Worldwide dementia prevalence is almost 25 million cases and is projected to reach more than 81 million cases by the year 2040(Reference Ferri, Prince and Brayne1). Alzheimer's disease comprises 60 to 80 % of cases of dementia(2). The construct mild cognitive impairment(Reference Petersen3) identifies individuals with elevated risk for dementia(Reference Chertkow, Nasreddine and Joanette4), and progression from mild cognitive impairment to Alzheimer's disease can be as high as 10 % per year(Reference Mitchell and Shiri-Feshki5). Further, there are indications that even age-associated memory impairment, originally conceptualised as benign forgetfulness(Reference Crook, Bartus and Ferris6, Reference Neilsen, Lolk and Kragh-Sorensen7), can reflect very early neurodegeneration. Older adult samples with subjective memory complaints who meet criteria for age-associated memory impairment show degradation in the medial temporal lobe that is similar, albeit not as extensive, as that observed in subjects with mild cognitive impairment and Alzheimer's disease(Reference Goldman and Morris8), and longitudinal investigation has shown a trebling of risk for those categorised as having age-associated memory impairment(Reference Reid and MacLullich9, Reference Saykin, Wishart and Rabin10). Such findings imply that memory complaints and associated manifestations in everyday functioning can be meaningful indicators of neurodegeneration. Preventive interventions initiated when early memory decline is evident have the potential to forestall progression, most likely at the final stage when such treatment might be effective(Reference Cotman, Stern and Carstensen11).
Regulation of inflammation generally is reduced with ageing(Reference Franceschi, Capri and Monti12), and accelerated inflammation is implicated in neurodegenerative disorders such as Alzheimer's disease(Reference Giunta, Fernandez and Nilolic13). Berry fruits contain polyphenol compounds, which have anti-inflammatory and antioxidant properties(Reference Ishige, Schubert and Sagar14). Polyphenols also induce neuroprotective effects and influence neuronal signalling involved in memory function(Reference Ishige, Schubert and Sagar14–Reference Olszanecki, Gebska and Kozlovski16), and specific constituents of grape juice have exhibited neuroprotective effects(Reference Conte, Pellegrini and Tagliazucchi17).
Concord grape juice contains a variety of flavonoids and antioxidants, among them anthocyanins and proanthocyanidins(Reference O'Byrne, Devaraj and Grundy18, Reference Rice-Evans, Miller and Paganga19) and comparatively high levels of total phenolics(Reference Mullin, Marks and Crozier20). Information concerning flavonoid transport into the central nervous system and absorption into brain tissue is emerging. A number of recent studies have indicated that certain of these compounds, in particular anthocyanins, cross the blood–brain barrier, although specific mechanisms have not been established(Reference Singh, Arseneault and Sanderson21–Reference Youdim, Qaiser and Begley23). In addition, anthocyanins have been identified in brain regions that mediate cognition, including the medial temporal lobe and cortex(Reference Andres-Lacueva, Shukitt-Hale and Galli24), and hippocampal distribution has been associated with behavioural enhancement in animal supplementation studies(Reference Andres-Lacueva, Shukitt-Hale and Galli24, Reference Williams, El Moshen and Vauzour25).
Human trials have shown that short- and moderate-term supplementation with grape juice produces benefit in individuals with CVD, including increased serum antioxidant capacity and reduced LDL oxidation(Reference Rice-Evans, Miller and Paganga19), improved endothelial function(Reference Chou, Keevil and Aeschlimann26) and reduced platelet aggregation(Reference Freedman, Parker and Li27). Such findings are pertinent with respect to age-related cognitive decline because of the strong relationship between CVD and neurodegeneration(Reference Kalaria28–Reference Swan, Carmelli and La Rue31). Epidemiological studies indicate that consumption of fruits and vegetables is associated with lower risk of neurodegenerative disorders and better cognitive performance in the elderly(Reference Dai, Bornstein and Wu32–Reference Letenneur, Proust-Lima and Le Gouge34), and these effects have been attributed to the intake of a variety of flavonoid compounds with antioxidant and anti-inflammatory properties. Recently, a preliminary animal study demonstrated that ingestion of Concord grape juice for 6–8 weeks induced enhancement of cognitive performance in aged rodents(Reference Shukitt-Hale, Carey and Simon35).
We sought to assess the effect of supplementation with Concord grape juice on memory performance in older adults with early age-related memory decline in a controlled trial as an initial assessment of potential benefit in an at-risk sample. We also obtained data on mood, anthropometrics and metabolic parameters.
Methods
Participants
Participants were recruited from the general community with newspaper advertising soliciting older adults with early memory decline but not dementia for a dietary intervention study. We enrolled twelve participants (eight men, four women) with acquired memory changes such as forgetfulness and prospective memory lapses. The mean age of the entire sample was 78·2 (sd 5·0) years and the mean educational level was 14·1 (sd 2·9) years.
Procedure
Prospective participants were assessed with structured interview instruments to determine eligibility for study inclusion. The Academic and Medical History Questionnaire(Reference Krikorian, Zimmerman and Fleck36) was used to obtain demographic information and information regarding academic attainment, current and past medical conditions, and medication and substance use. Those with diabetes, substance-abuse disorder, or diagnosed psychiatric or neurological condition were excluded. The level of memory impairment was determined with the Clinical Dementia Rating(Reference Hughes, Berg and Danziger37), which elicits information from the participant and an informant (typically, spouse or adult child) about the nature and extent of cognitive decline as manifested in activities at home and in the community. The domains memory, orientation, problem solving, community affairs, home activities and personal care were evaluated to determine a dementia staging classification. Scores for each domain contributed to a global Clinical Dementia Rating classification with the memory domain weighted most heavily. Clinical Dementia Rating classifications include no impairment, mild decline, and mild, moderate and severe dementia. We enrolled individuals with mild decline and excluded those with Clinical Dementia Rating classifications indicating no impairment and those with mild, moderate and severe dementia. A sum of boxes score also was derived(Reference Lynch, Walsh and Blanco38). This score represented the arithmetic sum of the category scores across the six domains of functioning and served to quantify level of functional decline.
Seven subjects were randomly assigned to receive the placebo beverage and five were assigned to receive 100 % Concord grape juice. Placebo and juice were provided for the research by Welch Foods, Inc. (Concord, MA, USA). The placebo beverage contained no juice or natural polyphenol but was formulated to look and taste like grape juice and to have the same carbohydrate composition and energy load (3·0 kJ/ml). The intervention involved 12 weeks of daily consumption of juice or placebo with assessments at pre-treatment baseline and during the final week of the intervention. Previous human trials examining antioxidant effects, endothelial function and cardioprotection in healthy subjects and those with CVD used briefer interventions, of the order of 2–4 weeks(Reference Conte, Pellegrini and Tagliazucchi17, Reference Mullin, Marks and Crozier20–Reference Singh, Arseneault and Sanderson21). We chose a longer intervention period because our outcomes concerned cognitive–cerebral function in older adults, and there are indications in pre-clinical studies with other berry fruits that several weeks may be required for accumulation in brain regions(Reference Willis, Shukitt-Hale and Joseph39). We instituted a dosing schedule determined by body weight to maintain daily consumption between 6 and 9 ml/kg, a range consistent with other human grape juice trials(Reference Conte, Pellegrini and Tagliazucchi17, Reference Mullin, Marks and Crozier20–Reference Singh, Arseneault and Sanderson21). Individuals weighing 54 to 64 kg were prescribed 444 ml/d, those weighing between 65 and 76 kg consumed 532 ml/d, and those weighing between 77 and 91 kg consumed 621 ml/d. Participants were instructed to take daily quantities in equal, divided dosages with the morning, midday and evening meals.
The primary outcomes were neurocognitive measures of memory function administered before and after the intervention. The California Verbal Learning Test(Reference Delis, Kramer and Kaplan40) was administered to assess verbal learning and retention, and the Spatial Paired Associate Learning Test(Reference Krikorian41) was used to evaluate non-verbal memory. The California Verbal Learning Test is a list-learning and recall task, and the Spatial Paired Associate Learning Test assesses memory for visual-spatial information that is not amenable to verbal encoding. Both list-learning and paired associate tasks have been used in the context of cognitive ageing and dementia and are among the more sensitive measures of memory decline associated with neurodegeneration(Reference Greenaway, Lacritz and Binegar42–Reference Sahakian, Downes and Eagger44). We also assessed mood as a potential covariate of the cognitive measures with the Geriatric Depression Scale(Reference Yesavage, Brink and Rose45). We performed weight and waist circumference measures and obtained fasting blood samples for determination of serum glucose and insulin values.
Analyses of covariance were performed for each outcome factor to isolate effects of the intervention while controlling for individual differences(Reference Sheeber, Sorensen and Howe46). The outcome score from the final visit was the dependent measure and the corresponding score from the baseline visit and the depressive symptom score were covariate measures. We used eta squared values to derive Cohen's f effect size estimates, which are characterised as small (0·10), medium (0·25) and large (0·40)(Reference Cohen47).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Cincinnati Medical Institutional Review Board. Written informed consent was obtained from all subjects.
Results
At pre-intervention baseline there was a modest, non-significant difference for age between the groups (80 v. 75 years; t(10) = 1·8; P = 0·10). There was no group difference for educational level (13·4 v. 15·2 years; t(10) = 1·02; P = 0·32), index of functional impairment (Clinical Dementia Rating sum of boxes score 1·0 v. 1·0; t(10) = 0·0; P = 1·0), weight (74·3 v. 79·4 kg; t(10) = 1·04; P = 0·32) and waist circumference (92·7 v. 96·7 cm; t(10) = 0·81; P = 0·43). There was a group difference for level of depressive symptoms (Geriatric Depression Scale score 7·8 v. 3·0; t(10) = 2·19; P = 0·05), with greater depressive symptomology among the placebo subjects. However, the symptom level was not clinically elevated for either group(Reference Olin, Schneider and Eaton48).
Both the juice and placebo beverage were generally well tolerated, and there was no consistently reported adverse effect. Discrete concerns included, for example, increased frequency of urination associated with greater fluid consumption and aversion to the taste of the juice or placebo that developed over time.
As shown in Fig. 1, analysis of covariance demonstrated a significant effect (P = 0·04) for item acquisition across learning trials on the California Verbal Learning Test, indicating improvement for subjects in the Concord grape juice group relative to those receiving placebo. The effect size was moderate (Cohen's f = 0·28). Also, there were trends toward improved performances for the grape juice subjects with respect to delayed verbal recall (P = 0·10; Cohen's f = 0·33) and spatial memory (P = 0·12; Cohen's f = 0·67), although these were not statistically significant (Fig. 2).

Fig. 1 List acquisition performance assessing verbal learning on the California Verbal Learning Test. Values are adjusted means, with standard errors represented by vertical bars. Subjects consuming Concord grape juice demonstrated significant improvement (F(1, 8) = 5·55; P = 0·04; Cohen's f = 0·28).

Fig. 2 Delayed recall performance for verbal material on the California Verbal Learning Test (F(1, 8) = 3·37; P = 0·10; Cohen's f = 0·35) and for visual-spatial material on the Spatial Paired Associate task (F(1, 8) = 3·23; P = 0·12; Cohen's f = 0·67). Subjects consumed either Concord grape juice (![]() ) or a placebo drink (
) or a placebo drink (![]() ). Values are adjusted means, with standard errors represented by vertical bars.
). Values are adjusted means, with standard errors represented by vertical bars.
There was no appreciable effect of the intervention on depressive symptoms (adjusted Geriatric Depression Scale scores 5·0 v. 7·2; F(1,8) = 2·56; P = 0·14) and no effect on weight (77·5 v. 77·8 kg, adjusted values; F(1, 8) = 0·31; P = 0·58) or waist circumference (94·9 v. 95·5 cm, adjusted values; F(1, 8) = 0·24; P = 063). Fasting glucose values were not affected by the intervention (1011 v. 975 mg/l, adjusted values; F(1, 8) = 0·42; P = 0·53), but fasting insulin at 12 weeks was significantly elevated for the subjects consuming grape juice (10·0 v. 13·7 μU/ml, adjusted values; F(1, 8) = 6·07; P = 0·03). Table 1 contains the unadjusted mean scores for the outcome measures and shows the changes in absolute values from the baseline to final assessment.
Table 1 Unadjusted mean values for memory, mood, anthropometric and metabolic measures by group*
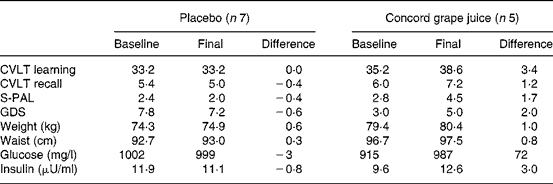
CVLT, California Verbal Learning Test; S-PAL, Spatial Paired Associate Learning Test; GDS, Geriatric Depression Scale.
* Baseline refers to measures obtained at the pre-intervention assessment. Final refers to measures obtained during the final week of the intervention. Difference = final score less baseline score.
Discussion
In this preliminary study we sought to assess the effect of moderate-term supplementation with 100 % Concord grape juice on cognition in older adults with early memory decline and found that memory function was improved with regular grape juice consumption. To our knowledge, this is the first controlled human trial examining neurocognitive response to this dietary intervention, and our findings are consistent with those of a recent animal study showing improvement in cognitive performance with grape juice supplementation in aged rodents(Reference Shukitt-Hale, Carey and Simon35). Our data do not provide information as to possible mechanisms leading to the beneficial effects. However, given the existing body of research concerning reductions of inflammatory and oxidative stress markers in human subjects with CVD and lower risk of age-related neurodegeneration with flavonoid consumption, these putative mechanisms would be primary considerations.
Recent preliminary data involving pharmaceutical TNF-α inhibition have suggested that acute functional improvement can be observed in patients with Alzheimer's disease(Reference Griffin49, Reference Tobinick and Gross50), indicating that suppression of a pro-inflammatory cytokine can ameliorate mental decline even in patients with substantially more advanced neurodegeneration than in our sample of mild cognitive impairment subjects. Accordingly, consistent application of a food product with anti-inflammatory effects over a brief to moderate timeframe also might be expected to induce cognitive–cerebral enhancement, especially in individuals with very early neurodegeneration. Pre-clinical data indicating absorption of anthocyanins in brain regions mediating cognition and associations with behavioural indices of cognitive function(Reference Andres-Lacueva, Shukitt-Hale and Galli24, Reference Williams, El Moshen and Vauzour25) also would support the notion that the demonstrated improvement in memory ability may reflect reduced inflammation and/or enhanced neural function in response to the intervention. While it is not yet clear to what extent and by what mechanism berry fruit constituents cross the blood–brain barrier, anthocyanins have been identified in specific brain tissues even when not detected in plasma(Reference Shukitt-Hale, Lau and Joseph51, Reference Kalt, Blumberg and McDonald52). And, it may be that consistent, moderate-term consumption is necessary to achieve sufficient concentrations in brain sites(Reference Willis, Shukitt-Hale and Joseph39). Further study of Concord grape juice supplementation for greater duration with memory and inflammatory marker outcomes will be important. Other putative mechanisms including reduction of oxidative stress and enhanced neuronal signalling also merit investigation, as these factors have been demonstrated to be important in animal studies with blueberry supplementation(Reference Casadesus, Shukitt-Hale and Stellwagen53, Reference Martineau, Couture and Spoor54).
Our preliminary data indicated increased fasting insulin for those who received grape juice in the absence of changes in weight and waist circumference. This finding was unanticipated and is provocative given the matched carbohydrate load in the placebo beverage and the fact that group differences in metabolic parameters were not observed before the intervention. It is possible that increased insulin secretion was induced by constituents of the grape juice other than sugars. There are data indicating that anthocyanins derived from blueberries influence metabolic function, in particular enhancing the actions of insulin(Reference Martineau, Couture and Spoor54, Reference Tsuda55). It may be that similar or related actions were induced in the present study, with consistent grape juice consumption resulting in increased insulin secretion. However, such notions are speculative, particularly given the small scale of this preliminary trial. Certainly, this issue warrants further investigation with respect to its reproducibility and the specific nature and basis for the effect.
The major limitation of the present study was the small sample size, which limited power to detect differences. However, the moderate to large effect sizes indicate that it would be worthwhile to conduct larger trials to evaluate the neurocognitive benefits and putative mechanisms of Concord grape juice supplementation in pre-dementia conditions. In view of the public health burden associated with neurodegeneration and Alzheimer's disease, in particular, safe, low-cost dietary interventions offer the possibility of inducing substantial benefit.
Acknowledgements
Funding and material support was provided by Welch Foods, Inc. (Concord, MA, USA).
R. K. conceived of the study and supervised the data collection, analyses, interpretation and manuscript preparation. T. A. N. and M. D. S. participated in data collection, interpretation and manuscript preparation. B. S.-H. and J. A. J. participated in manuscript preparation.
None of the authors has a financial interest in the supporting company or the outcome of the research activity.





