European Regulation (EC) no. 1925/2006(1) on the addition of vitamins and minerals to foods has been in force since 1 July 2007. It consists in establishing a harmonised Community basis on such practices in order to guarantee both consumer safety and free movement of goods. The main issue remaining is to set maximum safe levels of vitamins and minerals in fortified foods which take into account tolerable upper intake levels (UL), micronutrient intakes from all dietary sources and population reference intakes. With the growing use of supplements(Reference Kelly, Kaufman and Kelley2), it becomes necessary to also consider nutrient intakes from this source.
Different models have already been developed to set maximum safe levels either for fortified foods or for dietary supplements(Reference Flynn, Moreiras and Stehle3–Reference Domke, Großklaus and Niemann8). Apart from the German Federal Institute for Risk Assessment (BFR) model(Reference Domke, Großklaus and Niemann7, Reference Domke, Großklaus and Niemann8), these models lead independently to maximum safe levels either for fortified foods (MSLf) or for dietary supplements (MSLs). But the MSLf and MSLs are dependent on each other and have to be taken into consideration simultaneously. To our knowledge there is no approach based on these maximum safe levels which attempts to estimate the overall vitamin or mineral intakes and to check their safety.
In 2000, the French Food Safety Agency (AFSSA) developed a probabilistic risk assessment approach for assessing the safety of maximum safe levels of vitamins and minerals, but only for food fortification(9). This method simulated the consequences of food fortification in terms of food safety by comparing high nutrient intakes with the UL. The present study had already been used as a validation tool by Flynn et al. (Reference Flynn, Moreiras and Stehle3). Moreover, a public health approach had been suggested to evaluate the relevance of vitamins and minerals for basic food fortification(10). Foods contributing the most to vitamin and mineral intakes were also defined(Reference Touvier, Lioret and Vanrullen11). Derived from AFSSA's previous work, the present study proposes a new approach to estimate the impact of consumption of fortified foods and dietary supplements on total micronutrient intakes in adults. This approach permits testing and validation of the maximum safe levels of vitamins or minerals for fortification and supplementation set by different mathematical models for food safety (Fig. 1). These values were simultaneously tested on recent representative French consumption data. It should be pointed out that nutritional benefit has not been considered in this approach. Consequently the present study only takes into account consumer safety.
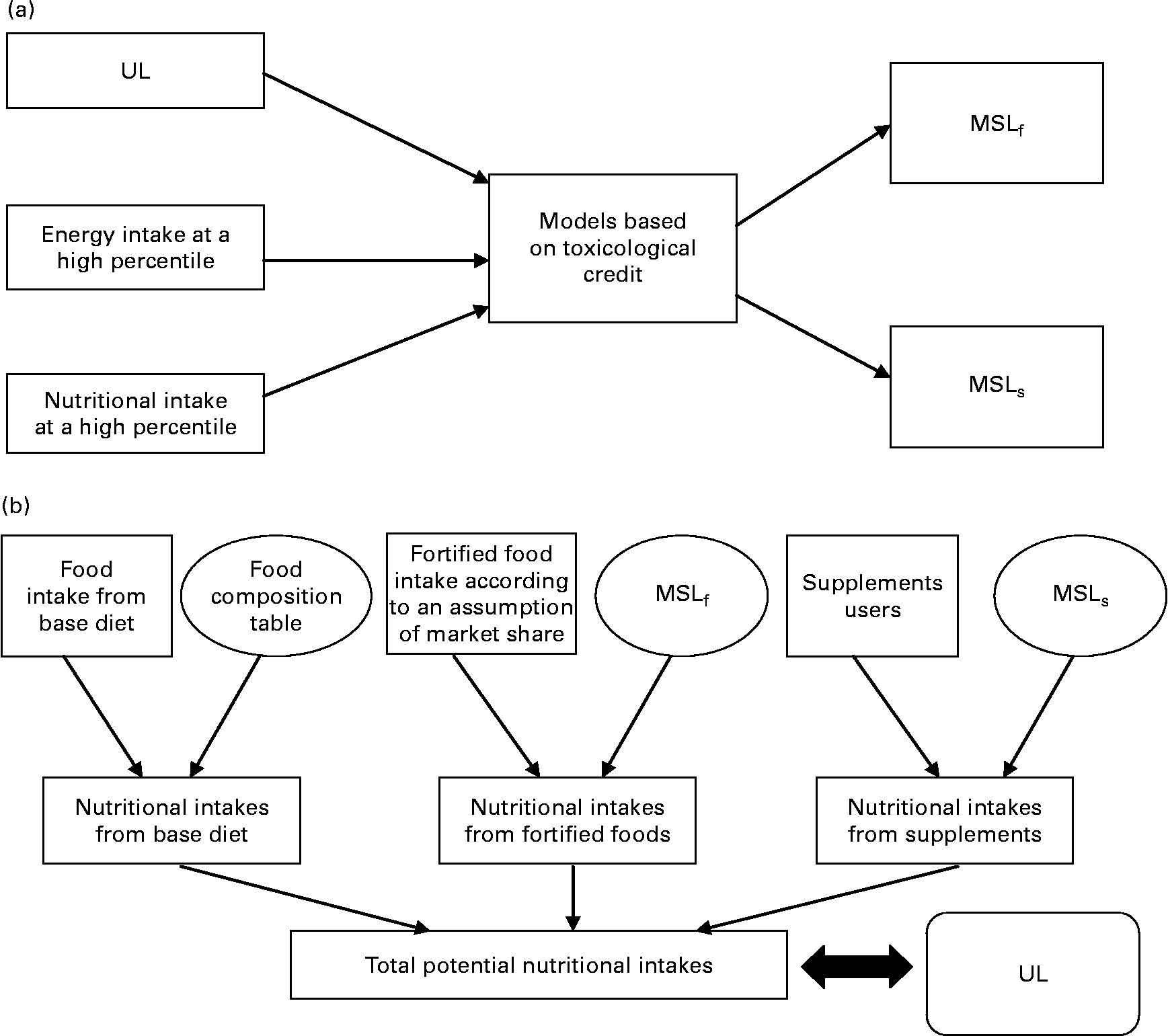
Fig. 1 Differences between principles of models which set maximum safe levels (a) and the French Food Safety Agency (AFSSA) risk assessment approach (b). UL, tolerable upper intake level; MSLf, maximum safe level for fortified foods; MSLs, maximum safe level for dietary supplements.
Subjects, methods and procedures used
Subjects and design
The present study used data from the second French Individual and National Study on Food Consumption (INCA2)(12). The survey was carried out by the Dietary Survey Unit–Nutritional Epidemiology (OCA-EN) of AFSSA between December 2005 and May 2007 in order to take into account seasonal variations in food intake. It involved 4079 participants aged 3–79 years (2624 adults and 1455 children aged 3–17 years) living in mainland France. The participants were selected using a three-stage cluster sampling technique stratified by region and size of urban area. The random selection of households was made from the French National Institute for Statistics and Economic Studies (INSEE) 1999 General Population Census(13) supplemented by the Survey Database for New Housing (BSLN) and Automated Processing and Information System for Basic Data on Housing and Premises (SITADEL) databases for homes built after 1999. The survey design and sampling frame were previously described in more detail in the INCA2 report(12) in particular.
To ensure the national representativeness of the final samples, data were weighted using a post-stratification algorithm(Reference Sautory14) according to sociodemographic criteria: region, size of urban area, size of household, sex, age, profession and social category of head of household, and season. Moreover, under-reporter adults (defined as participants who intentionally or unintentionally underestimate their energy intakes compared with their needs) were identified using the Goldberg et al. criterion and excluded from the analyses(Reference Goldberg, Black and Jebb15). The following analyses were based on a sample of 1918 adults considered as plausible reporters of energy intake.
The INCA2 survey was approved by the French data protection authority (Commission Nationale Informatique et Libertés) and the French national council for statistical information (Conseil National de l'Information Statistique).
Measurements
Consumption data
Dietary intakes were reported using a 7 d open-ended food record: food and beverages consumed at each main meal (breakfast, lunch and dinner) and between-meal snacks were described in detail. Portion sizes were estimated with the help of a validated picture booklet(Reference Hercberg, Deheeger and Preziosi16). Foods collected in the dietary records were codified according to a food list drawn up specifically for the study (containing forty-three food groups and 1342 food items). As the nomenclatures were compatible, consumption data were matched with the French food composition databank from the French Information Centre on Food Quality (CIQUAL)(17). Each food item is linked to a nutritional composition vector containing macronutrients, twelve vitamins and eleven minerals.
Food supplement data
For the first time in a nationally representative French dietary survey, consumption of dietary supplements was also assessed: firstly during the 7 d of the study using a 7 d dietary supplement record and secondly, during the previous 12 months, using a face-to-face interview. The definition of a food supplement used in the INCA2 study was not restricted to the regulatory definition but also included drugs containing micronutrients. Dietary supplements were therefore defined as vitamins, minerals, plant extracts or concentrates, amino acids, proteins, essential fatty acids, phyto-oestrogens, or any other supplement to the diet taken as pills, tablets, powder or syrup.
Maximum safe levels of vitamins and minerals in fortified foods and dietary supplements set by different models
Different institutions have developed models to estimate maximum safe levels of vitamins and minerals in fortified foods, as well as in dietary supplements. The Appendix summarises the different models and gives further details on the mathematical formulations. Flynn first proposed an original model (also called the International Life Sciences Institute (ILSI) model) for the safe addition of vitamins and minerals to foods(Reference Flynn, Moreiras and Stehle3, Reference Renwick, Flynn and Fletcher18). This model estimated the level of each nutrient that can be safely added to foods, to minimise the risk of excessive intakes. It was based on the following parameters: UL, current micronutrient intake from the base diet at the 95th percentile, energy intake at the 95th percentile and fraction of food on the market that is available for fortification. Another study from the Danish Institute for Food and Veterinary Research (DFVR)(Reference Rasmussen, Andersen and Dragsted5) proposed extending the model developed by Flynn et al. (Reference Flynn, Moreiras and Stehle3) by considering the common use of micronutrient supplements. The BFR proposed a method for deriving maximum safe levels of vitamins and minerals for both fortified foods and supplements(Reference Domke, Großklaus and Niemann7, Reference Domke, Großklaus and Niemann8). The toxicological credit or maximum amount for safe addition of vitamins and minerals to food (including dietary supplements) resulted from the difference between the UL and the highest percentile (95th or 99th percentile) of nutrient intake from the base diet. This residual amount constituted the total amount available for dietary supplements and fortified foods. The percentage of this amount allocated to dietary supplements or fortified foods may be between 0 and 100 %. However, the sum of the two percentages may not exceed 100 %. Richardson(Reference Richardson6) provided a system (also known as the European Responsible Nutrition Alliance (ERNA) model) for categorising nutrients according to the risk of exceeding their UL. The qualitative risk characterisation of nutrients was based on the concept of Population Safety Index. The micronutrients were classified into three groups: group A (no evidence of risk), group B (low risk) and group C (potential risk). This risk management model was applied to determine the maximum safe level for vitamins and minerals in dietary supplements.
Statistical analysis
French Food Safety Agency (AFSSA) simulation approach developed to estimate nutritional intakes taking into account the maximum safe levels for fortified foods and dietary supplements
The present study does not claim to add yet another mathematical model for setting the maximum safe levels for vitamins and minerals. The aim of this paper was to succeed in estimating the overall potential maximum nutritional intakes (Fig. 1). The MSLf and MSLs obtained using the different existing models were simultaneously tested according to different scenarios on the recent, detailed and representative French consumption data from the INCA2 survey(12). This estimation was based on a Monte Carlo probabilistic simulation. Total nutritional intakes in the population resulted from the sum of the three intake sources: base diet, i.e. ‘common’ foods, fortified foods and dietary supplements. Nevertheless, it should be pointed out that fortification and supplementation differ in terms of consumer decision process.
The estimation of nutritional intakes by the base diet is deterministic. It was obtained by linking French consumption data with the food composition table(17). However, the particular case of breakfast cereals should be pointed out. Indeed, since almost all breakfast cereals are already fortified in France, the food composition database of the base diet included these particular fortified foods.
Apart from breakfast cereals, since we did not have precise data on fortified food intake available to date, the method developed to estimate nutritional intakes from this source was probabilistic. First of all, a list of foods consumed in the INCA2 study that could be fortified was drawn up. Non-processed foods such as eggs, meat and poultry, offal, fruit, vegetables, traditional foods like some cheeses, beverages such as water and alcoholic drinks were excluded. The final list contained 743 items representing 55 % of the food items from the exhaustive INCA2 nomenclature. Then, for each individual, foods were randomly selected from this list based on a theoretically defined individual market share of the fortifiable foods. These were not mean market shares at the population level but maximum market shares that could be reached by consumers interested in vitamins and minerals. Market shares of 10 and 25 % appeared to be rational and realistic choices given existing information on fortification and 50 % represented a high assumption for the share of fortified foods that did not seem possible to exceed. The case of a market share of 0 % was also considered and corresponded to a situation with only supplements and no fortification in the diet. According to this method, each participant in the INCA2 study would therefore consume, in theory, some foods that were fortified and others that were non-fortified throughout the week. For randomly selected fortified foods, the nutritional intake was calculated using the MSLf estimated by the models previously presented instead of the nutritional content from the food composition table.
For the fraction of the population consuming dietary supplements, intakes through such products had to be estimated. The proportion of adult supplement users in INCA2 was about 11 % for the week and nearly 20 % considering the last 12 months(12). Our assumption was that, on every day of consumption, supplement users took the maximum safe level (defined by the maximum daily amount MSLs) for every nutrient considered. Consequently, the supplement composition table was not used in the present study to estimate the nutritional intake from supplements. Intakes via this source were estimated using the maximum safe levels set for supplements. Daily intakes from dietary supplements were then added to the intakes estimated from the base diet and fortified foods for the subpopulation of supplement users declaring consumption during the 7 d of the survey period.
The simulations to estimate the overall maximum daily intake from all sources were performed nutrient by nutrient. Among the twenty-three micronutrients available in our database, ten had a UL defined by the Scientific Committee on Food(19): five vitamins (retinol, vitamins D, E, B6, folic acid) and five minerals (Ca, Cu, I, Se and Zn). For these ten nutrients, it was possible to ascertain the proportion of individuals presenting a risk of exceeding the UL, according to the different scenarios and for a given market share of fortified foods.
Statistical analyses were conducted using the Statistical Analysis System software package version 9.1 (SAS Institute Inc., Cary, NC, USA).
Description of the different scenarios tested
To estimate the potential maximum daily nutritional intakes from the different dietary sources (base diet, fortified foods and dietary supplements) with the procedure described above, there were many conceivable scenarios based on the different calculated MSLf and MSLs.
For fortified foods, three series of MSLf were obtained from the ILSI model(Reference Flynn, Moreiras and Stehle3), the Danish Institute for Food and Veterinary Research (DFVR) model(Reference Rasmussen, Andersen and Dragsted5) and the BFR model(Reference Domke, Großklaus and Niemann7, Reference Domke, Großklaus and Niemann8), to which a fourth series has been added (conventional assumption of fortification up to 15 % of the European recommended daily allowance for 100 kcal (418 kJ) of food, which is not related to the regulation on nutritional labelling). For dietary supplements, there were two series of values (maximum daily intake) obtained from the ERNA(Reference Richardson6) and BFR(Reference Domke, Großklaus and Niemann7, Reference Domke, Großklaus and Niemann8) models, as well as an additional series based on the French regulatory values(20).
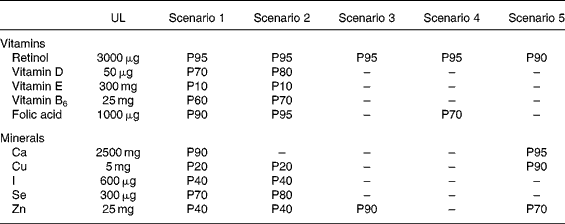
A selection of five scenarios summarised in Table 1 was explored out of the twelve possible combinations of the four series of MSLf and the three series of MSLs: the model presented by the BFR, setting the maximum safe level for both fortified foods and dietary supplements, is a scenario in itself (scenario 4); the combination of ILSI's fortification levels and ERNA's dietary supplement levels is a scenario presenting high levels in both cases (scenario 1); lastly, with no other data on dietary supplements, it was agreed to combine the three other models setting fortification levels with the values set by the French regulation in 2006 for dietary supplements (scenarios 2, 3 and 5). Table 2 summarises the different MSLf and MSLs values obtained by the models and considered in the five different scenarios.
Table 1 Selected scenarios combining maximum safe levels for fortified foods and for dietary supplements out of the twelve possible combinations
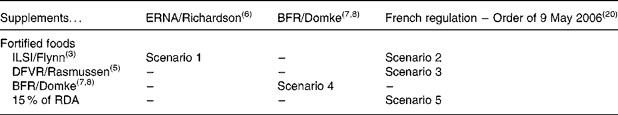
ERNA, European Responsible Nutrition Alliance; BFR, Federal Institute for Risk Assessment; ILSI, International Life Sciences Institute; DFVR, Danish Institute for Food and Veterinary Research; RDA, European Recommended Daily Allowance.
Table 2 Maximum safe level (MSL) values estimated using the different models for ten selected nutrients and applied to the five scenarios of simulation

MSLf, maximum safe level for fortified foods; MSLs, maximum safe level for dietary supplements.
* 100 kcal = 418 kJ.
Results
Nutrient intake from the base diet
Table 3 presents the daily intake distributions (mean, sd, percentiles) from the base diet (excluding fortified foods and dietary supplements) for the ten nutrients selected among non-under-reporter adults in the INCA2 study. This table also indicates for each micronutrient and for the adult population the estimated average requirement and the French population reference intake values as well as the UL set at European Union level (European Food Safety Agency and the Scientific Committee on Food). For some nutrients such as retinol, folic acid, Ca, Cu and Zn, the UL are not very far from the higher percentile of distribution (less than twice).
Table 3 Nutrient intake distributions from the base diet (excluding dietary supplements and fortified foods) from the second French Individual and National Dietary Survey (INCA2): non-under-reporter adults (n 1918)

ANC, French population reference intake; EAR, estimated average requirement; P, percentile; UL, European tolerable upper intake level.
* ANC: male, 1·8; female, 1·5. EAR: male, 1·5; female, 1·2.
† ANC: male, 330; female, 300. EAR: male, 234; female, 213.
‡ ANC: male 20–64 years and female 20–54 years, 900; others, 1200. EAR: male 20–64 years and female 20–54 years, 693; others, 924.
§ ANC: male 20–64 years, 2; others, 1·5. EAR: male 20–64 years, 1·5; others, 1·2.
∥ ANC: male 18–19 years and female 18–54 years, 50; male 20–64 years and female 55–75 years, 60; others, 70. EAR: male 18–19 years and female 18–54 years, 38·5; male 20–64 years and female 55–75 years, 46·2; others, 53·9.
¶ ANC: female 18–19 years, 10; male 65–75 years and female 55–75 years, 11; male 20–64 years, 12; male 18–19 years, 13. EAR: female 18–19 years, 7·7; male 65–75 years and female 55–75 years, 8·5; male 20–64 years, 9·2; male 18–19 years, 10.
Results with the different scenarios
Results regarding nutritional intakes using our approach do not represent actual current intakes in the French adult population, but rather potential nutritional intakes under hypotheses taking into account maximum safe levels for fortified foods and dietary supplements and assumptions about the proportion of fortifiable foods in the diet of consumers.
To illustrate the utilisation of the method developed, Table 4 summarises the results obtained for the ten nutrients according to the five selected scenarios, in the event that fortified foods would account for 25 % of fortifiable foods. This median, realistic assumption corresponds to a consumer for whom 25 % of the foods that could be fortified are actually fortified.
Table 4 Percentiles (P) beyond which the European tolerable upper intake levels (UL) may be exceeded: results for the ten nutrients according to the five different scenarios in an adult population (n 1918)*
* As an example, the UL set for Zn (25 mg) may be exceed by 60 % of the population with scenario 2 and by 30 % with scenario 5.
The first two scenarios (using both ILSI's maximum safe levels for fortified foods) are those for which the UL are exceeded for all the nutrients at different percentile levels depending on the nutrient. To be more precise, in terms of vitamins, with scenarios 1 and 2 the UL may be exceeded by 20 to 30 % of the population for vitamin D, 90 % for vitamin E, 30 to 40 % for vitamin B6 and 5 to 10 % for folic acid. Considering minerals, for the same two scenarios, the UL set for Cu intakes may be exceeded by 80 % of the population, for iodine and Zn intakes by 60 % of the population and for Se intakes by 20 to 30 % of the population. Scenarios 3 and 4 are those which incur the least risk of exceeding the UL for all ten nutrients. Scenario 5 has fairly similar results to scenarios 3 and 4. However, with scenario 4, 30 % of the population may still exceed the UL for folic acid and 5 % for retinol. With scenario 5, 5 to 30 % may exceed the UL for different minerals (Ca, Cu and Zn).
For each scenario, other assumptions on the individual market share have also been tested: 0 % (no fortification, only supplementation, results not shown), 10 %, and 50 %. Opting for a 50 % market share instead of 25 % provided the same classification within the different scenarios, depending on their more-or-less conservative options, but led naturally to less protective results. For example, in scenarios 1 and 2 with the market share of 50 %, the UL set for Zn intakes may be exceeded by 95 % of the population, whereas this limit was exceeded by ‘only’ 60 % in the event of a market share of 25 %. Conversely, if the market share of fortified foods is quite low (10 %), the UL may be exceeded for Zn by a lower proportion of adults in the first two scenarios (30 % instead of 60 %).
Discussion and limits
The present study aimed to test the acceptability of the MSLf and MSLs values set by different models to prevent consumers from exceeding the UL defined for vitamins and minerals. The maximum safe levels in fortified foods and dietary supplements result in nutritional intakes that may frequently (scenarios 1 and 2) or rarely (scenarios 3, 4 and 5) exceed the UL. When considering all ten nutrients simultaneously, this simulation approach demonstrated that scenarios 1 and 2 did not provide enough protection for consumers: the risk of exceeding UL varies depending on the nutrient but is real for all of them. Scenarios 3 and 4 seemed to be safer for consumers: the risk exists for only two nutrients (out of the ten) and for a small proportion of the population (5 or 10 % with scenario 3, 30 % for folic acid with scenario 4). The higher intakes observed with the first two scenarios can be explained mainly by the higher values of the maximum safe levels obtained for fortified foods (see Table 2). Indeed, an additional simulation considering only fortified foods shows that the UL are largely exceeded for certain vitamins and minerals (vitamin E, Cu, Zn, I). On the other hand, a simulation considering only supplements does not show that UL were exceeded to a significant extent. The initial mathematical model proposed by Flynn et al. (Reference Flynn, Moreiras and Stehle3) did not take into account the higher intakes from supplements and led to MSLf values that are quite high. For this reason we remain cautious about the use of this model.
It should be pointed out that the main goal of the study was to describe the simulation method and not to provide the real intakes of French consumers. Therefore, several assumptions and simplifications have been made.
First, the simulations performed in the present study concerned only adults and were therefore restrictive. In order to be comprehensive and offer more protection to the whole population, children should also be considered. Children could also consume the same fortified foods that adults consume and reach the UL more quickly because of lower values. But these simulations are more difficult to perform in the child population partly because all the parameters for setting the maximum safe level are not always available. For example, UL are not available for all nutrients for the different age ranges of children. The issue of which child age range should be taken into consideration is also raised. Some assumptions had to be made to remove some of these difficulties. Additionally, new simulations could be performed considering adults as well as children. In order to offer more protection, calculations of the MSLf should be carried out with the parameters of the 3- to 10-year-old children because they are more restrictive (UL for instance).
Second, we considered on one hand in our simulations that food supplement users consumed only one product per d and we assigned the maximum daily dose calculated (MSLs) for the ten nutrients considered to all users. This hypothesis concerning supplement users is not maximalist. Indeed, about one-third of adult supplement users consumed more than one product per d during the week in the INCA2 survey(12). Therefore, the hypothesis of only one supplement per d could underestimate the real intakes and the possible exceeding of UL among supplements consumers. On the other hand, we supposed that all the supplements consumed contained the ten nutrients, which tends to overestimate actual intakes. Besides, simulations did not take into account the probable interactions between nutrients. Because of the complexity of modelling these interactions we were not able to take them into account. We assumed that they might be capable of limiting or increasing absorption and bioavailability.
It should also be pointed out that we treated as supplement users any individuals who declared consumption of such products during the 7 d of the survey and filled in a supplement record. Consequently, the percentage of users considered is lower than the percentage of long-term users over the 12-month period (11 v. 20 %). This choice seemed more rational, as micronutrient intakes from the base diet were also estimated during a 7 d period with no correction for intra-individual variability. However, the number of days needed to correctly assess intakes in France was estimated at 5 d for Ca and 10 d for vitamin E(Reference Mennen, Bertrais and Galan21). Except for retinol, whose intake is linked to rarely consumed foods such as offal, one may consider that an overestimation of high percentiles is not that significant and that the method is conservative overall.
Intakes of vitamins and minerals from fortified foods were simulated without considering any correlation with nutrient intakes from the base diet or with supplement intakes, because of the lack of data to support this correlation in France. However, the specific food habits of supplement users were considered because diet and supplement use were assessed at the same time for the same individuals. Indeed, previous publications have shown that supplement users do not have the same food habits as the general population(Reference Touvier, Niravong and Volatier22).
Moreover, it should be stressed that fortification and supplementation differ in terms of the consumer decision process. The decision to consume dietary supplements is voluntary and individual while the consumption of fortified food can be completely unintentional. However, nutritional intakes are estimated based on all sources (including fortified foods or supplements). Taking into account all the sources is justified in order to prevent consumers from exceeding UL. By choosing individual random exposure to fortified foods, we assume independence in fortified food consumption which is a strong hypothesis. Some data on the preferential purchasing of fortified foods are available in the INCA2 survey, through a self-administered questionnaire. The results show that 60 % of the adults did not consider the fortified status of foods when choosing them. But, women chose fortified foods more frequently whereas men avoided them more frequently(12). However, as one can consume fortified foods without knowing it, we chose to assign consumption of fortified foods to every subject of the sample, which was a conservative option for the food safety of consumers. Nevertheless, the method proposed here could be improved in the future by taking into account additional information that is not yet available on detailed consumer behaviour regarding fortified foods.
Finally, the nutritional intakes are estimated based on all sources (i.e. fortified foods or supplements). Taking into account all the sources is justified in order to prevent consumers from exceeding UL. Although in France the consumption of dietary supplements is not very developed, in some other countries supplements may represent a non-negligible source of nutrients in the daily diet(Reference Balluz, Okoro and Bowman23, Reference Harrison, Holt and Pattison24).
The simulations performed in the present study were done after removing under-reporters who represent about one out of four individuals. In fact, some participants in dietary surveys tend to under-report their food intake. Consequently, most of dietary surveys report energy intake levels below needs. Therefore, we consider it justified to exclude under-reporters to more accurately describe nutritional intakes. However, in order to check the possible influence of this choice on results, we performed the same simulation without the exclusion of under-reporters. The results were very close and the conclusions were the same.
All breakfast cereals in the French food composition table are already fortified. Therefore, we did not include them in the list of fortifiable foods. This assumption may have underestimated the present results because breakfast cereals could be more highly fortified than they presently are. Later, it could be possible to consider them as fortifiable food and to assign them the MSLf values set by the different models if these values are higher.
With the names and brands of products collected in the INCA2 study, an ad hoc nutritional composition table of fortified foods will be soon developed. It will be then possible to take into account the true intakes from this source. However, the probabilistic method remains useful for making assumptions about the possible evolution of the market share of fortifiable foods.
Very recently in 2008, Flynn(Reference Flynn25) proposed an improvement of his model for setting the maximum safe levels in fortified foods by taking into account the supplement intake and not only the base diet intake. Testing the result obtained from this new model could be done in the future using our approach. It is also possible to modulate parameters from the model to integrate security factors in order to test other different options.
Conclusion
A rational risk assessment approach is useful to protect all consumers from the risk of exceeding UL. The simulation work conducted in the present study gives a clearer insight into this new situation. We assessed the maximum vitamin and mineral intakes in fortified foods and dietary supplements using different models.
The risk assessment approach developed to estimate the overall potential maximum nutritional intakes by integrating the maximum safe levels for fortified foods and dietary supplements set by different models is useful for ensuring consumer protection. Nevertheless, given the parameters considered, the results presented in this paper concern only the adult population. This method may be subsequently used to test any other maximum safe level for vitamins and minerals. In future, other hypotheses on parameters could be proposed to reinforce safety factors and lead to new maximum safe levels that could be tested with our method.
The probabilistic assessment approach is quite common in risk analysis in terms of food safety(Reference Sioen, Bilau and Verdonck26, Reference Cao, Suzuki and Sakurai27). However, this kind of approach is not used very often in the field of nutritional regulation, and so this kind of work is quite innovative. It allows scenarios to be tested in terms of public health and consumer protection. This method can be used with any other proposed maximum safe limits for fortified foods or dietary supplements, for example, values proposed directly by risk managers without the support of a model. Furthermore, this probabilistic risk assessment method has been applied to French intake data, but it could also be applied to other national dietary surveys with food consumption data and occurrences of supplement intake.
Acknowledgements
The INCA2 study was supported by the French Food Safety Agency (AFSSA).
We would like to thank the Institut de Sondage Lavialle (ISL) team for the collection of the data and all the participants for their cooperation in the study.
The contributions of the authors were as follows: A. D. performed the simulations and wrote the manuscript; J.-L. V. supervised the simulations and contributed to interpretation and discussion; S. W., J. G. and E. K. contributed to the interpretation of the data in the nutrient risk assessment unit directed by I. M.; J.-L. V. and L. L. coordinated and supervised the INCA2 study; C. D., S. L., G. C. T., M. T., A. D., J. L. V. and L. L. contributed to the conception, design and data collection of the INCA2 study. All authors critically reviewed and approved the manuscript in its final version.
The authors declare no conflict of interest.
Appendix Short presentation of the different models setting the maximum safe level for fortified foods (MSLf) and maximum safe level for dietary supplements (MSLs)

ILSI, International Life Sciences Institute; DFVR, Danish Institute for Food and Veterinary Research; ERNA, European Responsible Nutrition Alliance; BFR, Federal Institute for Risk Assessment.
* 100 kcal = 418 kJ.








