The prevalence of atopic manifestations, including atopic eczema, allergic rhinoconjunctivitis and asthma, has increased worldwide, especially in children in Westernised countriesReference Maziak, Behrens, Brasky, Duhme, Rzehak, Weiland and Keil1, Reference Beasley, Crane, Lai and Pearce2. Currently one-third of the children in Western societies show symptoms3.
In 1999 and 2006, studies were published showing that an anthroposophic lifestyle protected against atopic disease in 7–8-year-old childrenReference Alm, Swartz, Lilja, Scheynius and Pershagen4, Reference Alfven, Braun-Fahrlander and Brunekreef5. Due to the cross-sectional design of the studies, specific protective factors could not be identified. Among the potentially beneficial factors of an anthroposophic lifestyle in relation to atopic disease is the consumption of organically produced food. A rapidly growing group of consumers in our society prefers to buy organically grown and processed products, which are perceived as healthier and saferReference Magnusson, Arvola, Hursti, Aberg and Sjoden6, Reference Lockie, Lyons, Lawrence and Grise7. However, there are virtually no studies of any size that have evaluated effects of organic v. conventionally grown foods on human healthReference Williams and Hammitt8. Food or agricultural production certified as ‘organic’ is a system that excludes the use of synthetic inputs, such as synthetic fertilisers and pesticides, preventive veterinary drugs, genetically modified (GM) seeds and breeds, most preservatives, additives and irradiation (http://www.ifoam.org/sub/faq.html). In the Netherlands ‘organic’ products include both products from bio-organic as well as from biodynamic production, the latter being a method which strives even more strongly for a healthy ecosystem on the farmReference Mäder, Fliessbach, Dubois, Gunst, Fried and Niggli9. Both types of products carry the registered ‘EKO’ certification mark for organic products. From comparison studies between biodynamic- and bio-organic-grown products and conventionally grown products we know that these ‘organic’ products contain on average more antioxidants, especially vitamin CReference Williams10, and organic dairy products contain on average a higher amount of conjugated linoleic acid and n-3 fatty acidsReference Jahreis, Fritsche and Steinhart11, Reference Bergamo, Fedele, Iannibelli and Marzillo12. A preventive effect of antioxidants on wheezing complaints has been suggestedReference Litonjua, Rias-Shiman, Ly, Tantisira, Rich-Edwards, Camagro, Weiss, Gillman and Gold13, possibly through protection of the epithelium of the bronchial tubes against oxidative stress. Further, it is hypothesised that n-3 fatty acids might have anti-inflammatory properties and can contribute to the skin barrierReference Wijga, van Houwelingen, Kerkhof, Tabak, de Jongste, Gerritsen, Neijens, Boshuizen and Brunekreef14, Reference Oddy, Pal and Kusel15, thus potentially decreasing atopic reactions.
The present paper describes the first prospective study to evaluate the role of organic food consumption on human health. In a large birth cohort study in the Netherlands, we investigated whether early-life organic food consumption was associated with developing atopic manifestations in the first 2 years of life.
Experimental methods
Study subjects
A detailed description of the study design has previously been publishedReference Kummeling, Thijs and Penders16. In summary, the KOALA Birth Cohort Study started in October 2000. KOALA is (in Dutch) an acronym for Child, Parent and health: Lifestyle and Genetic constitution. Healthy pregnant women were recruited from an ongoing prospective cohort study on Pregnancy-related Pelvic Girdle PainReference Bastiaanssen, de Bie, Bastiaenen, Heuts, Kroese, Essed and van den Brandt17 at 34 weeks of gestation (‘conventional sub-cohort’). Additionally, pregnant women with so-called ‘alternative’ lifestyles were recruited through several specific recruitment channels, i.e. anthroposophic doctors, midwives, under-five clinics, Steiner schools, posters and flyers in organic food shops (‘alternative sub-cohort’). A total of 2834 participants were included in the KOALA study, of whom 2343 were recruited through the Pregnancy-related Pelvic Girdle Pain study and 491 through the specific ‘alternative’ recruitment channels. The study was approved by the medical ethical committee of the University of Maastricht and Academic Hospital Maastricht. All participants gave signed informed consent and completed the first questionnaire at the third trimester of pregnancy.
Three children were excluded for Down's syndrome and sixty-seven for prematurity (gestational age < 37 weeks), resulting in 2764 participants. Loss-to-follow-up rates were low: 93·4 % (n 2598) of all recruited participants were followed up until age 2 years, with information available on at least one of the health outcomes measured in the study (this was 93·5 % (n 2135) of the conventional and 96·3 % (n 463) of the alternative sub-cohort).
All parents were sent detailed questionnaires when the infants were 3, 7, 12 and 24 months of age. Parents in the second half of the cohort (n 1301) were also asked to consent to infant venous blood sampling at age 2 years. Of these, 815 (65 %) gave signed informed consent and were visited at home for blood sampling.
Exposure and follow up
Organic food consumption was measured at age 2 years, by parental reported diet of the infant in the second year of life. Parents were asked whether their infant had consumed meat, eggs, vegetables, fruit, dairy products, bread and/or dry products such as pasta, rice, beans and wheat. If yes, parents were further asked whether these products were conventionally or organically produced. Three diet categories were defined: ‘conventional’ (organic in < 50 % of the occasions that the food is eaten); ‘moderately organic’ (organic in 50–90 % of the occasions); ‘strictly organic’ (organic in >90 % of the occasions). Each of the seven food groups was given a score, depending on the percentage organic (‘conventional’, score = 0; ‘moderately organic’, score 0·7 (mean of 0·5–0·9); ‘strictly organic’, score = 1). The scores were added up and divided by the number of food groups that had been eaten. After multiplying by 100 a score between 0 and 100 % was achieved. Organic food consumption is more frequent among individuals with alternative lifestyle practices than in the Dutch general population. Therefore, we calculated cut-off points for three equal groups within our alternative sub-cohort only, which were 21·0 and 71·0. Then, for the whole cohort, we defined a conventional diet as a score below 21·0, a moderately organic food diet as a score between 21·0 and 71·0 and a diet strictly based on organic products as a score above 71·0. The production method of the foods consumed in the second year of life was considered to reflect the production method of foods consumed in the entire first 2 years of life.
Organic food consumption was measured in pregnant mothers at 34 weeks of gestation, by repeating the above-described procedure, using the same food products (ever consumed during pregnancy) and cut-off points to be able to compare the diets of the mothers with those of the infants. The information on the mother's diet was used as a proxy for the infant's at age 2 years when information was missing due to incomplete questionnaires (n 453; 16 %) since organic food consumption by mothers was strongly correlated with organic food consumption by infants (Pearson's R 0·85; P < 0·001).
Eczema and wheeze occurrence were assessed by questionnaires (from the International Study of Asthma and Allergies in Childhood; ISAAC)Reference Asher, Keil and Anderson18 adapted to the infant's age and spacing between questionnaires. At age 7, 12 and 24 months, parents were asked whether their child had ever had an itchy rash that was coming and going in the past months. If this question was answered affirmatively at least once, infants were classified as having developed eczema in the first 2 years of life. Infants for whom only diaper rash, rash around the eyes and/or scalp scaling was reported were not regarded as having developed eczema. Wheeze was also covered in the 7, 12 and 24 months postpartum questionnaires. ‘Recurrent wheeze’ was defined as reported presence of wheezing with at least four attacks ever in the first 2 years. ‘Prolonged wheeze’ was classified as ever having been awake due to wheezing in both the first and second year.
Assessment of sensitisation
Total IgE antibodies were measured using RIA in serum samples as described elsewhereReference Stallman and Aalberse19, Reference Akkerdaas, Wensing, Asero, Fernandez Rivas, Knulst, Bolhaar, Hefle, Aalberse and van Ree20. Results are expressed as international units (IU) IgE/ml; 1 IU is 2·4 ng IgE. For values < 150 IU/ml a sandwich RIA was usedReference Akkerdaas, Wensing, Asero, Fernandez Rivas, Knulst, Bolhaar, Hefle, Aalberse and van Ree20; for values >150 IU/ml, a competitive RIAReference Stallman and Aalberse19. Serum levels of specific IgE against hen's egg, cows' milk, peanut, birch pollen, grass pollen, cat, dog, and house dust mite were determined by the radioallergosorbent test as described previouslyReference Akkerdaas, Wensing, Asero, Fernandez Rivas, Knulst, Bolhaar, Hefle, Aalberse and van Ree20. Calculation was performed by means of a standard curve that was obtained by a radioallergosorbent test with a dilution series of a chimeric monoclonal IgE antibody against the major house dust mite allergen Der p 2 and sepharose-coupled recombinant Der p 2 (Schuurman et al. Reference Schuurman, Perdok, Lourens, Parren, Chapman and Aalberse21). The detection limit for total and specific IgE was < 0·50 IU/ml and < 0·13 IU/ml, respectively. We defined atopic sensitisation as a radioallergosorbent test value >0·3 IU/ml for one or more allergens.
Statistical analysis
Unadjusted associations between organic food consumption and atopic outcomes were examined using logistic regression. Multivariate logistic regression models were fitted to control for potential confounders (sex, maternal education, BMI in infant at age 1 year, parental history of allergy, sibling history of allergy, number of older siblings, breast-feeding, day-care attendance, pets, exposure to environmental tobacco smoke, vaccinations, antibiotic intake through breast-feeding or through oral medication, vegetarian diet), by simultaneously including them in the regression models. Differences in the geometric mean of total IgE levels were evaluated by linear regression analyses, controlling for the same confounders. In the multivariate analyses, we first tested the possibility of interaction between organic food consumption and parental history of atopy. Since none of these interaction terms were statistically significant at the α = 0·05 level they were eliminated from the regression models. Separate analyses of the conventional v. alternative sub-cohort and boys v. girls showed that the key findings were similar within these groups; therefore all infants were combined in the final models. Results are presented as unadjusted and adjusted OR with corresponding 95 % CI.
Results
Study subjects
Table 1 shows participants' characteristics and prevalence of eczema, wheeze outcomes and atopic sensitisation at age 2 years. Eczema lifetime prevalence at age 7, 12 and 24 months of age was 18, 23 and 32 % respectively. Lifetime prevalence of wheeze symptoms at age 7, 12 and 24 months of age was 4, 9 and 11 % respectively. Baseline characteristics and prevalence of eczema and wheeze manifestations did not differ between infants with and without blood samples, but the frequency of organic food consumption was slightly higher among infants with blood samples. In the first 2 years of life, 2306 (84 %) of 2764 infants had consumed a diet based on conventional food products, 283 (10 %) consumed a moderately organic diet, and 175 (6 %) consumed a strictly organic diet (Table 1). During pregnancy, 2369 (86 %) of 2764 mothers consumed a diet based on conventional foods, 255 (9 %) consumed a moderately organic diet, and 150 (5 %) consumed a strictly organic diet. Percentages for specific sensitisation in infants with blood samples were 6 % for hen's egg, 19 % for cows' milk, 5 % for peanut, 1 % for birch, 2 % for grass pollen, 3 % for cat, 2 % for dog and 6 % for mite allergens.
Table 1 Characteristics of infants in the study for the total cohort and stratified by category of organic food use

* Percentages add to 100 % in columns, excluding missing values.
† Delayed scheme or alternative composition of vaccine.
‡ Analyses conducted among infants with blood sample (n 815). Atopic sensitisation refers to at least one allergen-specific IgE ≥ 0·3 international units (0·72 ng)/ml (against hen's egg, cows' milk, peanut, birch pollen, grass pollen, cat, dog, or house dust mite).
Eczema, wheeze and atopic sensitisation by organic food consumption
Although a trend for an association between organic food consumption and lower eczema risk was observed, this did not reach statistical significance (Table 2). The adjusted OR were 0·95 (95 % CI 0·70, 1·28) and 0·76 (95 % CI 0·50, 1·17) for moderately organic food consumption and strict organic food consumption respectively in comparison with conventional food consumption. No statistically significant associations were observed between organic food consumption and recurrent wheeze (OR 0·51 (95 % CI 0·26, 0·99) and 0·85 (95 % CI 0·35, 2·05) for moderately organic food consumption and strict organic food consumption respectively in comparison with conventional food consumption), prolonged wheeze (OR 0·80 (95 % CI 0·41, 1·55) and 0·97 (95 % CI 0·35, 2·70) for moderately organic food consumption and strict organic food consumption respectively in comparison with conventional food consumption) and atopic sensitisation (OR 0·86 (95 % CI 0·51, 1·45) and 1·45 (0·77, 2·73) for moderately organic food consumption and strict organic food consumption respectively in comparison with conventional food consumption); the latter remained unchanged when using a lower cut-off point for sensitisation (specific IgE < 0·13 IU/ml). The geometric mean of the total serum IgE level at age 2 years was not associated with organic food consumption. We repeated the analyses using information on maternal diet during pregnancy instead of the infants' diet. The adjusted OR changed very little compared with those presented in Table 2; for eczema these were 0·88 (95 % CI 0·58, 1·19) and 0·68 (95 % CI 0·42, 1·12), for recurrent wheeze 0·45 (95 % CI 0·21, 0·89) and 0·79 (95 % CI 0·32, 1·96) and for prolonged wheeze 0·79 (95 % CI 0·33, 1·87) and 0·88 (95 % CI 0·27, 2·83), for moderately and strictly organic diets in comparison with conventional food consumption, respectively.
Table 2 Associations between infant organic food consumption and eczema, wheeze and atopic sensitisation (AS) in the first 2 years of life (Odds ratios and 95 % confidence intervals)

* Results from logistic regression analysis, adjusted for sex, maternal education, BMI, parental history of allergy, sibling history of allergy, number of older siblings, breast-feeding, day-care attendance, pets, exposure to environmental tobacco smoke, vaccinations, antibiotic exposure through oral medication and through breast milk, and vegetarian diet.
† Analyses conducted among infants with a blood sample (n 815). AS refers to least one allergen-specific IgE ≥ 0·3 international units (0·72 ng)/ml against hen's egg, cows' milk, groundnut, birch pollen, grass pollen, cat, dog, or house dust mite.
Finally, we evaluated the isolated effects of each of the following organic foods on atopy outcomes: dairy products, meat, fruit, vegetables and eggs (Table 3). Consuming organic dairy products was associated with a reduced risk of eczema. The OR for strictly organic dairy products hardly changed if we restricted the analysis to (1) infants who had been consuming dairy products, i.e. excluding infants who never consumed dairy products (n 226; adjusted OR 0·69 (95 % CI 0·48, 1·01)), or (2) infants who had never consumed raw or farm milk, i.e. excluding infants who ever consumed raw or farm milk in the first year (n 52; adjusted OR 0·63 (95 % CI 0·43, 0·93)). Moreover, the OR for strictly organic dairy products remained essentially unchanged, when the analysis was performed using information on the mothers' diet during pregnancy instead of the children's diet (adjusted OR 0·67 (95 % CI 0·46, 0·98), or even when the mothers who avoided dairy products were excluded from the analysis (n 40; adjusted OR 0·67 (95 % CI 0·46, 0·99)). No other statistically significant associations were observed.
Table 3 Associations between infant organic food consumption and eczema in the first 2 years of life (n 2583) (Odds ratios and 95 % confidence intervals)
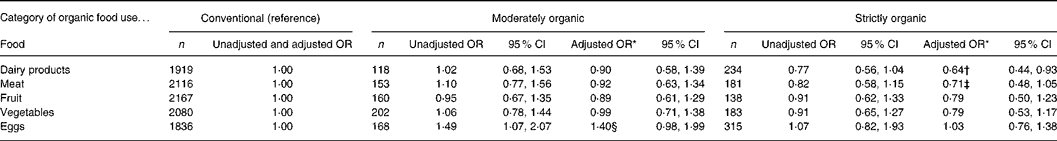
* Results from logistic regression analysis, adjusted for sex, maternal education, BMI, parental history of allergy, sibling history of allergy, number of older siblings, breast-feeding, day-care attendance, pets, exposure to environmental tobacco smoke, vaccinations, antibiotic exposure through oral medication and through breast milk, and vegetarian diet.
† P = 0·02.
‡ P = 0·10.
§ P = 0·06.
Discussion
In the present paper we show that the proportion of organic products within the total diet was neither associated with the development of eczema, nor with recurrent or prolonged wheeze during the first 2 years of life, in a statistically significant way. Furthermore, no relation could be found between the proportion of organic products within the total diet and atopic sensitisation at 2 years of age. However, we did find an association between consuming strictly organic dairy products and a reduced risk of eczema.
To date, we know of only one other study on the health effects of consuming organic food productsReference Floistrup, Swartz and Bergstrom22. This cross-sectional study performed in five European countries showed no difference in atopic outcomes in 5–13-year-old children who had been divided in three groups according to the origin of the corresponding diet: (1) conventional, (2) biodynamic, (3) or a mixture of conventional, biodynamic and organic foods. Since the latter group consumed foods from a mixture of production methods, the isolated effect of organic foods could not be assessed. In contrast, the present study is prospective, and comprises a population that strongly differs in the use of conventional and organic products due to the specific recruitment of participants. Children from conventionally eating mothers might be given organic foods because of the atopy symptoms that they had early in life (reverse causation). Organic food consumption by mothers was strongly correlated with organic food consumption by infants. The availability of information on organic food consumption by pregnant mothers (as proxy for the postnatal infants' diet) therefore gave us the opportunity to investigate an exposure that had preceded the outcome. This enabled us to exclude the possibility of bias in the associations found for infants. With the present data we cannot disentangle the potential influence from the mothers' and the infants' organic diet, because these were highly correlated. Therefore we cannot exclude the possibility that the lower risk of eczema in children who used organic dairy products was actually due to a high consumption of organic dairy products by the mother, conferring protection already starting in the intra-uterine period and during lactation. Interestingly we found that mothers with a high proportion of dairy intake from organic origin had higher levels of rumenic and trans-vaccenic acids in their breast milkReference Rist, Mueller and Barthel23.
The fact that we extensively adjusted for potential confounders such as vaccinations, use of antibiotics, breast-feeding duration and exclusivity, and BMI makes it highly unlikely that the observed associations are biased by an association between organic dieting and other healthy lifestyle characteristics.
Atopic outcomes such as infant eczema and wheeze may be fairly unspecific for infants aged 2 yearsReference Koopman, Brunekreef, de Jongste and Neijens24, and therefore subject to misclassification. Although not likely, we cannot completely disregard the possibility that parents who choose organic foods may report atopic symptoms differently from the other parents as they may be more health conscious. This would, however, tend to lead to over-reporting rather than underreporting in the organic-eating families, and therefore further strengthens the result for organic dairy consumption and eczema; nevertheless we cannot rule out that this differential reporting may have masked true effects of other organic food products on eczema (or organic food use as a whole) and effects on wheeze.
Eczema and wheeze at age 2 years may be weak indicators of an atopic development such as allergic asthmaReference Illi, von Mutius, Lau, Nickel, Gruber, Niggemann and Wahn25, Reference Henderson, North, Griffiths, Harvey and Golding26. Specific IgE or total serum IgE levels at this age are also only weakly associated with atopic symptomsReference Kusel, Holt, de Klerk and Sly27. Therefore, clinical symptoms and atopic sensitisation were evaluated as separate outcomes. The definitions of eczema and recurrent wheeze were based on questions adapted from The International Study of Asthma and Allergies in Childhood questionnaires3, Reference Asher, Keil and Anderson18 and included generally accepted characteristics, such as a chronically relapsing course and an itchy rash. Although we found a beneficial effect of the consumption of organic dairy products on the development of eczema, we must be aware that organic dairy products in the present study were always used in the context of an organic diet and not as an isolated product group within a conventional diet. It is therefore uncertain whether this finding represents a true association, and should be interpreted with some caution until it can be confirmed.
The mechanism by which organic dairy product consumption protects against the development of eczema is unknown. Dietary studies have indicated that raw (unpasteurised) or farm milk was associated with a protective effect against the development of atopic manifestationsReference Riedler, Braun-Fahrländer, Eder, Schreuer, Waser, Maisch, Carr, Schierl, Nowak and von Mutius28–Reference Perkin and Strachan30. However, when we repeated our analyses with raw and farm milk consumers excluded, the protective effect of organic dairy product consumption remained unchanged. Similarly to raw milkReference Suhren, Hesselbarth, Heeschen and Sudi31, organic dairy products can also contain more gram-negative bacteria and higher levels of the corresponding lipopolysaccharide than conventional, pasteurised dairy products. Therefore, one could hypothesise that the protective factor associated with the consumption of organic dairy products could be the ingestion of non-infectious microbial components with a possible effect on gut immunity development.
It has been hypothesised that n-3 long-chain PUFA have anti-inflammatory properties and can contribute to the maintenance of the skin barrier, due to the observation that n-3 fatty acids in maternal milk influence the risk of non-atopic eczema and asthma in infants, but not the risk of atopic sensitisationReference Wijga, van Houwelingen, Kerkhof, Tabak, de Jongste, Gerritsen, Neijens, Boshuizen and Brunekreef14, Reference Oddy, Pal and Kusel15. It has been shown that the levels of n-3 PUFA and conjugated linoleic acid are substantially higher in cows' milk from organic producers than from conventional producersReference Jahreis, Fritsche and Steinhart11, Reference Bergamo, Fedele, Iannibelli and Marzillo12, Reference Adriaansen-Tennekes, Bloksma, Huber, Baars, de Wit and Baars32. In the Netherlands, cows' milk from organic and conventional farms differed twofold in n-3 level (mean 10·6 v. 4·9 mg/g milk fat), whereas conjugated linoleic acid levels differed less (mean 6·3 v. 5·1 mg/g)Reference Adriaansen-Tennekes, Bloksma, Huber, Baars, de Wit and Baars32. In Germany, levels of conjugated linoleic acid were found to vary between 0·26 and 1·14 % of total milk fat methyl esters depending on season and production methods (intensive v. ecological)Reference Jahreis, Fritsche and Steinhart11.
In our most recent studies, we have reported that human breast milk of mothers consuming mainly organic dairy products contains higher levels of conjugated linoleic acid and trans-vaccenic acid compared with the milk of mothers who consume conventional dairy productsReference Rist, Mueller and Barthel23. In the Netherlands, the main sources of conjugated linoleic acid are of dairy originReference Voorrips, Brants, Kardinaal, Hiddink, van den Brandt and Goldbohm33. Dietary studies have indicated that margarine intake was linked to an increased risk of atopic diseasesReference Sausenthaler, Kompauer, Borte, Herbarth, Schaaf, von Berg, Zutavern and Heinrich34–Reference Dunder, Kuikka, Turtinen, Rasanen and Uhari36, while butter consumption and use of full-cream milk led to a decreased risk of developing these diseasesReference von Mutius, Weiland, Fritzsch, Duhme and Keil37–Reference von Ehrenstein, von Mutius, Illi, Baumann, Bohm and von Kries39. In the present study no discrimination was performed between a dairy diet comprising full-cream milk and one containing semi-skimmed milk, or between a margarine- v. a butter-based diet. We speculate that a high intake of n-3 fatty acids and/or conjugated linoleic acid from organic dairy products by the child is protective against eczema (independent of atopy), and that also the mother's intake of these fatty acids during pregnancy and lactation contributes to this protection.
In summary, the present prospective birth cohort study suggests that the consumption of organic dairy products, within the context of an organic diet, is associated with the development of eczema. Further studies to substantiate these results using more detailed and quantitative information are warranted. Information on the nutritional and non-nutritional composition of organic dairy products in relation to the development of eczema, separately from atopic disease, would also be valuable.
Acknowledgements
Dr A. P. Simões-Wüst and Dr R. Hooper are gratefully acknowledged for critically reading and helping to edit the manuscript. We would also like to thank all the parents and infants who participated in the present study.





