Adolescent pregnancy in Jamaica, as elsewhere, is a major health concern as it is associated with a high prevalence of low birth weight(Reference Jackson, Eggleston and Lee1, Reference Fraser, Brockert and Ward2). It has been proposed that the adolescent gives birth to a smaller baby because there is competition between mother and her foetus for a limited supply of nutrients to support her own growth plus growth of her reproductive tissues and her foetus(Reference Scholl and Hediger3). As pregnancy progresses, the requirement for amino acids rises in order to sustain faster rates of protein synthesis(Reference Duggleby and Jackson4) and gluconeogenesis(Reference Kalhan, Rossi and Gruca5). During pregnancy, the significant lowering of plasma amino acids after a brief fast suggests that the balance between maternal amino acid supply and utilisation is very tight, more so for the gluconeogenic amino acids(Reference Felig, Kim and Lynch6, Reference Fitch and King7). That is, the breakdown of body proteins plus de novo synthesis is not sufficient to meet the requirements for dispensable amino acids even after a brief fast.
In pregnancy, dispensable amino acids represent the largest source of maternal amino acid nitrogen transferred to the foetus(Reference Lemons, Adcock and Jones8). Apart from their unique individual biochemical functions, dispensable amino acids such as glycine are synthesised de novo because there is a high demand for these amino acids as precursors for the synthesis of proteins, peptides and numerous essential biochemicals and metabolites. For example, besides being a neurotransmitter and a 1-carbon donor, glycine is precursor for the formation of purines, porphyrins, creatine, glutathione and, through its interconversion to serine, phospholipids and cysteine(Reference Jackson9). During pregnancy, glycine supply becomes even more important because availability of 1-carbon is necessary for both the synthesis and methylation of DNA and, hence, for cell division and growth of the foetus. There is also a higher fetal demand for glycine as pregnancy progresses to late gestation when most glycine-rich collagen is synthesised(Reference Meier, Teng and Battaglia10). Further, stable isotope studies in the sheep have shown that fetal serine supply is exclusively provided by hepatic synthesis from maternal and placental glycine(Reference Geddie, Moores and Meschia11, Reference Chung, Teng and Timmerman12). It is therefore possible that adolescents give birth to more low birth weight babies because they cannot keep up with the increased demand for glycine as pregnancy progresses. At present it is not known whether pregnant adolescents can produce sufficient glycine to meet overall demands especially after brief periods of food deprivation. We propose that the pregnant adolescent will not be able to increase glycine production to the same extent as her adult counterpart as pregnancy progresses to late gestation. To test this hypothesis, glycine flux was measured in pregnant adult women and in adolescent girls at the end of the first and beginning of the third trimester of pregnancy. The present study is part of a larger study of glucose and amino acid metabolism in pregnancy.
Materials and methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethics Committee of the University of the West Indies and by the Institutional Review Board for Human Subject Research of Baylor College of Medicine & Affiliated Hospitals. Written informed consent was obtained from each study subject at recruitment.
Pregnant adolescents and adult women who were below 13 weeks of gestation and registered at the antenatal clinic at the University Hospital of the West Indies were invited to join this prospective study, and were enrolled consecutively. Women with chronic illnesses, such as diabetes mellitus, hypertension, heart disease or genetic abnormality such as sickle cell disease, or women with multiple gestation, were excluded. Sixteen subjects, eight adolescents and eight adult women with normal BMI (>18·5 and ≤ 25 kg/m2) were enrolled into the study. Maternal weight was measured to the nearest 0·01 kg using a Tanita digital scale (CMS Weighing equipment Ltd, London UK), and height was measured to the nearest 0·1 cm using a stadiometer (CMS Weighing equipment Ltd). Maternal weight was repeated at 28 weeks of gestation and at 36 weeks of gestation.
Once written informed consent was obtained, a questionnaire that provided information on demographics; socio-economic status; use of substances such as cigarette, alcohol, marijuana and cocaine; and menstrual details was administered to all the subjects. A socio-economic score was calculated using education and occupation of the mother and father, household possessions and a crowding index (calculated as the number of habitable rooms in the dwelling divided by the number of people living in that dwelling). A higher score denoted a better socio-economic status. Gestational age was determined by the last menstrual period and confirmed by an ultrasound measurement performed at the time of the first experimental study. Maternal weight gain from the first to the third trimester (12–36 weeks of gestation) was calculated. Birth weight was measured to the nearest 0·01 kg using a Tanita model 1583 digital baby scale (CMS Weighing equipment Ltd), crown–heel length was measured to the nearest 0·1 cm using a Harpenden infantometer (CMS Weighing equipment Ltd), and head circumference was measured with a fibre glass tape measure.
Tracer infusion protocol
All the subjects were studied after an 8 h fast on two occasions, at the end of the first trimester (12·8 (se 0·39) weeks of gestation) and the beginning of the third trimester (27·8 (se 0·4) weeks of gestation). Subjects were admitted to the obstetrics ward in the evening, and had their last meal at 22.00 hours. An intravenous catheter (Sesecure, 18 G. Morningside Pharmaceuticals Ltd, Oadby, Leicester, UK) was inserted into the antecubital vein of one arm 8 h later for the infusion of isotopes, while another catheter was inserted in an antiflow direction into the dorsal vein of the contralateral hand for drawing blood samples. This cannula was kept patent with intermittent small infusions of heparinised saline.
A sterile solution of 2H2-glycine (Cambridge Isotope Laboratories, Woburn, MA, USA) was prepared in sterile isotonic saline. After a baseline blood sample was collected, a primed, 6 h continuous infusion of 2H2-glycine (prime = 4 μmol/kg, infusion = 4 μmol/kg per h) was started. Blood was collected at 3, 4, 5 and 6 h of the infusion. At the end of the infusion, the catheters were removed, and the subjects were given lunch and discharged.
Laboratory analyses
Glycine and serine concentrations were measured by reverse-phase HPLC on a Hewlett-Packard 1090 HPLC equipped with a Model HP 1046A fluorescence detector (Hewlett-Packard, Avondale, PA, USA). To measure its tracer/tracee ratio, glycine was isolated from plasma by ion exchange (Dowex 200 × ) chromatography, and was converted to the n-propyl ester, heptafluorobutyramide derivative. The tracer/tracee ratio was determined by negative chemical ionisation GC-MS analysis by selectively monitoring ions at m/z ratios 293–295 using a Hewlett Packard 5890 quadrupole mass spectrometer (Hewlett Packard, Palo Alto, CA, USA).
Calculations
Endogenous flux (Q) of glycine, that is glycine derived from protein breakdown plus de novo synthesis, was calculated using its plasma plateau tracer/tracee ratio in the steady-state equation:
where Tr/TrInf is the tracer/tracee ratio of the infused tracer, Tr/Trpl is the tracer/tracee ratio of glycine in plasma at isotopic steady state and I is the tracer infusion rate.
Statistical analysis
Data are expressed as mean values with their standard errors. Differences in subject characteristics between the adolescent and adult groups were assessed by unpaired t test. Differences in glycine variables between the groups were analysed by mixed-model (repeated measures two-factor) ANOVA. This model included the two age groups (adult and adolescent) and time of pregnancy (first and third trimesters). Post hoc comparisons were performed using Bonferroni's test. Because each group had different body weights, total body glycine flux was not compared between the groups. Only within the group, comparisons were made from trimesters 1 to 3 using the paired t test. Tests were considered statistically significant if P < 0·05. Relationships between glycine flux and maternal and baby variables were sought using the Spearman correlation. Data analyses were performed with GraphPad Prism version 4 software (GraphPad Software, San Diego, CA, USA).
Results
There was no report of substance abuse such as cigarette, alcohol, marijuana or cocaine use during pregnancy among the sixteen subjects, and there was no difference in socio-economic scores between the adults and adolescents, 44·4 (se 3·8) and 36·3 (se 3·0), respectively. Maternal characteristics at the first and second studies are presented in Table 1. Maternal weight and BMI in the first study were significantly lower (P < 0·05) in the adolescent girls compared with their older counterparts. Weight gain from the first trimester to the end of the third trimester, 12–36 weeks of gestation, was significantly greater in the adolescents compared to the adults (P < 0·005). Mean Hb concentrations were at the lower end of the normal range in both the groups with three adolescents and two adults having values < 120 g/l.
Table 1 Subject characteristics
(Mean values with their standard errors)

Study 1, performed in trimester 1; Study 2, performed in trimester 3.
* Unpaired t test (P < 0·001).
Pregnancy outcomes and newborn characteristics are presented in Table 2. Among the sixteen participants in the study, there was one fetal loss in the adolescent group. Although there was no difference in gestational age between the groups, the adolescents had two premature deliveries while the adults had none. Though the birth weights were not significantly different, the mean value of the adolescent group was 8·6 % lower than that of the adult group. Each group had one low birth weight infant. Similarly, there were no differences in placental weight, newborn head circumference and crown–heel length, though the latter trended shorter (by 6·1 %) in the adolescent group.
Table 2 Pregnancy outcome and newborn characteristics
(Mean values with their standard errors)

* For the adolescent group, all newborn values are for n 7.
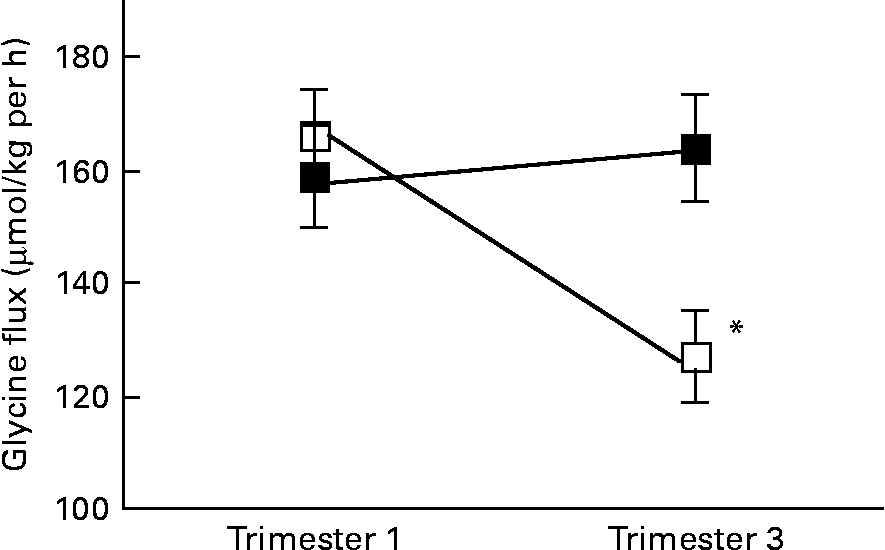
There was a significant interaction between age group and time of pregnancy (P = 0·02), as weight-specific glycine flux decreased by 39 % from trimesters 1 to 3 in the adolescents but increased by approximately 5 % in the adults (Fig. 1). This represented a significant decrease in glycine flux in the adolescent group (P = 0·005). When expressed per whole body, the decrease in glycine flux remained significant (P < 0·05) in the adolescent group (Table 3). Similarly, plasma glycine concentration decreased significantly (P < 0·05) from trimesters 1 to 3 in the adolescents but rose by 7 % in the adults (Table 3). Whereas the plasma concentration of serine increased significantly (P < 0·05) from trimesters 1 to 3 in the adults, it remained unchanged in the adolescents.
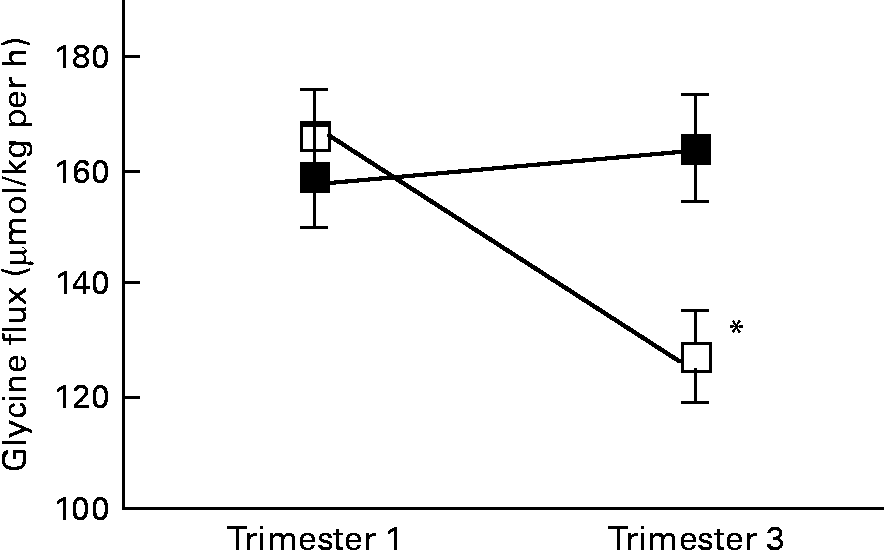
Fig. 1 Weight-specific glycine flux in pregnant adolescents (n 8) and adult women (n 8) at 12·8 (se 0·39) and 27·8 (se 0·4) weeks of pregnancy. * Significant interaction between age group and time of pregnancy (P < 0·005), as flux decreased significantly from trimesters 1 to 3 in the teenage group (repeated measures two-factor ANOVA). ■, Adult; □, teenager.
Table 3 Whole body glycine flux, glycine and serine plasma concentrations in adolescents and in adult women at the end of the first and beginning of the third trimester of pregnancy
(Mean values with their standard errors)

* Different v. trimester 1 value, P < 0·05, paired t test.
† Different v. trimester 1 value P < 0·005, Bonferroni's post test.
The pooled data of the fifteen subjects who had successful pregnancies were used to look for correlations between glycine flux and maternal and baby variables. In trimester 3, there was a positive correlation between glycine flux and subject's age (r 0·63, P = 0·008) indicating that the flux was slower in younger subjects (Fig. 2).
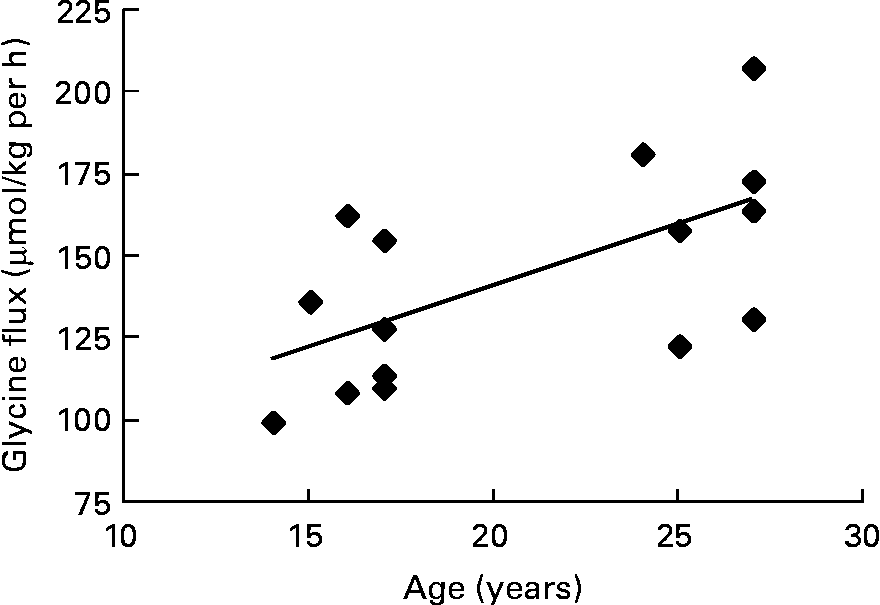
Fig. 2 The association between third trimester glycine flux and subject's age in seven pregnant adolescents and eight adults. Spearman r 0·63; P = 0·008.
Discussion
To test the hypothesis that the pregnant adolescent will not be able to increase glycine production to the same extent as her adult counterpart as pregnancy progresses to late gestation, endogenous glycine flux was measured in adult women and adolescent girls after an overnight fast at the end of the first and beginning of the third trimester of pregnancy. Our results show that as pregnancy progressed from trimesters 1 to 3, there was a decrease in weight-specific glycine flux in the adolescents, whereas in the adults there was a modest increase. Whole body glycine flux also decreased significantly in the adolescent group from trimesters 1 to 3, and this was associated with a reduction in plasma glycine concentration. In trimester 3, there was a positive correlation between glycine flux and subject's age indicating that flux was slower in the younger subjects. These findings suggest that after a brief period of food deprivation, the pregnant adolescent cannot maintain glycine production as her adult counterpart in late pregnancy. This inability to maintain glycine production seems to be related to her younger age.
The present study showed for the first time that whereas overnight fasted pregnant adolescent girls produce glycine at the same rate as their adult counterparts at the end of the first trimester, they produce it at a 39 % slower rate in the third trimester. This finding has important implications with respect to the development of the foetus because of the numerous critical functions of glycine in new tissue formation. First, besides its numerous biochemical functions, glycine is a primary source of 1-carbon which is necessary for both the synthesis and methylation of DNA and, hence, for cell division and growth(Reference Lamers, Williamson and Theriaque13). Secondly, there is evidence of a high fetal demand for glycine especially in late gestation to synthesise glycine-rich collagen(Reference Meier, Teng and Battaglia10). Thirdly, studies in the sheep have shown that there is an absence of serine transport from the maternal circulation to the foetus and that fetal serine supply is exclusively provided by hepatic synthesis from maternal and placental glycine(Reference Geddie, Moores and Meschia11, Reference Chung, Teng and Timmerman12). This is important because in human subjects, serine seems to be the dominant provider of 1-carbon for homocysteine remethylation(Reference Davis, Stacpoole and Williamson14). If this is also true for the foetus, then maternal glycine supply is critical for provision of 1-carbon for fetal growth and development. In the present study of well-nourished adolescents, the slower glycine flux after an overnight fast did not seem to have a marked negative effect on pregnancy outcome as there were no significant differences in baby's birth weight and length between the two groups. Nevertheless, it is worth noting that both baby's birth weight and length of the adolescent group were 8·6 and 6 % less compared with the mean values of the adult group.
Although such a marked reduction in glycine supply after a short period of food deprivation did not seem to have a pronounced adverse effect on fetal growth in the well-fed adolescents of the present study, it will likely do so in situations of intermittent prolonged maternal food deprivation because glycine produced from protein breakdown plus de novo synthesis may not be sufficient to maintain growth of the foetus. For example, earlier studies have shown a significant lowering of plasma amino acids after a brief fast in pregnant women(Reference Felig, Kim and Lynch6, Reference Fitch and King7), suggesting that the balance between maternal amino acid supply and utilisation is very tight, more so for the gluconeogenic amino acids(Reference Fitch and King7). The same will be true in acute pathological stresses, say the stress of an infection, when anorexia is associated with an increased catabolism of amino acids and a negative protein balance. In such situations, it is conceivable that glycine produced by the adolescent mother will deteriorate rapidly, and such events may contribute to the higher prevalence of low birth weight infants among adolescents. Our present data show that the pregnant adolescents are unable to maintain glycine supply even after an overnight fast as their plasma glycine pools decreased significantly from trimesters 1 to 3. On the other hand, the opposite occurred in the adult women as there was a trend towards an increase in plasma glycine concentration from trimesters 1 to 3 and even serine concentration increased significantly suggesting that the adults had adequate supplies of 1-carbon and glycine for serine synthesis. This may not have been the case in the adolescents, as serine concentration remained exactly the same from trimesters 1 to 3.
Although our data do not provide an explanation for the slower glycine production by the adolescents in the third trimester, the strong association between maternal age and glycine flux is intriguing. Because the endogenous flux of a dispensable amino acid consists of its release from whole body protein breakdown plus its de novo synthesis, the slower glycine flux of the adolescents could have been due to a decrease in either one or both the mechanisms. Evidence from studies in pregnant normal weight healthy adult women suggest that the extra amino acids required for increased maternal protein synthesis is provided by increased release from body protein breakdown and an overall decrease in amino acid catabolism(Reference Duggleby and Jackson15–Reference Kalhan, Rossi and Gruca17). Duggleby & Jackson(Reference Duggleby and Jackson15) also reported that protein breakdown rose to a greater extent in pregnant women whose BMI exceeded 25 kg/m2 compared with those with lower BMI, suggesting that the amino acid supply is directly related to maternal BMI. However, although the adolescent group in the present study had a lower BMI than the adult group at trimester 1, because the adolescents gained more weight from study 1 to study 2, the BMI of the two groups were not different in study 2 when glycine flux was slower in the adolescent group. An alternative explanation is that the pregnant adolescent may not be able to downregulate the rate of amino acid oxidation to conserve nitrogen for the de novo synthesis of dispensable amino acids such as glycine. A definitive answer to this question can only be provided by more detailed studies of protein and amino acid metabolism in pregnant adolescents and adults.
Acknowledgements
All authors contributed to the production of this manuscript, from the design of the study, data collection, analysis and interpretation and writing of the manuscript. None of the authors have any conflict of interest with the funding agency. We are also grateful to the nursing staff of the Obstetrics ward at the University Hospital of the West Indies for their care of the subjects and to the technical staff for their excellent work in the laboratory analyzing the samples. This research was supported with federal funds from the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6001.