As the development of bone mass in childhood seems to be associated with the risk of osteoporosis and bone fractures in later adulthood, prevention of osteoporosis should ideally begin in childhood(Reference Faulkner and Bailey1, Reference Bachrach2). One modifiable factor that is discussed to play an important role in osteoporosis prevention is an adequate diet providing a sufficient nutrient intake (e.g. Ca, but also protein and vitamin D)(Reference Ilich and Kerstetter3). In this context, former recommendations(Reference Murray4) for an optimal diet for bone development in childhood often focused on milk as a food group and especially Ca as a nutrient. However, a positive impact of higher intakes of Ca on bone health has not been unequivocally demonstrated. Accordingly, the sole supplementation of Ca does not seem to significantly reduce the risk of fracture in childhood or later life(Reference Winzenberg, Shaw and Fryer5).
Recent findings from cohort studies showed significant associations between bone mineral content (BMC) or bone mineral density and other nutrients, e.g. protein(Reference Alexy, Remer and Manz6, Reference Vatanparast, Bailey and Baxter-Jones7), and dietary acid load(Reference Alexy, Remer and Manz6, Reference New, MacDonald and Campbell8). The question arises whether these dietary components might perhaps be equally or even more important for bone status in childhood than Ca. However, previous corresponding studies primarily focused on the impact of single or only a few dietary components, but did not consider a wide range of potentially relevant dietary factors. Thus, it is currently not possible to compare the relevance of dietary factors for the bone status in childhood.
Furthermore, the importance of potential dietary effects in comparison with known predictors of bone development (e.g. anthropometrical variables and androgens) remains to be evaluated. It is well known that muscularity(Reference Schonau, Schwahn and Rauch9) and a favourable body composition(Reference Taes, Lapauw and Vanbillemont10), i.e. the contribution of lean and fat mass, have a high beneficial impact on parameters of bone size. Additionally, results from a recent examination of the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study showed that the prepubertal level of the sex steroid 5-androstene-3β,17β-diol (androstenediol) predicts juvenile diaphyseal BMC and polar strength strain index in healthy children(Reference Remer, Manz and Hartmann11).
Hence, the objective of the present study was to identify the strongest long-term dietary predictors of prepubertal diaphyseal bone status of healthy children participating in the DONALD study and to investigate how important these dietary effects were in relation to the impact of muscularity-related anthropometrical variables (e.g. muscle area) and androstenediol levels. The objective of the present study was to compare for the first time the effects of dietary components with those of anthropometrical variables and sex steroids on radial bone status of healthy prepubertal children.
Material and methods
Study sample and design
The study sample consisted of a subcohort of participants from the DONALD study. The DONALD study is an ongoing open cohort study that started in 1985 in Dortmund, Germany, and investigates the relationship between nutrition, development and metabolism in subjects between infancy and early adulthood. Until now, more than 1200 subjects have participated in the DONALD study. About forty subjects are enrolled in the DONALD study each year. The regular, non-invasive assessments that take place in intervals of 1 year include 3 d weighed dietary records, anthropometry, urine sampling, as well as interviews on lifestyle and medical assessments. Tanner stages are assessed by a study paediatrician. Details of the study protocol have previously been described(Reference Kroke, Manz and Kersting12).
For the present examination, we considered those subjects who participated in a subproject of the DONALD study between July 1998 and June 1999 that included a single peripheral quantitative computed tomography (pQCT) measurement of the non-dominant forearm(Reference Neu, Rauch and Rittweger13, Reference Schoenau, Neu and Rauch14). Overall, the pQCT was carried out in 371 participants. Of these, 191 participants had at least three of five possible plausible 3 d dietary records in the 4 years before bone analysis and a 24 h urine sample at the time of the pQCT. Implausible dietary records were excluded using age- and sex-specific cut-off values for the ratio of reported total energy intake and predicted BMR(Reference Sichert-Hellert, Kersting and Schoch15) that was calculated by equations using measured height and weight(Reference Schofield16). Finally, 107 prepubertal (Tanner stage 1) children were included in the examination.
Ethical approval
The DONALD study was approved by the ethical committee of the Rheinische Friedrich-Wilhelms-Universität Bonn and with respect to bone analysis by the Federal Office for Radiation Protection (Salzgitter, Germany). All examinations and assessments are performed with parental, and later on with the children's written consent.
Dietary survey
In general, 3 d weighed dietary records are used for the assessment of food consumption in the DONALD study. Details of the dietary survey are provided elsewhere(Reference Kroke, Manz and Kersting12). In short, the parents of the children or the older subjects themselves weigh and record all foods and beverages before consumption as well as leftovers on 3 consecutive days. The first day of dietary recording can be chosen by the participant within a given period of time. Individual energy and nutrient intakes are calculated as arithmetic means of the three recorded days using our in-house nutrient database LEBTAB(Reference Sichert-Hellert, Chahda and Schaefer17), which contains detailed data on the energy and nutrients content of all recorded food items and is continuously updated.
For the present examination, we calculated the long-term consumption as the mean value of each dietary record in the 4 years before pQCT for dietary factors that are discussed to have a potential impact on bone parameters: Ca, protein, vitamin D and dietary potential renal acid load (PRAL) (all are given as densities, i.e. in relation to total energy intake). Dietary PRAL was calculated according to Remer et al. (Reference Remer, Dimitriou and Manz18) using the following equation:
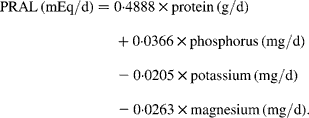
The approach of PRAL calculation omits Ca intake and therefore allows to separately assess the association of Ca with bone status. Furthermore, the model considers the different absorption rates of minerals and mean values of S-containing amino acids in proteins. The PRAL model has already been validated not only in adults(Reference Remer and Manz19) but also in children and adolescents(Reference Remer, Dimitriou and Manz18).
Anthropometric measurements
According to the study protocol, anthropometric measurements are performed from the age of 2 years onwards at each annual visit by trained nurses, with the children dressed in underwear only and barefoot. Standing height is measured to the nearest 0·1 cm using a digital telescopic stadiometer (Harpenden; Holtain Ltd, Crymych, UK). Weight is measured to the nearest 0·1 kg using an electronic scale (Seca 753E; Seca Gmbh & Co. KG, Hamburg, Germany). Triceps and subscapular skinfolds are measured on the right side of the body to the nearest 0·1 mm using a Holtain caliper (Holtain Limited, Crymych, UK). The sum of both skinfolds was used for the estimation of body fat percentage according to the equations of Slaughter et al. (Reference Slaughter, Lohman and Boileau20). Sex- and age-independent BMI standard deviation scores were calculated using the German national reference data(Reference Kromeyer-Hauschild, Wabitsch and Kunze21).
Steroid hormone analysis
GC–MS analyses were performed to detect individual 24 h urinary excretion rates of steroid hormones. Besides 5-androstene-3β,17β-diol (androstenediol), we quantified further androgen metabolites such as dehydroepiandrosterone (DHEA), 16α-hydroxy-DHEA and 5-androstene-3β,16α,17β-triol(Reference Remer, Boye and Hartmann22). The sum of DHEA and its 16-hydroxylated downstream metabolites represents the main direct metabolites of DHEA and DHEA sulphate(Reference Remer, Boye and Hartmann22). Androstenediol was considered as a potential exposure variable in linear regression models, because prepubertal levels of this sex steroid(Reference Mo, Lu and Simon23, Reference Lardy, Marwah and Marwah24) have already been identified as a strong predictor of bone status in late puberty in a previous examination of the DONALD study(Reference Remer, Manz and Hartmann11).
Peripheral quantitative computed tomography
A XCT-2000 device (Stratec, Inc., Pforzheim, Germany) equipped with a low-energy X-ray tube (38 keV) was used for the pQCT measurement of bone and muscle variables of the non-dominant forearm at the maximum circumference, i.e. at a distance to the ulnar styloid process of 65 % of the forearm length proximal to the radial endplate(Reference Neu, Rauch and Rittweger13). A 2 mm-thick single tomographic slice was sampled at a voxel size of 0·4 × 0·4 × 2 mm. Image processing and the calculation of numerical values were performed using the manufacturer's software package (software version 5.40).
Cortical area (CA), i.e. the cross-sectional area of cortical bone, was determined by detecting the outer and inner cortical bone contour at a threshold of 710 mg/cm3. The same threshold was also used for the identification of BMC, which is defined as the mass of mineral (in mg) per unit of axial bone length (in mm). For the determination of the periosteal circumference (PC), a cylindrical bone shape was assumed, whereby the outer bone radius was calculated as follows:
Strength strain index as an indicator of bone stability was calculated as the product of section modulus and cortical density normalised to the maximal physiological cortical density of human bones(Reference Schoenau, Neu and Rauch14). Besides these bone variables, we also measured the cross-sectional muscle area at 65 % of the ulnar length using a built-in software algorithm to separate muscle from bone and fat tissue.
Statistical analysis
All the statistical tests were performed using SAS® procedures (version 91.3, 2002–3; Statistical Analysis Systems, Cary, NC, USA). In all statistical tests, a P value < 0·05 was considered as significant.
Descriptive data are given as median and interquartile range. Sex differences in anthropometrics, hormones and dietary variables were tested using the Wilcoxon rank sum test. Stepwise linear regression analyses in three stages were applied to identify predictors of prepubertal bone variables (CA, BMC, PC and strength strain index). All variables, i.e. outcome and predictor variables, were checked for normality and log transformed when required before entering the regression models. In model 1, the anthropometrical variables at the time of the pQCT, i.e. local muscle area, age, sex and BMI standard deviation scores and body fat percentage as indicators of body composition, were considered as potential predictors. Only those variables with P values < 0·1 for the association with the respective bone variable were kept in model 2, which also accounted for 24 h urinary excretion of androstenediol at the time of the pQCT. Apart from relevant variables that were identified in steps 1 and 2, the final model tested for the effects of long-term dietary variables, i.e. dietary protein intake, Ca, vitamin D and PRAL. Standardised β-values were computed for the comparison of the effect sizes of the predictor variables on bone status. A post hoc two-tailed power analysis with α = 0·05 was performed that yielded a power of 0·80 002 for protein density.
Additionally, least-square means and 95 % CI of BMC and CA were computed for categories of muscle area, androstenediol excretion, dietary protein and Ca in order to graphically illustrate the impact of these potential predictor variables. Therefore, muscle area, androstenediol excretion, dietary protein density (g protein intake/MJ energy intake) and dietary Ca density (mg Ca intake/MJ energy intake) were subdivided into three categories, respectively (low, < 25th percentile; middle, ≥ 25th percentile and < 75th percentile; high, ≥ 75th percentile). Each of the least-square means was adjusted for the respective three other (continuous) predictor variables, i.e. muscle area, dietary protein and Ca in the case of the androstenediol categories.
As regression analyses mostly did not indicate any interaction between sex and the association of anthropometrical variables with bone variables in the basic models, data from girls and boys were pooled for analyses.
Results
Median and interquartile ranges of anthropometrical variables, steroid hormones and long-term dietary data including all the dependent and independent variables are given in Table 1. Despite a comparable BMI, the prepubertal body composition differed by sex as body fat was significantly lower in boys in comparison with girls. Additionally, we observed a higher excretion of androstenediol, DHEA and its 16-hydroxylated downstream metabolites in boys, and also higher values of muscle area and all bone variables except for PC.
Table 1 Anthropometrical variables, steroid hormones, bone characteristics and dietary characteristics in a sample of 107 healthy prepubertal children at the time of the peripheral quantitative computed tomography (pQCT) measurement
(Medians and quartiles)

Q, quartile; BMI-SDS, BMI standard deviation scores; DHEA, dehydroepiandrosterone; PRAL, potential renal acid load.
* Sex differences were tested using the Wilcoxon rank sum test.
† Long-term dietary characteristics in the 4 years before the pQCT measurement.
Long-term intakes of total energy and most absolute values of dietary variables were greater in boys compared with girls. However, relative values related to total energy intake showed no sex differences in dietary densities. In both sexes, long-term Ca intake was slightly below the recommended 800 mg/d for children aged 4–8 years proposed by the National Institute of Medicine(25). Vitamin D intake did not reach the recommended 5 μg/d, but was not much below the proposed adequate intake level of 1·9–2·5 μg that should be sufficient when sun exposure or skin pigmentation limits vitamin D skin synthesis(25). In contrast, both absolute intake and intake per kg body weight of protein were twice as high as the reference values(26). Median PRAL values indicated a modest dietary acid load.
Results from the first step of the linear regression models showed that log values of the forearm muscle area were strongly associated with all bone variables (Table 2). Additionally, age predicted all bone outcomes except for PC in model 1. BMI standard deviation score was significantly associated with BMC and CA in model 1. Sex and log values of body fat percentage were not associated with any bone variable. The associations for BMI standard deviation scores and age disappeared after consideration of androstenediol and dietary variables in the following models. Androstenediol levels were significantly positively associated with all bone parameters except for PC. These associations remained significant after consideration of dietary variables in model 3. Of all dietary variables, only protein showed a positive trend with BMC and also with CA. None of the other dietary variables entered the models.
Table 2 Predictors of bone variables in a sample of 107 healthy prepubertal children*

BMI-SDS, BMI standard deviation scores; PRAL, potential renal acid load.
* Results from stepwise linear regression analysis.
† Model 1 considered local muscle area, BMI-SDS, percentage body fat, age and sex.
‡ Model 2 considered variables identified in model 1 and androstenediol.
§ Model 3 considered variables identified in model 2 and intakes of protein, Ca, vitamin D and PRAL (all given in g/MJ).
With regard to all considered variables, forearm muscle area was the strongest predictor of all bone variables, with standardised β-values ranging from 0·64 to 0·71 in the fully adjusted models. Androstenediol secretion was found to be the second most important predictor with standardised β-values between 0·18 and 0·27. The standardised β-value of protein was 0·11.
The importance of protein was shown in Fig. 1, which illustrates the means of BMC and CA according to the categories of muscle area, androstenediol excretion, protein density and Ca density. Both BMC and CA increased especially between the lowest and the middle categories of protein intake. The slope of BMC and CA between these two groups was comparable to the increase between the lowest and the middle categories of androstenediol excretion. However, the P value for bone parameter differences by categories of protein density showed only a trend and was not statistically significant.

Fig. 1 Bone mineral content and cortical area by categories of muscle area (mm2) ((a) P diff < 0·001, (e) P diff < 0·001), urinary 24 h androstenediol excretion (μg/d) ((b) P diff = 0·013, (f) P diff = 0·029), protein density (g protein intake/MJ energy intake) ((c) P diff = 0·074, (g) P diff = 0·073) and Ca density (mg Ca intake/MJ energy intake) ((d) P diff = 0·874, (h) P diff = 0·963). All the variables were subdivided into three categories, respectively (low, < 25th percentile; middle, ≥ 25th percentile and < 75th percentile; high, ≥ 75th percentile). Data are least-square means (95 % CI) adjusted for the respective three other (continuous) predictor variables, i.e. muscle area, dietary protein and Ca in the case of androstenediol categories. Q, quartile.
Discussion
The main finding of the present examination was the positive trend between long-term protein intake and both BMC and CA in healthy prepubertal boys and girls, which was independent from the bone anabolic effect of muscularity and androgens. Bone-related muscle area was found to be the most important predictor of diaphyseal bone variables followed by the sex steroid androstenediol. The impact of a protein increase from the lowest to middle intake category was comparable with that of the sex steroid androstenediol varying between its lowest and medium excretion categories. None of the other considered dietary variables showed a trend with any of the bone variables.
The observed positive trends for protein intake support the increasing evidence of a bone anabolic effect of dietary protein(Reference Alexy, Remer and Manz6, Reference Vatanparast, Bailey and Baxter-Jones7, Reference Ginty27, Reference Conigrave, Brown and Rizzoli28). Recently, a positive effect of dietary protein was already demonstrated for adults in a meta-analysis of randomised controlled trials(Reference Darling, Millward and Torgerson29). In the past, an increase in protein intake was supposed to have a detrimental influence on bone parameters(Reference Rizzoli and Bonjour30) due to the acidifying impact of S-containing amino acids that leads to higher urinary Ca losses. Today, it is well known that an increase in protein intake also stimulates insulin-like-growth factor 1 secretion and thus may cause bone anabolism in total despite its existing acidifying effect(Reference Rizzoli and Bonjour30, Reference Bonjour, Ammann and Chevalley31).
A higher protein intake at a constant PRAL level has been recently shown to be significantly associated with stronger bone parameters in an examination of the DONALD study including children and adolescents(Reference Alexy, Remer and Manz6). The lack of significance for dietary protein in the present examination might be due to the overall relatively high protein intake in our prepubertal study population. The present results (Fig. 1) suggest that there may be no further bone anabolic effect of protein in the highest intake category and therefore no linear cause-and-effect relationship. The stimulating effect of protein on insulin-like-growth factor 1 secretion may become weaker at high protein intake levels and could be even levelled by the acidifying impact of S-containing amino acids. This assumption is supported by Ilich & Kerstetter(Reference Ilich and Kerstetter3) who supposed that diets which are particularly high in protein could even have a detrimental effect on bone. In their opinion, a protein intake of 1·0–1·5 g/kg body weight may be optimal for bone health(Reference Ilich and Kerstetter3). The present results indicate that even protein intakes of 2·0 g/kg (median in our sample) could be beneficial for bone health at least in prepubertal children. This beneficial effect of high protein intakes is in line with findings from Chevalley et al. (Reference Chevalley, Bonjour and Ferrari32) who observed that an increase in physical activity at a protein intake of 2·0 g/kg was associated with a higher BMC in prepubertal boys in comparison to a similar increase in physical activity at a protein intake of 1·5 g/kg body weight. However, the optimal protein intake for bone strength probably depends on protein sources(Reference Heaney and Layman33), Ca intake(Reference Ginty27), consumption of alkali-rich foods (fruits and vegetables)(Reference Alexy, Remer and Manz6, Reference Ginty27) and physical activity(Reference Chevalley, Bonjour and Ferrari32).
The American Academy of Pediatrics recommends adequate intakes of Ca in childhood and adolescence for the promotion of bone health and the prevention of osteoporosis(Reference Greer and Krebs34). In this context, Ilich & Kerstetter(Reference Ilich and Kerstetter3) suggested that bone variables would only respond to increases in Ca intakes if the baseline supply was deficient, i.e. additional Ca intake would have no further relevant effect on bone if the intake levels were already sufficient. The general good supply with nutrients in our sample could therefore be one reason for the missing association between Ca intake and bone parameters in the present examination, although mean long-term Ca intake was slightly lower than the recommended values. Heaney(Reference Heaney35) postulated that vitamin D status has to be considered together with Ca intake. As the DONALD study is not invasive, we were unable to include serum 25-hydroxy-vitamin D concentrations in our data analysis, but we did include dietary intakes of vitamin D. Skin vitamin D biosynthesis was not factored into the estimated intake of vitamin D. Therefore, data from the DONALD study might not be fully appropriate to evaluate the specific contribution of Ca and vitamin D for prepubertal bone status. However, based on the results of the present study, we hypothesise that differences in protein intake may have a stronger effect on diaphyseal bone parameters than increases in Ca intake in prepubertal children with a good nutrient supply.
In a previous data analysis of the DONALD study, a significant negative association for PRAL with several bone parameters was observed in a sample of 229 prepubertal and pubertal children(Reference Alexy, Remer and Manz6). The inverse relationship between dietary acid load and bone parameters, which was confirmed in further studies(Reference Macdonald, New and Fraser36, Reference Welch, Bingham and Reeve37), could rely on a higher bone resorption due to osteoclast stimulation(Reference New, MacDonald and Campbell8, Reference Macdonald, New and Fraser36). In contrast, we did not observe a significant association between PRAL and bone parameters in the present examination. The discrepancy with the above-mentioned analysis of the DONALD study could be due to the smaller sample size in our examination. Another reason for the missing association could be the small variation in dietary PRAL in the present study sample of prepubertal children. However, we cannot exclude that a higher sample size might have resulted in clearer associations for dietary PRAL and perhaps also for other nutrients with bone parameters.
For all examined bone parameters, muscle area of the forearm was the most important predictor variable. The strong relationship between bone and muscle area is the basis of the mechanostat theory, which posits that bone mass and architecture changes result from muscle-dependent stimuli(Reference Frost38). Accordingly, encouragement of physical activity to increase muscle mass from childhood onwards could be the most powerful tool for osteoporosis prevention.
The main limitation of the present study was the fact that only one pQCT measurement was carried out in each subject. Thus, we were not able to estimate effects of changes in dietary habits on the concurrent bone development. Accordingly, our data are not suitable to prove a causality link, but they are capable of identifying associations. Another limitation of our examination was the missing information on physical activity. However, we were able to indirectly adjust for physical activity using pQCT information on muscle area at the analysed bone site. We also had no reliable information on long-term sunlight exposure for the present study sample. Therefore, we are only able to discuss the role of dietary vitamin D, but not the importance of vitamin D in total.
An advantage of our approach could be the application of pQCT instead of the dual-energy X-ray absorptiometry method that is often used in other cohort studies. The pQCT method is known to provide a sensitive and more specific measurement of bone quality in children(Reference Leonard39–Reference Loud and Gordon41). Weighed dietary records, which were used in the DONALD study, are regarded as the ‘gold standard’ for dietary surveys(Reference Biro, Hulshof and Ovesen42). Another advantage is the consideration of long-term dietary data over 4 years of study participation, which produces a more accurate estimation of dietary behaviour than a single survey.
The present examination compared for the first time the effects of dietary components with those of anthropometrical variables and sex steroids on bone status of healthy prepubertal children. The present results suggest that muscle area has the strongest effect followed by the sex steroid androstenediol and protein intake, which was found to be the strongest dietary predictor of diaphyseal bone in prepubertal boys and girls with a good general nutrient supply. The bone anabolic impact of protein, increasing from the lowest to middle intake category, was found to be comparable with that of the sex steroid androstenediol, varying between its lowest and medium excretion categories in prepuberty. An adequate protein intake appears to be one of the most important components of osteoporosis prevention.
Acknowledgements
We are very grateful to the staff of the Research Institute of Child Nutrition for carrying out the anthropometric measurements and for collecting and coding the dietary records. The DONALD study is funded by the Ministry of Science and Research of North Rhine Westphalia, Germany. The contributions of the authors were as follows: L. L. and T. R. designed the research and performed the initial statistical analyses; L. L. conducted further analyses and wrote the manuscript; S. A. W. and E. S. provided critical input on the data analyses and on the early versions of the manuscript; T. R. supervised the study. None of the authors had any personal or financial conflicts of interest.





