid (Mean values with their standard errors for ten birds)

Mean values were significantly different from those of the fed group: * P < 0·05, *** P < 0·001.
Metabolic response of plasma metabolites to lipoic acid infusion
Changes in plasma glucose during intravenous infusion of ALA are shown in Fig. 5. Infusion of saline into controls hardly altered the plasma glucose level, regardless of the feeding condition. In fed chickens, ALA infusion transiently increased the plasma glucose at 30 min, but its concentration returned to the basal level. Under the fasting condition, this response also occurred at 30 min and was maintained until 60 min. A significant increase in the area under the curve was shown in chickens that received the ALA infusion, regardless of feeding status (control (n 10), − 47·7 (se 26·1) mmol/l × min; ALA (n 10), 81·4 (se 22·4) mmol/l × min; P < 0·01). However, the response of plasma glucose to ALA infusion had no significant interaction with feeding status as shown by the ANOVA. In the chickens infused with ALA, plasma NEFA gradually increased from basal levels until 90 min (Fig. 6). As shown by the area calculated (Table 3), the responsiveness of plasma NEFA to ALA did not interact with the feeding condition but did significantly increase compared with controls (control (n 10), − 3106 (se 1589) μmol/l × min; ALA (n 10), 17 913 (se 3474) μmol/l × min; P < 0·001). Plasma TAG was unchanged by ALA infusion for 90 min (Fig. 7), and the area under the curve also was not statistically significant. On the other hand, plasma hydroxybutyrate in fed chickens was gradually decreased until 60 min (Fig. 8 (a)), but an apparently marked reduction in plasma hydroxybutyrate during ALA infusion was observed in the fasted condition (Fig. 8 (b)). This rapidly intensive response of plasma hydroxybutyrate occurred in the first 30 min. The area calculated showed a significant interaction between feeding status and ALA infusion by ANOVA (P < 0·01). In fed chickens, ALA infusion diminished plasma hydroxybutyrate significantly (control (n 5), − 2·2 (se 1·4) mmol/l × min; ALA (n 5), − 7·6 (se 1·7)) mmol/l × min; P < 0·05). Under the fasting condition, the area of plasma hydroxybutyrate was intensively lowered in the ALA-infused group compared with the controls (control (n 5), 49·85 (se 29·96) mmol/l × min; ALA (n 5) − 93·41 (se 18·0) mmol/l × min; P < 0·05). This result specifically indicates that the inhibition of plasma hydroxybutyrate by ALA infusion was apparently greater in the fasted chickens than in the fed birds.

Fig. 5 Changes in plasma glucose concentration with a continuous infusion of α-lipoic acid (ALA) in broiler chickens either diet-fed (a) or fasted (b). Values are means for five birds with standard errors represented by vertical bars. (●), Controls; (■), 100 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significance levels from ANOVA as a 2 × 2 factorial arrangement are shown in Table 3.

Fig. 6 Changes in plasma NEFA concentration with a continuous infusion of α-lipoic acid (ALA) in broiler chickens either diet-fed (a) or fasted (b). Values are means for five birds with standard errors represented by vertical bars. (●), Controls; (■), 100 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significance levels from ANOVA as a 2 × 2 factorial arrangement are shown in Table 3.
Table 3 Areas under the curve and above the baseline of plasma metabolite responses in fed or fasted chickens during continuous infusion of α-lipoic acid (ALA)† (Mean values with their standard errors for five birds)
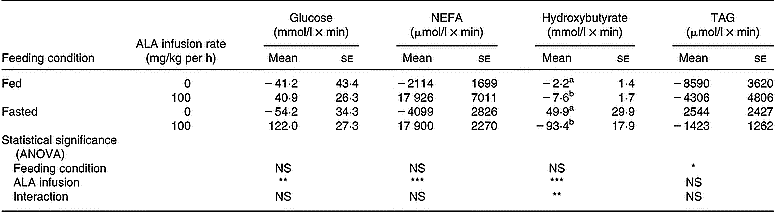

Fig. 7 Changes in plasma TAG concentration with a continuous infusion of α-lipoic acid (ALA) in broiler chickens either diet-fed (a) or fasted (b). Values are means for five birds with standard errors represented by vertical bars. (●), Controls; (■), 100 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significance levels from ANOVA as a 2 × 2 factorial arrangement are shown in Table 3.

Fig. 8 Changes in plasma hydroxybutyrate concentration with a continuous infusion of α-lipoic acid (ALA) in broiler chickens either diet-fed (a) or fasted (b). Values are means for five birds with standard errors represented by vertical bars. (●), Controls; (■), 100 mg ALA/kg per h. The response areas under the curve and above the baseline (concentration × min) with significance levels from ANOVA as a 2 × 2 factorial arrangement are shown in Table 3.
Discussion
Metabolic response of plasma glucose to lipoic acid infusion
ALA has been well documented to decrease plasma glucose in rats (Khamaisi et al. Reference Khamaisi, Potashnik, Tirosh, Demshchak, Rudich, Tritschler, Wessel and Bashan1997; Bustamante et al. Reference Bustamante, Lodge, Marcocci, Tritschler, Packer and Rihn1998). This action is closely associated with stimulation of both insulin-mediated and -independent glucose uptake in skeletal muscle (Henriksen et al. Reference Henriksen, Jacob, Streeper, Fogt, Hokama and Tritschler1997; Streeper et al. Reference Streeper, Henriksen, Jacob, Hokama, Fogt and Tritschler1997). A similar response of plasma glucose to ALA was previously observed in broiler chickens (Hamano, Reference Hamano2002, Reference Hamano2006). Insulin sensitivity, as estimated by the euglycaemic clamp technique, in chickens as well as in mammals is stimulated by dietary ALA administration (Hamano, Reference Hamano2006). In contrast, the continuous infusion of ALA in experiment 2 resulted in rapid enhancement of the plasma glucose level (response area for 90 min) independent of feeding condition, but there was no response of plasma glucose to ALA in experiment 1. These different responses might be associated with dose level. The transiently hyperglycaemic response similar to the result in experiment 2 has occurred when a β-agonist, isoproterenol, was continuously infused in ALA-fed chickens (Hamano et al. Reference Hamano, Sugawara, Kamota and Nagai1999). Thus, the present study showed at least that ALA not only reduces plasma glucose (Hamano, Reference Hamano2006) but also possesses hyperglycaemic action in chickens.
Metabolic response of plasma lipids to lipoic acid infusion
Previous studies have found that dietary administration of ALA to broiler chickens initially increases plasma NEFA (Hamano et al. Reference Hamano, Sugawara, Kamota and Nagai1999; Hamano, Reference Hamano2002). Likewise, significantly increased plasma NEFA levels, shown as area under the curve, occurred rapidly in the chickens that received the continuous infusion of ALA in the present study (experiments 1 and 2). Plasma NEFA level is generally considered as an index of lipolytic status. With regard to lipolysis, dietary ALA increased the rate of free glycerol release from in vitro adipose tissue slices in chickens (Hamano, Reference Hamano2006). However, the author has never confirmed the reduction of adipose tissue accretion due to ALA administration, as an available benefit for broiler production, in in vivo studies (Hamano et al. Reference Hamano, Sugawara, Kamota and Nagai1999; Hamano, Reference Hamano2002, Reference Hamano2006). The present study additionally monitored plasma free glycerol, together with plasma NEFA, to assess lipolytic responsiveness more accurately. No significant changes in plasma free glycerol were detected in the ALA-infused birds (experiment 1). Hence, these findings suggest that the ALA-enhanced plasma NEFA did not depend on the stimulated lipolysis of adipose tissue.
In experiment 2, the lowered plasma NEFA observed in the fasted chickens, compared with their fed counterparts, would be attributed to enhanced fatty acid utilisation because the plasma hydroxybutyrate, which is produced mainly from fatty acids (Madsen, Reference Madsen and Riis1983), was markedly greater than it was in the fed chickens (Table 2). Fasting, which has been shown to increase the plasma hydroxybutyrate level (Emmanuel et al. Reference Emmanuel, Berzins and Robblee1982), would result in accelerated fatty acid mobilisation with adipose tissue lipolysis. Under this metabolic state of fatty acids, if plasma NEFA increased by ALA infusion resulted from stimulated lipolysis, plasma hydroxybutyrate (ketogenesis) would also be increased. However, the ALA infusion distinctly decreased plasma hydroxybutyrate in spite of a concomitant increase in plasma NEFA. Thus, these findings would be associated rather with a hepatic fatty acid response to ALA than with adipose tissue lipolysis, as discussed later.
The concentration of a blood metabolite is generally dependent on the difference (balance) between the rates of its release from and uptake by the tissues. In relation to the fatty acid metabolism of the liver, the present study focused on the effect of ALA on ketone body production. In fasted normal rats, Khamaisi et al. (Reference Khamaisi, Rudich, Potashnik, Tritschler, Gutman and Bashan1999) observed increases in plasma NEFA and acylcarnitine levels without elevation of plasma hydroxybutyrate, suggesting inhibition of hepatic fatty acid oxidation. Ketogenesis occurs primarily in the hepatocytes (Madsen, Reference Madsen and Riis1983). The blood level of ketone bodies is markedly high in newly hatched chicks and decreases with ageing (Ohtsu et al. Reference Ohtsu, Sato, Nishida and Akiba2003). Although the number of birds used in the present study was small, a significant reduction in plasma hydroxybutyrate was observed following ALA infusion, even when its basal concentration was relatively low in the fed chickens (Tables 1 and 3). Under a starved condition especially, the ALA infusion markedly diminished the intensively enhanced plasma hydroxybutyrate. Blumenthal (Reference Blumenthal1984) reported that ALA supplementation inhibits in vitro ketogenesis with antiglyconeogenesis in rat hepatocytes. Taken together with the lack of plasma glycerol response in the present experiment, the organ tissue most involved in the ALA-induced fatty acid metabolism must be liver tissue. Dietary ALA supplementation has been noted to increase the rate of hepatic O2 consumption in rabbits with experimental atherosclerosis (Ivanov, Reference Ivanov1974) and normal broiler chickens (Hamano et al. Reference Hamano, Sugawara, Kamota and Nagai1999). However, it remains uncertain how ALA inhibited fatty acid utilisation from β-oxidation to acetyl-CoA distribution in the citrate cycle or ketogenesis in the liver. Therefore, the increase in plasma NEFA during ALA infusion might be a subsequent response attributable to a lowered hepatic fatty acid uptake or oxidation.
Dietary ALA supplementation has been shown to decrease serum cholesterol and lipoproteins in rabbits with experimental atherosclerosis (Ivanov, Reference Ivanov1974). Previous studies found that dietary ALA not only reduced plasma TAG and total cholesterol in chickens (Hamano et al. Reference Hamano, Sugawara, Kamota and Nagai1999, Reference Hamano, Kamota and Sugawara2000) but also caused an increase in plasma TAG (Hamano, Reference Hamano2002, Reference Hamano2006). Fasting lowered plasma TAG, indicating that the metabolic flux of acetyl-CoA for fatty acid synthesis would be limited in experiment 2 (Table 2). Under such a condition, ALA infusion did not alter the plasma TAG level (Fig. 7; Table 3), even though fatty acid catabolism, as shown in the responses of plasma NEFA and hydroxybutyrate, was possibly reduced.
Conclusions
The present data found a manifest action of ALA, which has been previously suggested to inhibit hepatic fatty acid mobilisation. Here, in chickens, the increase in plasma NEFA following ALA treatment was not attributable to facilitation of lipolysis. On the other hand, the present study established that ALA decreased plasma hydroxybutyrate, possibly inhibiting hepatic ketogenesis even with fasting-stimulated fatty acid mobilisation.
Acknowledgements
The present study was supported by a grant-in-aid for young scientists (B) (no. 13760202) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The author is grateful to R. Hodokuma, Y. Fujiwara, N. Koizumi and K. Watanabe for their technical assistance in the experiments.













