Long-chain PUFA (LC-PUFA), such as EPA (20 : 5n-3), docosapentaenoic acid (DPA, 22 : 5n-3), DHA (22 : 6n-3) and arachidonic acid (ARA, 20 : 4n-6), play unique roles in controlling and regulating growth performance, cell membrane fluidity, lipid metabolism and immune function in fish( Reference Higgs and Dong 1 – Reference Tian, Ji and Oku 4 ). Among these fatty acids, ARA and EPA are the two main C20 LC-PUFA, which are the precursors for one of the most important groups of bioactive lipid mediators, the eicosanoids( Reference Bell and Sargent 5 – Reference Yin, Zhou and Zhu 7 ). There exists three enzymatic routes for the synthesis of eicosanoids: the cyclo-oxygenase (COX) pathway, the lipoxygenase (LOX) pathway and the cytochrome 450 pathway( Reference Funk 8 , Reference Wang and DuBois 9 ). ARA shows similar structure with EPA but lacks one double bond located at the third carbon from the n end, and it is believed that these two fatty acids compete for binding to the eicosanoid synthesis enzymes such as COX or LOX( Reference Tocher 6 , Reference Hamre, Moren and Solbakken 10 ). In mammals, ARA is the chief precursor of eicosanoids, which generate two-series prostanoids (PG) and 4-series leukotrienes (LT), whereas EPA converted to 3-series PG and 5-series LT; these metabolites from EPA are generally less biologically active than the corresponding metabolites from ARA( Reference Tocher 6 , Reference Halver and Hardy 11 ). However, some recent studies have shown a similar activity of ARA-derived PGE2 and EPA-derived PGE3 such as in endothelial cells( Reference Moreno 12 , Reference Rodríguez-Lagunas, Ferrer and Moreno 13 ). Moreover, the sn-2 position of fish phosphoinositides (PI) has a distinct preference for C20 PUFA, and the existence of a competition between ARA and EPA during phospholipid esterification has also been suggested, especially in PI( Reference Halver and Hardy 11 , Reference Fountoulaki, Alexis and Nengas 14 ). However, ARA and EPA are also very susceptible to be attacked by O2 radicals and other organic radicals, resulting in serious consequences for cell membrane structure and fluidity( Reference Tocher 6 ). Several enzyme systems exist for the possible protection of membrane LC-PUFA in fish, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px)( Reference Tocher 6 ).
Studies have shown that ARA has unique roles in regulating stress resistance( Reference Atalah, Hernández-Cruz and Ganuza 15 – Reference Montero, Terova and Rimoldi 18 ), immune response( Reference Xu, Ai and Mai 19 – Reference Shahkar, Yun and Lee 21 ), reproductive performance( Reference Norambuena, Estévez and Mañanós 22 – Reference Xu, Cao and Zhang 24 ), pigmentation( Reference Villalta, Estévez and Bransden 25 – Reference Wishkerman, Boglino and Darias 27 ), morphogenesis( Reference Lie, Kvalheim and Rasinger 28 , Reference Boglino, Darias and Estévez 29 ) and fatty acid metabolism( Reference Norambuena, Morais and Emery 30 , Reference Norambuena, Rombenso and Turchini 31 ) of fish. Recently, we have shown that moderate dietary ARA (0·30 %) decreased lipid accumulation and lipogenic gene expression levels in grass carp (Ctenopharyngodon idellus)( Reference Tian, Ji and Oku 4 ). It has already been further demonstrated that the COX pathway is responsible for the improvement of lipid catabolic genes rather than lipogenic genes by using COX inhibitor acetylsalicylic acid( Reference Tian, Lei and Ji 32 , Reference Tian, Lei and Ji 33 ). In addition, it has also been demonstrated that n-3 LC-PUFA, including EPA, could decrease the lipid deposition and increase the capacity of lipolysis in this fish species( Reference Ji, Li and Liu 3 , Reference Liu, Li and Huang 34 , Reference Li, Liu and Ji 35 ). These studies indicated that both n-6 and n-3 LC-PUFA played peculiar roles in regulating lipid metabolism of grass carp, whereas the efficacy of these two fatty acids, such as ARA and EPA, remains largely unknown.
Grass carp, a typical herbivorous freshwater fish species that is widely cultured in China, easily accumulates excessive fat in the abdominal cavity and suffers from fatty liver in aquaculture( Reference Tian, Lei and Ji 32 , Reference Tian, Lu and Ji 36 ). It is worth noting that grass carp could synthesise LC-PUFA from linoleic acid (LA) and α-linolenic acid (LNA) via its desaturase and elongase multienzyme complexes( Reference Glencross 37 , Reference Tocher 38 ). ARA is an end product of the n-6 series fatty acids, whereas EPA is easily converted to DHA, which is the end product of the n-3 series fatty acids( Reference Tocher 6 , Reference Tian, Lei and Ji 39 ). Thus, ARA and EPA should have different patterns for deposition in tissues of this fish species, which might further differently affect physiological functions, such as lipid metabolism. Therefore, the aim of this study was to compare the efficacy of dietary ARA and EPA on the lipid accumulation in grass carp. Four purified diets containing different fatty acids (ARA or EPA) were designed to feed grass carp for 10 weeks, and the growth, tissue fatty acid composition, antioxidant response and lipid metabolism were evaluated, with the purpose of providing reference for the use of these two fatty acids in aquaculture industry.
Material and methods
Experimental diets
Four isonitrogenous and isoenergetic semipurified diets containing 36·0 % crude protein and 6·0 % crude lipid were formulated, based on the method of Lavell with some modifications( Reference Lavell 40 ). Soyabean oil (Kerry Oils & Grains Co.) and linseed oil (Hoval Seasons Bio-Sci Co.) were added to satisfy the 1 % LA and 1 % LNA requirements, respectively( Reference Glencross 37 ) (Table 1). The control diet was ARA-free, whereas the other three diets were added with ARA-enriched oil (ARA content, approximately 40·8 % of total fatty acid; in the form of ARA-methylester; Hubei Fuxing Biotechnology Co., Ltd) or EPA-enriched oil (EPA content, approximately 62 % of total fatty acid; Xunda Marine Bio-Pro Co.) at the expense of lard oil (purchased from Kangle Market) to meet the 0·30 % ARA content, 0·30 % EPA content and 0·30 % ARA+EPA (equivalent), respectively. A measure of 0·1 % butylated hydroxytoluene (Sigma-Aldrich) was added as the antioxidant (Table 1). The fatty acid compositions of the experimental diets were determined by GC and are given in Table 2.
Table 1 Formulation and chemical composition of the experimental diets (g/kg DM)
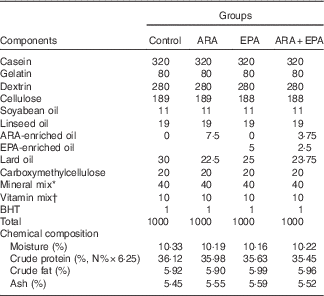
ARA, arachidonic acid ; BHT, butylated hydroxytoluene.
* The mineral mix contained the following (g/100 g of the total mineral): KAl(SO4), 0·159; CaCO3, 18·101; Ca(H2PO4)2, 44·601; CoCl, 0·070; MgSO4, 5·216; MnSO4.H2O, 0·070; KCl, 16·553; KI, 0·014; ZnCO3, 0·192; NaH2PO4, 13·605; Na2SeO3, 0·006; CuSO4.5H2O, 0·075; ferric citrate, 1·338.
† The vitamin mix contained the following (mg/1000 g of diet): vitamin C, 200; thiamine, 10; riboflavin, 20; vitamin A, 7·5; vitamin E, 500; vitamin D3, 0·5; menadione, 10; pyridoxine HCl, 10; cyanocobalamin, 0·02; biotin, 1·0; calcium pantothenate, 40; folic acid, 5; niacin, 20; inositol, 400; choline chloride, 2000; and cellulose was used as a carrier.
Table 2 Fatty acid composition of the experimental diets (percentage of total fatty acids)
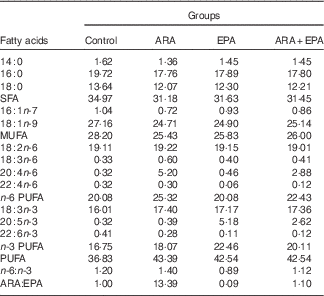
ARA, arachidonic acid.
Ingredients were mixed by hand and approximately 70 % water was added to make a dough, and the dough was then pressed to obtain noodle-like pellets with a diameter of 2 mm. The pellets were dried under forced air at room temperature for 24 h, which were then kept at –20°C until use.
Experimental procedure
Juvenile grass carps were obtained from the Ankang Fisheries Experimental and Demonstration Station of Northwest A&F University (Ankang, China). Fish were reared in the aquaria and fed a commercial diet (protein 36·54 %, fat 4·99 % dry weight and soyabean oil as a lipid source) for two weeks to acclimatise to the experimental condition.
Before the feeding experiment, fish were fasted for 24 h. A total of 216 fish (10·21 (sd 0·10) g body weight) were randomly distributed into twelve aquaria (0·73×0·46×0·60 m; 18 fish/aquarium). Each diet was randomly assigned into three aquaria. Fish were hand-fed to apparent satiation twice daily (at 09.00 and 16.00 hours) for 10 weeks. Satiation feeding was achieved by allowing fish to eat until feeding activity stopped, with no feed remaining in the aquaria. The feed intake was recorded. During the feeding experiment, water was circularly filtered and renewed by 1/5 daily to maintain acceptable water quality. Water temperature was controlled at 28°C. The dissolved O2 was approximately at saturation (9 mg/l) through continuous aeration. The photoperiod was 12 h light–12 h dark (from 08.00 to 20.00 hours). Dead fish were weighed and recorded.
Sampling procedure
After 10 weeks of feeding, the fish were fasted for 24 h. All of the fish were anaesthetised with tricaine methanesulfonate (MS222; 0·06 g/l). Fish were weighed and their body lengths were measured. Six fish per aquarium were randomly selected for blood collection from the caudal vein, and sampled blood was allowed to clot at 4°C for 6 h. Serum samples were collected after centrifugation of the clot for 10 min (825 g , 4°C), and then they were pooled two by two in each aquarium, frozen in liquid N2 and stored at −80°C until analysis. The remaining fish were killed and dissected. After weighing the total visceral weight, the hepatopancreas, intraperitoneal fat (IPF), kidney, spleen and intestine were stripped and weighed. Afterwards, samples of hepatopancreas and IPF from three fish/aquarium were stored at −20°C for fatty acid composition analyses; those from another six fish/aquarium were frozen in liquid N2 and then stored at −80°C for gene expression or enzyme activities measurements; and those from the other three fish/aquarium were fixed in paraformaldehyde solution for histology analysis. All experimental animal procedures were approved by the institutional animal care and use committee and performed in accordance with national and institutional regulations on the care and use of experimental animals.
Specific growth rate (SGR), feed conversion ratio (FCR), hepatopancreas index (HI), intraperitoneal fat index (IPFI), relative kidney weight (RKW), relative spleen weight (RSW), relative intestine weight (RIW), viscera index (VI), condition factor (CF) and survival rate (SR) were calculated using the following formulae:
 $$\eqalignno{ & {\rm SGR}\, {\equals}\, \left( {{\rm Ln}\,{\rm final}\,{\rm weight}-{\rm Ln}\,{\rm initial}\,{\rm weight}} \right)\, {\times}\, {{100} \mathord{\left/ {\vphantom {{100} {70{\rm d}}}} \right. \kern-\nulldelimiterspace} {70{\rm d}}} \cr & {\rm FCR}\, {\equals}\, {{{\rm amount}\,{\rm of}\,{\rm feed}\,{\rm given}} \mathord{\left/ {\vphantom {{{\rm amount}\,{\rm of}\,{\rm feed}\,{\rm given}} {{\rm weight}\,{\rm gain}\,\left( {\rm g} \right)}}} \right. \kern-\nulldelimiterspace} {{\rm weight}\,{\rm gain}\,\left( {\rm g} \right)}} \cr & {\rm HI}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm hepatopancreas}\,{\rm weight}\, {\times} \,100} \mathord{\left/ {\vphantom {{{\rm hepatopancreas}\,{\rm weight}\,\, {\times}\, \,100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm IPFI}\,\left( \,\%\, \right){\rm }\, {\equals}\, {\rm }{{{\rm IPF}\,{\rm weight }\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm IPF}\,{\rm weight }\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm RKW}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm kidney}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm kidney}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm RSW}\,\left( \,\%\, \right){\rm }\, {\equals}\, {\rm }{{{\rm spleen}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm spleen}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm RIW}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm intestine}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm intestine}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm VI}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm viscera}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm viscera}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm CF}\,\left( {{{\rm g} \mathord{\left/ {\vphantom {{\rm g} {{\rm cm}^{3} }}} \right. \kern-\nulldelimiterspace} {{\rm cm}^{3} }}} \right)\, {\equals}\, {{{\rm body}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm body}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm length}^{3} }}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm length}^{3} }} \cr & {\rm SR}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm final}\,{\rm number}\,{\rm of}\,{\rm fish}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm final}\,{\rm number}\,{\rm of}\,{\rm fish}\, {\times}\, 100} {{\rm initial}\,{\rm number}\,{\rm of}\,{\rm fish}}}} \right. \kern-\nulldelimiterspace} {{\rm initial}\,{\rm number}\,{\rm of}\,{\rm fish}}}. $$
$$\eqalignno{ & {\rm SGR}\, {\equals}\, \left( {{\rm Ln}\,{\rm final}\,{\rm weight}-{\rm Ln}\,{\rm initial}\,{\rm weight}} \right)\, {\times}\, {{100} \mathord{\left/ {\vphantom {{100} {70{\rm d}}}} \right. \kern-\nulldelimiterspace} {70{\rm d}}} \cr & {\rm FCR}\, {\equals}\, {{{\rm amount}\,{\rm of}\,{\rm feed}\,{\rm given}} \mathord{\left/ {\vphantom {{{\rm amount}\,{\rm of}\,{\rm feed}\,{\rm given}} {{\rm weight}\,{\rm gain}\,\left( {\rm g} \right)}}} \right. \kern-\nulldelimiterspace} {{\rm weight}\,{\rm gain}\,\left( {\rm g} \right)}} \cr & {\rm HI}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm hepatopancreas}\,{\rm weight}\, {\times} \,100} \mathord{\left/ {\vphantom {{{\rm hepatopancreas}\,{\rm weight}\,\, {\times}\, \,100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm IPFI}\,\left( \,\%\, \right){\rm }\, {\equals}\, {\rm }{{{\rm IPF}\,{\rm weight }\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm IPF}\,{\rm weight }\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm RKW}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm kidney}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm kidney}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm RSW}\,\left( \,\%\, \right){\rm }\, {\equals}\, {\rm }{{{\rm spleen}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm spleen}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm RIW}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm intestine}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm intestine}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm VI}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm viscera}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm viscera}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm weight}}}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm weight}}} \cr & {\rm CF}\,\left( {{{\rm g} \mathord{\left/ {\vphantom {{\rm g} {{\rm cm}^{3} }}} \right. \kern-\nulldelimiterspace} {{\rm cm}^{3} }}} \right)\, {\equals}\, {{{\rm body}\,{\rm weight}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm body}\,{\rm weight}\, {\times}\, 100} {{\rm body}\,{\rm length}^{3} }}} \right. \kern-\nulldelimiterspace} {{\rm body}\,{\rm length}^{3} }} \cr & {\rm SR}\,\left( \,\%\, \right)\, {\equals}\, {{{\rm final}\,{\rm number}\,{\rm of}\,{\rm fish}\, {\times}\, 100} \mathord{\left/ {\vphantom {{{\rm final}\,{\rm number}\,{\rm of}\,{\rm fish}\, {\times}\, 100} {{\rm initial}\,{\rm number}\,{\rm of}\,{\rm fish}}}} \right. \kern-\nulldelimiterspace} {{\rm initial}\,{\rm number}\,{\rm of}\,{\rm fish}}}. $$
Proximate composition analysis
The proximate compositions of the diets were determined according to the methods of Association of Official Analytical Chemists Procedures (1995)( 41 ). In brief, samples were dried to a constant weight to determine moisture at 105°C. Crude protein was determined by measuring N (N×6·25) of the samples using the Kjeldahl method. Crude lipid was measured by diethyl ether extraction using the Soxhlet method. Crude ash was determined by combustion at 550°C in a muffle furnace.
Fatty acid composition analysis
Lipid extraction of tissues (hepatopancreas and IPF; 3 individuals/aquarium) and diets was performed by using chloroform–methanol (2:1, v/v) based on the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 42 ). Extracts were dried under vacuum, and then the tissue lipids were dissolved in chloroform and separated into polar lipids (PL) and non-polar lipids (NPL) using Silica Cartridges according to the method of Juaneda & Rocquelin( Reference Juaneda and Rocquelin 43 ). A volume of 20 ml of chloroform was used to elute the NPL fraction and 30 ml of methanol was added to elute the PL fraction. Extracts were then dried under vacuum again. The preparation of fatty acid methyl esters (FAME) was performed based on the method described previously( Reference Tian, Ji and Oku 4 , Reference Tian, Lei and Ji 39 ). In brief, 1 ml of hexane was added to dissolve the lipid fractions, and methyl esterification was performed for 1 h after adding 1 ml of potassium hydroxide methanol (0·4 m). Then, 2 ml of distilled water was added to separate the mixture into two layers. The upper layer was separated and used for GC analysis. The FAME were determined by an Agilent 7820a Series GC (Agilent Technologies) equipped with a flame ionisation detector and capillary column (HP-88, length 100 m, internal diameter 0·25 mm, film thickness 0·20 μm; Agilent Technologies). Individual methyl esters were identified through comparison with known standards (47015-U; Sigma-Aldrich, Inc.). The results of identified fatty acids were presented as percentage of total fatty acids.
Antioxidant status and lysozyme activity
Serum SOD activity, CAT activity, GSH-Px activity, malondialdehyde (MDA) content and the activity of lysozyme (LZM) were measured with the use of assay kits (Jiancheng Biotech Co.).
Histological processing and morphological evaluation
The samples of fixed hepatopancreas and IPF were washed in tap water for 12 h, followed by a routine dehydration in a graded series of ethanol (30, 50, 70, 80, 90, 95 and 100 % twice). The samples were then equilibrated in xylene and embedded in paraffin based on the standard histological techniques, as described previously( Reference Liu, Ji and Li 44 ). Afterwards, sections were cut at 5 μm with the use of a rotary microtome (RM2235; Leica) and mounted in glass slides, which were then stained with haematoxylin–eosin. Histological samples were observed and photographed by using an upright microscopy (Leica Biosystems). The average adipocyte size per image was quantified using Photoshop (Adobe), as previously described by Osman et al. ( Reference Osman, Selway and Kępczyńska 45 ). An average value across five non-overlapping images (five/section) was calculated for each group.
NEFA and TAG assays
NEFA from serum and hepatopancreas and the TAG content from hepatopancreas were measured by using the NEFA assay kit or TAG assay kit (Jiancheng Biotech Co.). These two indexes were measured based on the user’s manual step by step. All assays were performed in triplicate in each aquarium.
Hepatopancreatic lipid metabolism enzyme activities
Lipid-metabolism-related enzymes, including the lipoprotein lipase (LPL), hepatic lipase (HL) and malate dehydrogenase (MDH), in the hepatopancreas were measured by using the assay kits (Jiancheng Biotech Co.) according to the method in the specification.
Real-time quantitative RT-PCR
Hepatopancreas and IPF from three fish per aquarium were used for the detection of gene expression. RNA extraction, complementary DNA synthesis and gene expression measurements were performed as described previously(
Reference Tian, Ji and Oku
4
). The primer sequences for β-actin, LPL, fatty acid synthase (FAS), diacylglycerol O-acyltransferase (DGAT), apoE, PPARα, adipose TAG lipase (ATGL), PPARγ, CCAAT enhancer-binding protein α (C/EBPα), hormone-sensitive lipase (HSL) and carnitine palmitoyltransferase 1 (CPT-1) are listed in Table 3. After the PCR reaction, melting curves were analysed to confirm that single products were obtained in these reactions. A relative quantification method, the comparative CT method (
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
), was used to calculate the gene expression values, as described in the literature(
Reference Livak and Schmittgen
46
,
Reference Pfaffl
47
).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
), was used to calculate the gene expression values, as described in the literature(
Reference Livak and Schmittgen
46
,
Reference Pfaffl
47
).
Table 3 Primers used in real-time quantitative PCR

Statistical analysis
All data were expressed as means and standard deviations. Percentage data were arcsine-transformed before analysis. One-way ANOVA was used to compare differences among the experimental groups, followed by Duncan’s post hoc tests. All analyses, including the principle component analysis (PCA) of the fatty acids, were conducted using PASW Statistics 18 (SPSS). A significance level of P<0·05 was used for all tests.
Results
Growth performance and biological parameters
The growth of fish doubled compared with the initial weight (Table 4). Fish fed 0·30 % EPA exhibited significantly higher final weight and SGR in comparison with fish fed the control diet (P<0·05). HI in the ARA, EPA and ARA+EPA groups presented significantly lower values than those in the control group (P<0·05). The IPFI value was inhibited in the ARA group compared with the control (P<0·05). Interestingly, fish subjected to ARA+EPA had higher RSW than those subjected to control (P<0·05). No statistically significant difference was found in other indices such as FCR, RKW, RIW, VI, CF and SR (P>0·05).
Table 4 Effects of dietary arachidonic acid (ARA) and EPA on the growth performance, feed utilisation, survival and biological parameters of juvenile grass carp (Mean values and standard deviations; n 3/group)

a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Fatty acid composition
Lipids in the hepatopancreas and IPF were separated for NPL and PL fractions, and then fatty acid compositions were analysed (Tables 5 and 6). ARA and EPA presented higher levels in the PL (3·56–5·86 % for ARA; 0·40–1·46 % for EPA) than NPL (0·41–0·98 % for ARA; 0·25–0·50 % for EPA) of the hepatopancreas, whereas there was no apparent difference in the IPF (0·75–2·47 % in NPL and 0·63–1·61 % in PL for ARA; 0·47–1·30 % in NPL and 0·51–1·20 % in PL for EPA).
Table 5 Effects of dietary arachidonic acid (ARA) and EPA on the fatty acid composition (percentage of total fatty acids) in the non-polar lipid (NPL) and polar lipid (PL) fraction of hepatopancreas in juvenile grass carp (Mean values and standard deviations; n 3/group)

a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Table 6 Effects of dietary arachidonic acid (ARA) and EPA on the fatty acid composition (percentage of total fatty acids) in the non-polar lipid (NPL) and polar lipid (PL) fraction of intraperitoneal fat in juvenile grass carp (Mean values and standard deviations; n 3/group)

a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P<0·05).
To be detailed, in the hepatopancreas, the highest ARA contents were presented in fish fed ARA both in NPL and PL, followed by ARA+EPA (P<0·05). However, significantly different contents of EPA were only found in the PL, which showed significantly higher level in the EPA group, followed by the ARA+EPA group (P<0·05). Moreover, EPA feeding fish also showed increased 22 : 5n-3 content in the PL compared with the control (P<0·05). These resulted in the increase of the total n-3 PUFA. ARA:EPA ratio exhibited a similar trend with those in the diets (P<0·05) (Table 5).
In the IPF, dietary ARA significantly increased the ARA proportion both in NPL and PL (P<0·05); paralleled trends also applied to 22 : 4n-6 in the NPL and PL, and 18 : 3n-6 in the NPL (P<0·05). Similarly, EPA content increased in fish fed EPA and ARA+EPA in these two lipid fractions (P<0·05). Moreover, dietary EPA also significantly increased 18 : 3n-3 in the PL, 22 : 5n-3 in the NPL, as well as the DHA content both in the NPL and PL fractions (P<0·05). Interestingly, SFA proportion decreased and MUFA proportion increased in the PL of fish fed EPA (P<0·05) (Table 6).
The PCA demonstrated different patterns of fatty acid distribution in NPL and PL of these two tissues (Fig. 1). Overall, there exists more distinct difference of the fatty acid composition between these two fractions in the hepatopancreas than those in the adipose tissue. Specifically, in the first component of score plot (Fig. 1(a)), all the hepatopancreas-PL samples are located to the right of the diagram, whereas the adipose tissue NPL are located to the left. The corresponding component plot (Fig. 1(b)) suggests that SFA, ARA and DHA (on the right of the diagram) correspond to the hepatopancreas-PL samples, whereas MUFA and C18 PUFA (on the left right of the diagram), in particular, correspond to the adipose tissue NPL. Furthermore, the second component of the corresponding component plot (Fig. 1(b)) shows that MUFA is located on the negative axis of the second component plot and could correspond to the hepatopancreas NPL samples, whereas the other types of fatty acids could correspond to the adipose tissue PL. Besides, the component plot also demonstrates that adipose tissue is likely to deposit C18 PUFA.

Fig. 1 Score plot (a) and component plot (b) from principal component analysis on fatty acid composition of the non-polar lipid (NPL) and polar lipid (PL) of the hepatopancreas and intraperitoneal fat in juvenile grass carp fed different diets. a: ![]() , Control;
, Control; ![]() , arachidonic acid (ARA);
, arachidonic acid (ARA); ![]() , EPA;
, EPA; ![]() , ARA+EPA.
, ARA+EPA.
Antioxidant responses and lysozyme activity in serum
Table 7 shows the status of antioxidant (SOD, CAT and GSH-Px activities and MDA contents) and LZM activity in serum of fish fed different diets. The serum SOD activities and CAT activities were significantly increased in fish fed ARA and EPA diets (P<0·05), whereas fish fed these LC-PUFA showed declined trend of serum GSH-Px activities (P<0·05). MDA contents also decreased in fish fed ARA, EPA and ARA+EPA, and significant differences are shown in EPA and ARA+EPA groups (P<0·05). As for the serum LZM activities, the treatment groups had higher levels than the control group; EPA and ARA+EPA reached significant levels (P<0·05).
Table 7 Effects of dietary arachidonic acid (ARA) and EPA on the serum antioxidant responses and lysozyme activity in juvenile grass carp (Mean values and standard deviations; n 3/group)
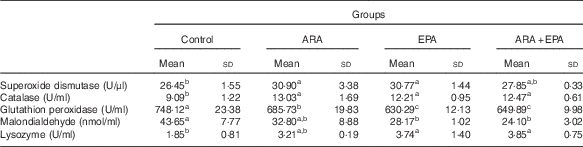
a,b,c Mean values with unlike superscript letters were significantly different (P<0·05).
Lipid accumulation
Fig. 2 shows the phenotype of lipid accumulation of the fish fed various diets. Dietary ARA and EPA treatment groups showed decreased TAG content in the hepatopancreas (P<0·05) (Fig. 2(A) and (C)). On the other hand, in the IPF, adipocyte size in the ARA and ARA+EPA groups presented significantly lower values compared with that in the control (P<0·05) (Fig. 2(B) and (C)). Interestingly, LC-PUFA treatment groups showed higher serum NEFA levels, and ARA significantly increased the serum NEFA in comparison with the other groups (P<0·05).

Fig. 2 Effects of dietary arachidonic acid (ARA) and EPA on the lipid accumulation in juvenile grass carp. (A) Histological morphology of the hepatopancreas; (B) histological morphology of intraperitoneal fat; (C) TAG content of hepatopancreas, adipocyte size, serum NEFA levels. Values are means (n 3), and standard deviations represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
Lipid-metabolism-related enzyme activities in hepatopancreas
The LPL activity of the hepatopancreas was lower in fish fed ARA compared with fish fed control (P<0·05). However, both ARA and EPA groups presented significantly lower HL activities than the ARA+EPA group (P<0·05). MDH activities exhibited significantly higher levels in all the LC-PUFA treatment groups compared with those in the control group (P<0·05) (Fig. 3).

Fig. 3 Effects of dietary arachidonic acid (ARA) and EPA on the activities of lipid-metabolism-related enzymes in the hepatopancreas of juvenile grass carp. Values are means (n 3), and standard deviations represented by vertical bars. LPL, lipoprotein lipase; HL, hepatic lipase; MDH, malate dehydrogenase. a,b Mean values with unlike letters were significantly different (P<0·05).
Lipid-metabolism-related gene expression in hepatopancreas and intraperitoneal fat
For the hepatopancreas (Fig. 4), dietary ARA, EPA and combined ARA and EPA groups presented significantly lower mRNA expression of FAS, DGAT and apoE (P<0·05). Dietary ARA significantly increased the mRNA expression of ATGL (P<0·05), whereas this gene was decreased in fish subjected to EPA and ARA+EPA.

Fig. 4 Effects of dietary arachidonic acid (ARA) and EPA on the lipid-metabolism-related gene expression of the hepatopancreas in juvenile grass carp. Values are means (n 3), and standard deviations represented by vertical bars. FAS, fatty acid synthase; DGAT, diacylglycerol O-acyltransferase; ATGL, adipose TAG lipase; CPT-1, carnitine palmitoyltransferase 1. a,b,c Mean values with unlike letters were significantly different (P<0·05).
In the IPF (Fig. 5), ARA treatment significantly decreased C/EBPα and LPL, and dietary ARA and ARA+EPA decreased the expression of FAS (P<0·05). The PPARα and CPT-1 mRNA level significantly increased in the ARA group compared with the other groups (P<0·05); EPA and ARA+EPA groups also showed a significant increase in these gene levels compared with the controls, whereas they showed lower levels compared with the ARA group. Dietary ARA significantly increased the expression of ATGL and HSL (P<0·05), whereas these genes were not increased under EPA or ARA+EPA treatments.

Fig. 5 Effects of dietary arachidonic acid (ARA) and EPA on the lipid-metabolism-related gene expression of intraperitoneal fat in juvenile grass carp. Values are means (n 3), and standard deviations represented by vertical bars. C/EBPα, CCAAT enhancer-binding protein α; LPL, lipoprotein lipase; fatty acid synthase; ATGL, adipose TAG lipase; HSL, hormone-sensitive lipase; CPT-1, carnitine palmitoyltransferase 1. a,b,c Mean values with unlike letters were significantly different (P<0·05).
Discussion
ARA and EPA exhibit a competitive relationship for binding to some enzymes in fish, such as the eicosanoid synthesis enzymes, phospholipid esterase or fatty acid elongase( Reference Tocher 6 , Reference Halver and Hardy 11 ). The inverse relationship between EPA and ARA has been reported in fish tissues. Some enzymes have preference for these two fatty acids. ARA is the preferred substrate for eicosanoids, whereas EPA is the preferred substrate for phosphoglycerides( Reference Tocher 6 , Reference Halver and Hardy 11 ). Moreover, the affinity of the elongase 2/5 is higher for EPA than for ARA( Reference Tocher 6 , Reference Halver and Hardy 11 , Reference Fountoulaki, Alexis and Nengas 14 , Reference Tocher 38 ). Hence, there must be a complex and competitive regulation of fish physiological processes by these two fatty acids. However, our early studies have suggested a similar function of ARA and n-3 LC-PUFA in the lipid accumulation, and the mechanism were also analogous( Reference Ji, Li and Liu 3 , Reference Tian, Ji and Oku 4 , Reference Tian, Lei and Ji 32 , Reference Liu, Li and Huang 34 , Reference Tian, Lu and Ji 36 ). Thus, in this study, we aimed to compare the effect of dietary ARA and EPA on the performance of grass carp in detail. Our data demonstrated that dietary ARA to some extent has a different role from EPA in controlling growth performance and lipid metabolism of grass carp.
In this study, dietary ARA had no significant effect on the growth performance of grass carp, similar to early studies( Reference Tian, Ji and Oku 4 , Reference Tian, Lei and Ji 33 ). However, a positive effect on the growth was found in fish fed 0·3 % EPA. The result that EPA promoted slightly higher growth than ARA has also been found in Atlantic salmon (Salmo salar)( Reference Norambuena, Morais and Emery 30 , Reference Norambuena, Rombenso and Turchini 31 ). Actually, moderate dietary n-3 LC-PUFA (approximately 0·3 % EPA and approximately 0·2 % DHA) has been reported to increase growth in juvenile grass carp, suggesting that EPA concentration at 0·3 % or a high ratio of n-3:n-6 LC-PUFA might benefit the growth of juvenile grass carp( Reference Ji, Li and Liu 3 ). The decreased HI in fish fed ARA, EPA or a combination of ARA and EPA was consistent with lipid accumulation in the hepatopancreas. These results highlighted the growth-promoting effects of dietary supplementation of EPA compared with ARA.
We also demonstrated different accumulation patterns of EPA and ARA in the hepatopancreas and IPF. As a prerequisite, given that the fatty acid compositions of hepatopancreas and IPF reflected those of the diets, dietary ARA and EPA were effectively incorporated into the fish lipid. In the hepatopancreas, ARA and EPA are more likely to retain in PL; in particular, ARA content was 7-fold higher in PL than that in NPL. A similar result was reported in the hepatopancreas, muscle and gill in gilthead bream (Sparusaurata L.)( Reference Fountoulaki, Alexis and Nengas 14 ). However, in IPF there was no apparent preference of these two fatty acids on NPL and PL, suggesting that adipose tissue might be an ‘inert organ’ that has lower ability for allotting ARA and EPA at a subcellular level. In the ARA+EPA group, EPA showed less levels than ARA did in tissues, although ARA and EPA were equally provided in the diet. This might be due to the conversion of EPA to DPA and DHA, as suggested by the higher content of these two derivatives in fish fed EPA. There was a slightly inverse relationship between ARA and EPA content in hepatopancreas, but no such phenomenon was found in IPF. This was not consistent with early studies, which have observed an apparent decrease in EPA with increased dietary ARA levels in gilthead bream and Atlantic salmon( Reference Fountoulaki, Alexis and Nengas 14 , Reference Bessonart, Izquierdo and Salhi 48 , Reference Bell, McVicar and Park 49 ), possibly because of the difference in fish species. It has been suggested that SFA and MUFA are preferentially located in the sn-1 and sn-3 position of the TAG and sn-1 in the phosphoglycerides, whereas PUFA are preferentially located in the sn-2 position( Reference Halver and Hardy 11 ). Roughly similarly, the fatty acid composition and PCA scores demonstrated that SFA and LC-PUFA are readily retained in PL, whereas MUFA is retained in NPL, suggesting that SFA have more affinity in PL and MUFA in NPL in grass carp. Besides, C18 PUFA presented higher content in IPF than in hepatopancreas. This might be because IPF is an ‘entrepot’ that accumulates dietary lipid for energy storage, whereas hepatopancreas is an important intermediary metabolism tissue that has higher capacity for fatty acid metabolism such as desaturation/elongation and β-oxidation( Reference Tian, Lei and Ji 39 , Reference Turchini, Torstensen and Ng 50 ).
Because of the high unsaturation, ARA and EPA are easy to be attacked by free radicals, which cause lipid peroxidation and impair the functional integrity of cell membranes and enzyme activity( Reference Gray 51 , Reference Halliwell and Gutteridge 52 ). MDA is the end product of lipid peroxidation and its content could reflect the degree of lipid peroxidation( Reference Surapaneni and Venkataramana 53 ). SOD is considered to play a pivotal antioxidant role and catalyses O2 − to H2O2, and H2O2 can be removed by the activities of CAT or GSH-Px( Reference Tocher 6 ). It has been shown that moderate dietary ARA could increase the antioxidant response in fish such as Japanese seabass (Lateolabrax japonicus)( Reference Xu, Ai and Mai 19 ), Synechogobius hasta ( Reference Luo, Tan and Li 54 ) and Japanese eel (Anguilla japonica)( Reference Shahkar, Yun and Lee 21 ). Similar results have also been found in fish fed appropriate n-3 LC-PUFA( Reference Li, Liu and Ji 35 , Reference Zuo, Ai and Mai 55 ). However, high levels of dietary ARA or n-3 LC-PUFA were reported to cause severe oxidative stress( Reference Ji, Li and Liu 3 , Reference Xu, Ai and Mai 19 , Reference Luo, Tan and Li 54 , Reference Todorčević, Kjær and Djaković 56 ). In the present study, we have shown that dietary 0·3 % ARA and EPA decreased the serum MDA content, and increased the SOD and CAT activities, suggesting that these two fatty acids improved the antioxidant status at this concentration in diet of grass carp. Interestingly, GSH-Px activities were decreased in fish fed ARA and EPA, possibly because of an adaptive down-regulation of this enzyme resulting from lower free-radical levels. Our study also demonstrated that ARA and EPA increased the LZM activity, similar to the study in Japanese seabass( Reference Xu, Ai and Mai 19 ), Japanese eel( Reference Shahkar, Yun and Lee 21 ) and large yellow croaker (Larmichthyscrocea)( Reference Zuo, Ai and Mai 55 ). Overall, these results demonstrate that ARA and EPA exhibited analogous function in regard to the antioxidant and antibacterial response.
In early studies, it was observed that dietary ARA decrease the lipid accumulation in grass carp( Reference Tian, Ji and Oku 4 , Reference Tian, Lei and Ji 32 , Reference Tian, Lei and Ji 33 ) as in other fish species( Reference Fountoulaki, Alexis and Nengas 14 , Reference Xu, Ai and Mai 19 , Reference Luo, Tan and Li 54 ). On the other side, considerable studies have shown that n-3 LC-PUFA, including EPA, have the capacity to decrease lipid content in fish, including grass carp( Reference Ji, Li and Liu 3 , Reference Li, Liu and Ji 35 , Reference Tian, Lu and Ji 36 , Reference Todorčević and Hodson 57 ). In the present study, we have shown that 0·3 % ARA and EPA decreased the TAG level in the hepatopancreas, whereas a combination of ARA and EPA had no such function, which might be due to the low concentration or the competition for binding enzymes. However, in regard to the dietary n-6:n-3 LC-PUFA ratios, high (ARA group, 8·2) or low (EPA group, 0·10) ratios presented a positive relationship with the inhibition of lipid accumulation compared with the moderate ratios (control group, 0·88; ARA+EPA group, 1·09), indicating that an imbalance of n-6 and n-3 LC-PUFA might result in the decrease of lipid retention in hepatopancreas, but this needs further confirmation. Interestingly, regarding the adipose tissue, although ARA blocked the development of adipocyte as indicated by the IPFI and adipocyte sizes, EPA had no such efficacy, suggesting that dietary EPA had weaker ability in repressing adipogenesis than ARA in this study. Taken together, these results demonstrated that dietary ARA and EPA played similar roles in repressing lipid accumulation in the hepatopancreas but only ARA acted as the inhibitor of adipose tissue development. Consistent with the possible inhibitory effects on adipogenesis, ARA but not EPA increased the serum NEFA level, possibly because of the up-regulation of the lipolytic genes in adipose tissue( Reference Frühbeck, Méndez-Giménez and Fernández-Formoso 58 ).
LPL is considered as a key enzyme in the lipid accumulation and metabolism of many tissues, which participates in the removal of lipoprotein TAG from the circulation (mainly chylomicron and very LDL)( Reference Eckel 59 , Reference Nilsson-Ehle, Garfinkel and Schotz 60 ). A decrease in LPL activity or gene expression with increasing dietary ARA has been suggested in S. hasta and grass carp( Reference Tian, Lei and Ji 33 , Reference Luo, Tan and Li 54 ), which was also shown in this study. The low LPL activity was consistent with the low TAG level in hepatopancreas. EPA also inhibited this enzyme but to a lesser extent, and our early study has observed an increase in LPL activity in grass carp fed n-3 LC-PUFA. The effects of PUFA on LPL-mediated lipid metabolism might need further investigation( Reference Ji, Li and Liu 3 ). HL is primarily synthesised in liver and involved in chylomicron-remnant and HDL metabolism( Reference Connelly 61 ). It should be noted that the ARA+EPA group presented significantly higher HL activity, which might be because of the moderate ratio of n-6:n-3 LC-PUFA and may possibly be the reason for the relatively higher lipid accumulation in this group. MDH plays a key part in the malate/aspartate shuttle across the mitochondrial membrane and in the tricarboxylic acid cycle within the mitochondrial matrix( Reference Minarik, Tomaskova and Kollarova 62 ). In our study, dietary ARA, EPA and a combination of ARA and EPA increased the hepatopancreatic MDH activities, indicating that these C20 LC-PUFA could increase the active status of tricarboxylic acid cycle.
In this study, both ARA and EPA decreased the de novo lipogenesis capacity in a similar manner as suggested by the down-regulation of FAS and DGAT mRNAs in hepatopancreas, which is consistent with the low TAG levels. Many studies have demonstrated that ARA and n-3 LC-PUFA could decrease the lipogenic gene expression in grass carp( Reference Tian, Ji and Oku 4 , Reference Tian, Lei and Ji 33 , Reference Li, Liu and Ji 35 , Reference Tian, Lu and Ji 36 ). ApoE is mainly produced by the liver and combines with lipids in the body to form lipoproteins, acting as a key regulator of plasma lipid levels( Reference Phillips 63 ). Interestingly, all dietary LC-PUFA treatments resulted in the decrease of the expression of apoE in this study, suggesting a lower capacity of packaging lipoproteins in these groups. However, these gene expressions seem to have no relevance to the ratio of n-3:n-6 LC-PUFA, as proved in the ARA+EPA group. A clear difference between ARA and EPA was found in lipid catabolism, which is also an important factor influencing lipid accumulation. Only dietary ARA increased the expression of ATGL that catalyses the first step for the sequential hydrolysis of TAG( Reference Sun, Ji and Li 64 ). There is one previous study showing similar results( Reference Tian, Lei and Ji 33 ). Considering that the hepatopancreatic TAG contents were similar in the ARA and EPA groups, lipid anabolism would play the primary role in controlling TAG level in hepatopancreas. Alternatively, compared with EPA, dietary ARA may decrease lipid incorporation and enhance mobilisation in the hepatopancreas (as suggested by LPL activity and ATGL mRNA levels), resulting in the apparently similar TAG levels.
ARA decreased the expression of the adipogenic marker genes C/EBPα, LPL and FAS, and increased lipid catabolic mRNA levels of PPARα, ATGL, HSL and CPT-1 in IPF. The EPA group showed less effects on these genes, although it has been suggested that EPA also could trigger the inhibition of adipogenesis and promotion of lipolysis( Reference Liu, Li and Huang 34 , Reference Li, Liu and Ji 35 , Reference Todorčević and Hodson 57 ). The increased serum NEFA levels in fish fed ARA but not EPA also demonstrated the higher lipolysis-enhancing effect of ARA compared with EPA. In the present study, EPA level in the IPF was less than that in ARA, partly because of the conversion to DPA or DHA. The low EPA concentration might account for its low influence on lipid metabolism genes. In another study, we observed that EPA content at 0·6 % could effectively inhibit lipogenic and increase lipolytic genes (unpublished results). Moreover, it has been suggested that the metabolites of ARA from the COX pathway might be responsible for the improvement of lipid catabolic capacity in grass carp( Reference Tian, Lei and Ji 32 , Reference Tian, Lei and Ji 33 ). The EPA-derived COX eicosanoids are less active possibly because of the lower level of substrate( Reference Tocher 6 , Reference Halver and Hardy 11 ). It is difficult in in vivo studies to exactly match intracellular levels of the target fatty acids. Detailed mechanisms underlying the difference between ARA and EPA will be addressed in further in vitro studies using model systems.
In conclusion, the present result showed that diet supplemented with EPA was beneficial on the growth performance, whereas ARA exhibited more potent ability in controlling lipid metabolism in IPF. These fatty acids showed similar effects on lipid metabolism in the hepatopancreas. ARA and EPA are more likely to be esterified in PL in the hepatopancreas than in IPF, and presented similar effects on the antioxidant capacity.
Acknowledgements
The authors thank Ankang Fisheries Experimental and Demonstration Station of the Northwest Agriculture and Forestry University for the experimental fish.
This study was supported by the National Natural Science Foundation of China (grant nos 31372538, K3050215105) and the earmarked fund for China Agriculture Research System (grant no. CARS-46-16). The funders had no role in the design, analysis or writing of the article.
J.-j. T., H. J. and J. X. conceived and designed the experiments. J.-j. T. and C.-x. L. performed the experiments and contributed to the analysis of data. J.-j. T. and G. K. co-wrote the manuscript. C.-x. L., H. J., J.-s. Z., H.b. Y., Y. L. and E.-m. Y. contributed to the revision.
The authors declare that there are no conflicts of interest.














