Brain ageing is associated with both a gradual and irreversible loss of brain cells and synapses, which can lead to dementia in human subjects and a syndrome of cognitive dysfunction (CDS), a condition similar to dementia in human subjects, in companion animals(Reference Pan1–Reference Hof and Morrison3). Recent work has established that aged cats, like both dogs and human subjects, develop β-amyloid pathology(Reference Brellou, Vlemmas and Lekkas4, Reference Head, Moffat and Das5) and develop plaques that are immunopositive for AB42, as they are in the human brain(Reference Cummings, Pike and Shankle6). Aged cats have also been reported to develop hyperphosphorylated τ protein(Reference Gunn-Moore, McVee and Bradshaw7) and decrease in cholinergic-positive neurons(Reference Zhang, Sampogna and Morales8). In the human brain, these age-associated changes are known to link to cognitive decline and dementia. Despite evidence to the contrary(Reference McCune, Stevenson and Fretwell9, Reference Levine, Lloyd and Fisher10), we have recently reported that neuropsychological test performance in cats shows age-dependent changes that parallel those seen in dogs and in human subjects(11). In cats, the clinical symptoms of CDS includes disorientation, altered interactions with pet owners and other pets, sleep–wake cycle abnormality, loss of housetraining and altered activity levels and patterns(Reference Gunn-Moore, Moffat and Christie12, Reference Landsberg, Denenberg and Araujo13). CDS affected 28 % of 11- to 14-year-old cats, and 50 % of cats over 15 years showed one or more signs of CDS(Reference Gunn-Moore, Moffat and Christie12, Reference Landsberg, Denenberg and Araujo13).
Attempts to treat dementia in human subjects and CDS in companion animals are complicated by the fact that once brain cells are lost, they cannot be replaced in sufficient quantities to provide normal brain functions(Reference Pan1–Reference Hof and Morrison3, Reference Pugliese, Gangitano and Ceccariglia14, Reference Siwak-Tapp, Head and Muggenburg15). This suggests that there should be a greater focus on preventive strategies, such as using nutrients or bioactives that target reducing the rate of brain cell loss in both human subjects and pets to promote healthy brain ageing and reduce the risk of dementia. We have hypothesised that the best option to manage brain ageing successfully is to retard ageing-induced changes, especially brain atrophy, by reducing or eliminating risk factors associated with brain ageing and dementia(Reference Pan1). Brain ageing, stroke and dementia have also been linked to several risk factors that include DHA deficiency, high homocysteine, low status of vitamin B6, vitamin B12 and folic acid, high blood pressure, cerebral vascular lesion, increased oxidative stress and chronic inflammation(Reference Cole, Ma and Frautschy16–Reference Taupin24).
Based on earlier assumptions, we have proposed a strategy for promoting healthy brain ageing that focuses on nutritional modifications targeting metabolic changes and risk factors associated with brain ageing and dementia(Reference Pan1). Because of the multiple factors, we hypothesised that no single nutrient or bioactive compound is likely to be sufficient for retarding brain ageing and reducing the risk of dementia and CDS. We have consequently developed a blend of nutrients, referred to as the brain protection blend (BPB), which were selected based on their ability to minimise or eliminate the risk factors associated with brain ageing and dementia. The BPB includes fish oil, arginine, B vitamins and selected antioxidants. Fish oil containing DHA and EPA was selected to prevent and correct DHA deficiency and offer anti-inflammatory benefits(Reference Ciubotaru, Lee and Wander25, Reference Wall, Ross and Fitzgerald26). Arginine was selected to enhance NO synthesis, which has been linked to circulation, blood pressure control and cognition(Reference Dong, Qin and Zhang27, Reference Yamada, Noda and Nakayama28). B vitamins prevent and correct any B vitamin deficiency and minimise the risk of high homocysteine(Reference Selhub, Bagley and Miller18, Reference Bryan, Calvaresi and Hughes29, Reference Smith, Smith and de Jager30). Antioxidants, including vitamins E and C and Se, offer protection against oxidative damage and inflammation-induced damage in both brain tissue and blood vessels(Reference Frank and Gupta31–Reference Schwenke and Behr36).
The primary objective of the present study was to assess the effectiveness of long-term maintenance on the BPB on cognitive functions in middle-aged and old cats. The cognitive assessments included a landmark discrimination learning test, which was designed to assess visuo-spatial learning and had proved sensitive to age in dogs(Reference Milgram, Adams and Callahan37, Reference Milgram, Head and Muggenburg38). The second test protocol was designed to assess egocentric spatial ability and was initially described by Christie et al. (Reference Christie, Studzinski and Araujo39); this task also included a reversal learning component, which provides a measure of executive function. The third test protocol examined performance on a size discrimination and reversal learning task. These tasks have also previously been used in cognitive evaluation of dogs(Reference Head, Callahan and Cummings40, Reference Tapp, Siwak and Estrada41) and the reversal task provides a useful measure of executive function(Reference Tapp, Siwak and Estrada41). The final protocol examined performance on the delayed non-matching-to-position (DNMP) task, which had initially been used in group stratification and was designed to evaluate learning and short-term memory.
Materials and methods
Cats and housing
The present study used thirty-two short-haired cats with sixteen cats per group, and the age range of the cats was between 5·5 and 8·67 (6·65 (sd 0·72)) years at the start of the study. The study protocol was approved by the CanCog Technologies Institutional Animal Care Committee, and followed the guidelines of the Ontario Ministry of Agriculture. Whenever possible, cats were group housed based on compatibility in separate rooms provided with environmental enrichment consisting of toys, beds and the opportunity to play outside on a daily basis. Housing temperature and humidity were held relatively constant by automated temperature control and continuous ventilation. Room environmental conditions have design specifications as follows: single-pass air supply with 2200 cubic feet filtered air changes per min, relative humidity of 60 ± 10 %, temperature of 21 ± 3°C and a natural light–dark cycle.
Test and control diets
The control diet was a commercial super premium-type product for adult cats. Both diets were isoenergetic, manufactured by Nestlé Purina PetCare, Inc. and contained the same levels of protein, fat and carbohydrates. Dietary ingredients, chemical composition and the levels of individual ingredients of the nutrient blend are provided in Table 1. Diet samples were sent to Nestlé Purina Analytical Laboratories (Nestlé Purina Petcare) for chemical analyses. Ash, crude fat, crude fibre, crude protein, moisture and fatty acid profile were measured based on the Association of Official Agricultural Chemists methods 942.05, 922.06, 962.09, 990.03, 930.15 and 996.06, respectively.
Table 1 Ingredients and chemical composition of diets

BPB, brain protection blend; ME, metabolisable energy.
* Including addition of DHA, EPA, vitamin C and elevated levels of arginine, B vitamins, Se and α-tocopherol.
† Calculated based on the predictive equation for ME in cat foods(55).
Baseline cognitive testing and randomisation
All the cats assigned to the present study had previously been trained on a programme of cognitive testing that included, at a minimum, testing on discrimination and reversal learning and training on a two-component version of the DNMP task(Reference Head, Mehta and Hartley42). A total of twelve cats had received extensive training on a variety of cognitive tasks and constituted an experienced group. The other twenty cats were trained only on discrimination learning, reversal and the DNMP, and were characterised as an inexperienced group. Two factors were used in grouping: (1) baseline cognitive performance on the DNMP task over five consecutive sessions and (2) extent of previous cognitive experience. The cats were first separated into experienced and inexperienced groups. Each group was then separately ranked based on baseline cognitive performance. Table 2 illustrates that, after group assignment, the two treatment groups were equivalent in body weight, age and cognitive performance.
Table 2 Age, body weight and cognitive performance at baseline (Mean values with their standard errors, n 16)

BPB, brain protection blend; DNMP, delayed non-matching-to-position.
* Including addition of DHA, EPA, vitamin C and elevated levels of arginine, B vitamins, Se and α-tocopherol.
Cognitive testing apparatus
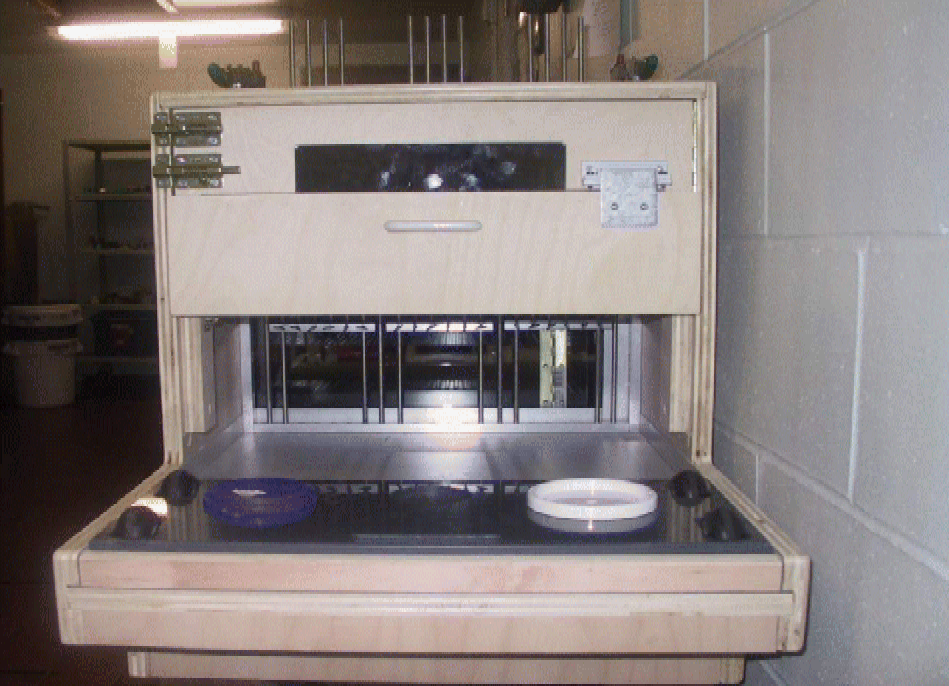
All cognitive testing utilised the feline general test apparatus, which is a modified version of the Toronto general test apparatus developed for use in cognitive testing of dogs(Reference Milgram, Head and Weiner43). The apparatus illustrated in Fig. 1 consisted of an enclosure, where the cat was placed during the cognitive tests, with a front consisting of three adjustable gates, which were set such that the cat could extend its head through the opening to reach the food well and obtain the reward. The cats were in the enclosure for 5–15 min, depending on the cognitive tests. The technologist was separated from the cat by a partition containing a one-way mirror and a hinged door through which the tray was presented. The tray contained one medial and two lateral food wells. The two lateral wells were for the landmark discrimination, size discrimination and DNMP. The egocentric test protocol used all three food wells. The food reward was Friskies Chef's Dinner Pate, Turkey and Giblets Dinner Pate or Chicken and Turkey Dinner in Gravy Senior, depending on the preference of the cats. Approximately, 1 g of the above-mentioned food was used as the food reward for each test.

Fig. 1 Test apparatus for cognitive assessment of felines. The apparatus consists of an enclosure where the cat was placed for 5–15 min during the cognitive test and a sliding tray with three food wells. (A colour version of this figure can be found online at www.journals.cambridge.org/bjn).
Feeding and cognitive test schedule
The cats were fed ad libitum and given free access to water via a wall-mounted automatic system and/or water bowls. The cats were weighed weekly during the study and the food provided was adjusted in order to maintain relatively constant body weights. All cats were fed the control diet during the recruitment phase and baseline cognitive test phase.
The assessment phase commenced after a 30 d wash-in period. The cats were tested on four cognitive test protocols (landmark discrimination learning, egocentric learning and reversal, size discrimination and reversal and a DNMP task). The study proceeded over 345 d, following the schedule outlined in Table 3.
Table 3 Cognitive test schedule

DNMP, delayed non-matching-to-position.
All behavioural testing was carried out in the morning or early afternoon, with each animal tested at about the same time every day. All cognitive tests were administered by trained behavioural technicians who were blinded with respect to diets.
The delayed non-matching-to-position task
The DNMP task provides a test of both visuo-spatial learning and short-term visuo-spatial memory, and the test protocol was based on a protocol originally developed for the assessment of dogs(Reference Head, Mehta and Hartley42). The cats were given a series of paired trials, with each containing both a sample and a test component. For the sample component, the cats were presented with a single object covering a food reward over either the left or right food well. For the test component, the cats were then presented with two identical objects covering both the left and right food wells. To obtain a reward, the cat had to respond to the well that was not covered with an object during the sample phase.
All cats included in the present study had been trained on the DNMP prior to starting the study. At baseline, they were all given five test sessions, which was used in group allocation. During the test phase of the study, the cats were tested for relearning of the DNMP task at a delay, with the delay between sample and test phase set at 5 s. To successfully acquire the task, the cats were required to successfully complete a two-stage learning criterion, as described previously for dogs(Reference Head, Mehta and Hartley42).
Egocentric test protocol
The egocentric task is a spatial discrimination task in which cats were required to use their body position to locate a reward by responding to an object based on proximity to either its left or right side(Reference Christie, Studzinski and Araujo39). The test protocol for the egocentric task consisted of a 1 d preference test phase, an egocentric discrimination phase and an egocentric reversal phase. The reversal task is used to assess executive function, a higher-level cognitive function that includes the ability to switch response sets.
During the preference test phase, the cats were given a preference test consisting of ten presentations of two identical objects, one of which covered the right food well and the other the left. The cats were rewarded for responding to either object. The side that the cat responded to most frequently was designated the preferred side. If both sides were selected equally often, the preferred side was decided by a coin toss. The cats' preferred orientation was used as the correct orientation for the egocentric discrimination phase. Thus, if the preferred side was the one to the cat's left side, then during the egocentric discrimination phase, the cat was rewarded for responding to the object.
For the egocentric discrimination phase, the cats were given twelve trials per d using all three food wells, with two wells used for each trial. There were three possible configurations: left v. centre, left v. right or right v. centre. Each configuration was presented to the cat on exactly four trials. Cats were tested daily until they successfully achieve a two-stage learning criterion. The first stage was achieved when they either obtained eleven or more correct choices on 1 d, twenty of twenty-four over two consecutive days or twenty-nine of thirty-six over three consecutive days. To complete the second stage, the cats were required to respond correctly on at least twenty-six of thirty-six trials over three successive sessions.
After completion of the egocentric discrimination phase, the correct location was switched to the opposite side and the cats were now rewarded for responding to the originally incorrect side. This constitutes the initial egocentric reversal task. After completing the first reversal task, the cats were given additional reversal training until they complete a total of forty sessions following the egocentric discrimination. For the first and second reversal tasks, a two-stage learning criterion was used. The first stage was achieved when they either obtained eleven or more correct choices on 1 d, twenty of twenty-four over two consecutive days or twenty-nine of thirty-six over three consecutive days. To complete the second phase, the subjects were required to respond correctly on at least twenty-six of thirty-six trials over three successive sessions. For the subsequent reversals, a one-stage criterion was used in which the cats were assumed to learn if they either responded correctly on 90 % or more of the trials on a single day or if they were correct on 80 % of the trials over two successive days.
Landmark discrimination task
This task provides a measure of allocentric spatial learning, in which the cats were required to utilise the location of an external landmark to determine the location of a food reward. The protocol had two parts. In the first, referred to as land-0, a yellow rod, the landmark, was attached to the middle of one of two coasters and the cats were required to respond directly to the landmark to obtain a reward. The cats were tested once daily for ten trials per d until they either successfully completed a two-stage learning criterion or failed to complete the criterion within thirty training days. To successfully complete the criterion, the subjects had to first respond correctly on at least nine of ten trials on a single day or on sixteen of twenty trials over a 2 d period. To successfully complete the second phase, the cats were required to respond correctly on 70 % of the trials over three consecutive days. Thus, a minimum of 4 d was required to complete the criterion. Cats that failed to successfully complete the task within the 30 d were given a programme of remedial training. If a cat did not pass land-0 after completing remedial training, it was not tested further on the subsequent landmark tasks. After completing the first task, the landmark was moved to a position 2·5 mm from the edge of the coaster and the cats were then presented with the choice of responding to one of the two coasters. This task, referred to as land-1, required the cat to respond to the coaster that was closest to the landmark.
Size discrimination and reversal task
The size discrimination task provides a measure of visual discrimination learning ability, a basic function that depends on associative learning, and the reversal task provided an additional assessment of executive function. For this task, the cats were first trained to selectively respond to one of the two objects that differed in size, but were otherwise identical. The protocol started with a 1 d preference test, in which the cats were allowed to respond to either of the two objects over ten trials, with all responses associated with reward. The object chosen most often was considered the preferred object and was the object associated with reward during the discrimination learning phase.
After completing the preference test, the cats were given up to forty sessions to successfully complete a two-stage learning criterion on the size discrimination task. Training on the reversal learning task started after a cat completed the size discrimination task. The procedure was identical to that used for the size discrimination learning test, except that the reward contingencies were reversed (i.e. the object that was positive on the initial training was negative on the reversal training and vice versa). A maximum of forty sessions was allowed on the reversal task. For both phases of the task, the learning criterion was the same as that used for the landmark test.
Body weight, fatty acid profile of erythrocytes, vitamin B12, folic acid and total antioxidant status
Prior to the start of the study, baseline jugular blood samples were collected by a veterinary technician for measurements of the fatty acid profile in erythrocytes, folic acid, B12, homocysteine and total antioxidant status. These measures were repeated after 200 or 345 d of treatment. Body weight was recorded at 2-week intervals. Erythrocyte samples for fatty acid profiles were sent to the Lipid Chemistry and Molecular Biology Laboratory (Department of Nutritional Sciences, University of Connecticut, Storrs, CT, USA) and were analysed based on a protocol published in a previous paper(Reference Watkins, Allen and Hoffmann44). Blood samples were sent to the Nestlé Purina Petcare PTC Clinical Laboratory for the analyses of total antioxidant status, homocysteine, vitamin B12 and folic acid. Plasma levels of total antioxidant status and homocysteine were measured photometrically with a Cobas c311 Clinical Chemistry Analyzer (Roche Diagnostics). Plasma levels of vitamin B12 and folic acid were measured by an electrochemiluminescence immunoassay with a Cobas e411 Immunochemistry Analyzer (Roche Diagnostics).
Statistical analysis
The original plan for analysis of cognitive data was to calculate errors to achieve a criterion level of responding, with response failures constituting 0·5 errors. However, there were several occasions in which individual cats did not achieve the a priori learning criterion because of too frequent response failures. This had the effect of elevating the total number of errors in individual cats that were very likely to respond correctly when they did respond. Accordingly, we examined a second target variable, that of percentage correct response out of total attempted responses, in which trials with response failures were ignored. Thus, if an animal responded correctly on five trials out of ten, and failed to respond on the other five trials, its score would be 100 %, despite the fact that a priori it would have been scored as making 2·5 errors. In some instances, it also became necessary to drop individual animals from specific tasks. Thus, for the BPB group, two animals were dropped from the landmark test, two from the size test and one from the DNMP test.
Prior to statistical analysis, the data were tested for normality using the Kolmogorov–Smirnov test, and in every case, normality was confirmed. The data were then analysed statistically using either ANOVA or two-tailed t tests.
For the statistical analyses, effects were considered marginally significant if a statistical significance of 0·10 was achieved and statistically significant if a level of significance of 0·05 was obtained. Repeated-measures ANOVA was used to test for differences between groups in body weight, fatty acid profiles of erythrocytes, vitamin B12, folic acid and total antioxidant status. Values are means with their standard errors, except for the cognitive data in the figures.
Results
Effect on performance of the egocentric test
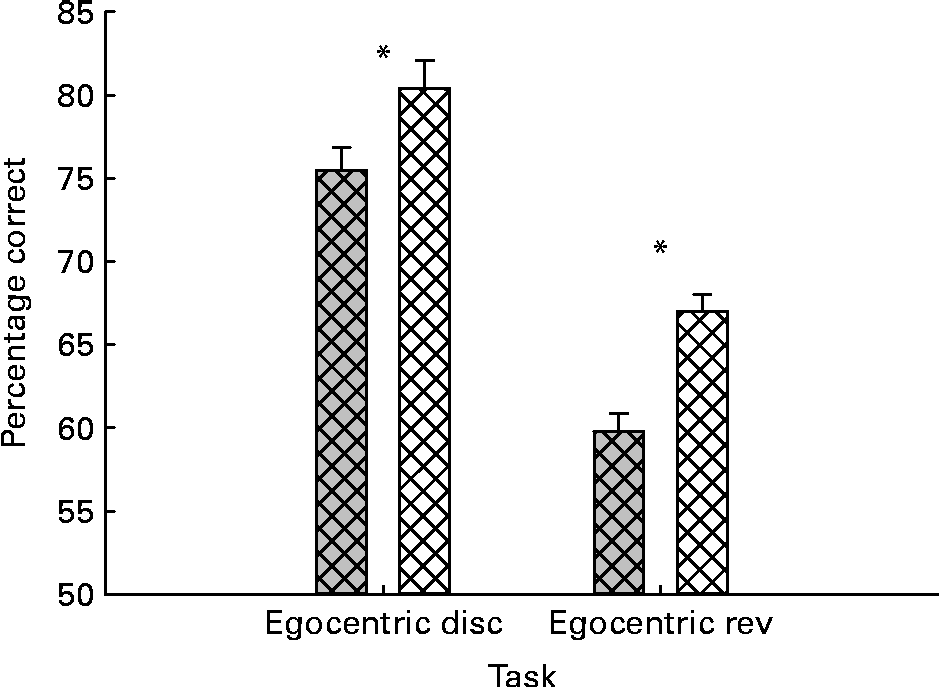
The results for the initial learning and reversal were first analysed with repeated-measures ANOVA, with task as the within-subject variable and both treatment (control v. BPB) and experience as the between-subject variables. For the percentage correct measure, the analysis revealed highly significant main effects of treatment and task (P= 0·000 and 0·000, respectively). As shown in Fig. 2, the significant treatment effect reflected the BPB group performing more accurately on both tests. Multiple comparisons using Tukey's method revealed significant differences between groups on both original learning (P= 0·042) and reversal learning (P= 0·002).

Fig. 2 Effects of brain protection blend (![]() ) supplementation on cats’ performance in egocentric tests. The data are mean values with their standard errors and n 16 for both groups. The performance was expressed as percentage correct choices. * Mean values were significantly different in both egocentric discrimination (disc) learning and reversal (rev) tests (P< 0·05).
) supplementation on cats’ performance in egocentric tests. The data are mean values with their standard errors and n 16 for both groups. The performance was expressed as percentage correct choices. * Mean values were significantly different in both egocentric discrimination (disc) learning and reversal (rev) tests (P< 0·05). ![]() , Control.
, Control.
The initial analysis also included experience, and the results were not statistically significant (see Table 4). Similar data were obtained for all tasks. Consequently, the experience variable was dropped for all subsequent analyses.
Table 4 Performance (% correct) on egocentric learning and reversal as a function of pre-test experience (Mean values with their standard errors)

There was considerable variability in the repeated reversal phase, with some animals completing only a single reversal, while others were able to successfully complete eight reversals. There were also differences between groups (P= 0·04), with the control group completing an average of 3·19 (sd 0·42) reversals and the BPB group completing an average of 4·67 (sd 0·55) reversals. Fig. 3 shows the percentage of animals from each group that successfully completed each reversal.

Fig. 3 Acquisition of multiple reversal tasks as a function of food group. The y axis shows percentage of total animals that successfully acquired each reversal. The x axis shows number of reversals that were successfully completed. —, Brain protection blend group; ![]() , control.
, control.
Effect on performance of the landmark test
Two of the animals in the BPB group were dropped before completing the land-0 phase of the study because of inconsistent responding and were not included in statistical analysis. The data from land-0 and land-1 were analysed with repeated-measures ANOVA, with treatment group and previous experience as between-subject variables and task as a within-subject variable. With the percentage correct measure, the results revealed a significant effect of task (P= 0·009) and no other significant effects or interactions. The results are summarised in Table 5, which illustrates that the significant effect of task was due to the animals performing more accurately on the land-0 task than on the land-1 task. Table 5 also shows that the control subjects performed less accurately than the animals on the BPB diet on both tasks, but the difference between the control and BPB groups was non-significant.
Table 5 Performance on landmark, size discrimination and reversal and delayed non-matching-to-position (DNMP) tests (Mean values with their standard errors)

BPB, brain protection blend.
* Including addition of DHA, EPA, vitamin C and elevated levels of arginine, B vitamins, Se and α-tocopherol.
† n 15 for DNMP test.
Effect on performance of the size discrimination learning and reversal tests
The data were first analysed with repeated-measures ANOVA, with treatment group and experience as between-subject variables and task (size discrimination and reversal) as the within-subject variable. The results of the percentage correct analysis are summarised in Table 5 and revealed a significant effect of group and a marginally significant effect of task. As illustrated in Table 5, the significant group effect reflects more accurate learning on both tasks by the BPB group. The task effect is due to less accurate performance on the reversal learning phase. Post hoc analysis with Tukey's test revealed that the group effect was largely driven by significant group differences on the size discrimination phase (Tukey's P= 0·010).
Effect on performance of the relearning of delayed non-matching-to-position test
The data were first analysed using a factorial ANOVA (percentage accuracy), with diet and previous experience as within-subject variables. The analysis revealed a significant effect of diet (P= 0·0149), and no other significant effects or interactions. Table 5 compares performance during baseline with performance in the treatment phase, and indicates that the significant diet effect reflects more accurate performance of the group on the BPB diet.
Effects on body weight, fatty acid profile of erythrocytes, vitamin B12, folic acid and total antioxidant status
The effects of the nutrient blend on the fatty acid profiles of erythrocytes are summarised in Table 6. Cats fed the BPB diet had significantly higher DHA, EPA, total n-3 fatty acids, lower LA and total n-6 fatty acids than the cats fed the control diet at the end of the study. There was no significant difference in fasting blood levels of vitamin B12, homocysteine, total antioxidant capacity and folic acid between the two groups at baseline (data not shown). The test diet did not significantly affect vitamin B12, homocysteine and total antioxidant capacity (data not shown). Cats fed the test diet had significantly (P< 0·05) higher fasting blood levels of folic acid (10·18 (sd 1·41) v. 15·65 (sd 1·51) ng/ml) at 200 d after the feeding started. Body weight did not differ significantly between the control and the BPB groups at baseline (Table 2) and at the end of the study (4·10 (sd 0·27) v. 4·03 (sd 0·18) kg).
Table 6 Effects of the nutrient blend on fatty acid profile of cat erythrocytes (Mean values with their standard errors, n 16)

BPB, brain protection blend.
* Mean values were significantly different from control (P< 0·05).
† Including addition of DHA, EPA, vitamin C and elevated levels of arginine, B vitamins, Se and α-tocopherol.
Discussion
The primary purpose of the present experiment was to examine the effects on cognitive function of long-term maintenance of cats on a BPB diet. The testing protocol examined performance on a battery of cognitive tests over a 1-year period.
Baseline cognitive ability was initially used in placing the cats into two cognitively equivalent groups. Over the course of the test phase, the cats were tested on four test protocols: (1) landmark discrimination learning, (2) egocentric learning and reversal, (3) size discrimination learning and reversal and (4) relearning of the DNMP task.
On all but the landmark discrimination protocol, the animals on the BPB diet showed significantly better performance than the controls. In the egocentric task, the animals in the treatment group showed significantly greater accuracy in responding in the initial discrimination and reversal task and completed more reversal learning problems than did the controls. On the size and reversal protocol, there was a highly significant effect of diet, with the SPB group performing more accurately on both tasks and with the group differences on the size discrimination task being highly significant. The final protocol that showed significant differences was the DNMP task, again with the animals on the BPB diet performing more accurately than the animals on the control diet. It is important to note, however, that we used a percentage accuracy measure in which an animal's score on the task was calculated by dividing the number of correct responses by the total responses. We did not include trials on which the subjects did not respond. This measure was used because the training protocol counted a non-response as an error and a large proportion of the cats showed occasional or frequent non-responses, which increased the total number of trials confounding the usefulness of the error measure as an index of performance.
Prior cognitive experience represented another potential confound. A total of twelve cats used in the study had an extensive previous training, while the previous experience of the other twenty was more limited. We controlled for this in group placement. In addition, we did not find differences linked to prior experience. We suspect that, although the groups differed in prior experience, the inexperienced group had been tested on three different cognitive test protocols prior to starting the study, which may have blunted effects of experience on cognitive performance.
Collectively, these data indicate that the BPB diet has global cognitive benefits. The nutrient blend may exert cognition-enhancing benefits or neuroprotective benefits, or possibly both. The suggestion that the blend has cognition-enhancing properties is supported by the fact that significant group differences were observed on the egocentric task, which started within a month of the start of treatment, which is probably too short a period for demonstration of neuroprotective effects. On the other hand, better DNMP performance at the end of the 1-year study suggests that the blend may not only enhance brain function, but also retard brain ageing. In this context, it is important to note that the control cats performed at the same level at the end of 1 year as they did at baseline. However, as they had previously been trained on the same task, we normally expected at least a small improvement in performance, reflecting savings. The absence of any savings effect may be indicative of an age-dependent cognitive decline in the control group over the 1-year period of the study. Of course, this is only a hypothesis, which would be supported by evidence that younger cats, which do not show cognitive decline, will show improvement in DNMP relearning after 1 year. The results from the present study support the hypothesis that the best option to manage brain ageing successfully is to retard ageing-induced changes in the brain by reducing or eliminating risk factors associated with brain ageing and dementia(Reference Pan1).
Our decision to test the effects of a nutrient blend on cognitive function in cats was based on our hypothesis that no single nutrient or bioactive compound is likely to be sufficient for retarding brain ageing and reducing the risk of dementia and CDS, and that the best option to manage brain ageing successfully is to retard ageing-induced changes, especially brain atrophy, by reducing or eliminating risk factors associated with brain ageing and dementia(Reference Pan1). The selection of the ingredients of the BPB was based on their ability to reduce or eliminate risk factors associated with brain ageing and dementia(Reference Selhub, Bagley and Miller18, Reference Ciubotaru, Lee and Wander25–Reference Pocernich, Lange and Sultana35). We developed a test diet formulation for the cat study, including the addition of fish oil and ascorbic acid, and elevated levels of arginine, B vitamins and antioxidant levels (Table 1). The levels of B vitamins and antioxidants in the BPB diet are present in commercially available, highly nutrient-dense products for adult cats. With the exception of the inclusion of fish oil and ascorbic acid, and elevated levels of other BPB ingredients, the control and BPB diets were formulated to make sure that both diets have the same levels of protein, fat, carbohydrates and similar levels of essential fatty acids (Table 1), Ca, choline, K, Mg, taurine, Zn and amino acid profile (data not shown). More importantly, all the essential nutrients in the control diet met the daily nutrient requirement recommended for cats by the Association of American Feed Control Officials.
The observed beneficial effects of the nutrient blend on brain functions in cats are consistent with the observation of a Mediterranean diet improving cognitive function in older adults(Reference Feart, Samieri and Barberger-Gateau45). In fact, all the ingredients in the BPB are present in the fruits, vegetables, cereals, seeds, legumes, vegetable oils and fatty fish of the Mediterranean diet. The beneficial effects could be attributable to various components of the nutrient blend. Our selection of B vitamins was further supported by a recent study showing that B vitamin supplementation reduced blood total homocysteine and slowed down the decline in cognition in people with mild cognitive impairment(Reference De Jager, Oulhaj and Jacoby46). As the B vitamins came from many of the food ingredients in both the control and test diets, the control diet contained all the B vitamins higher than the daily requirements for cats. Vitamin premix was used to further increase the B vitamins in the test diet; cats fed the test diet had significantly higher levels of folic acid, but did not significantly affect vitamin B12 and homocysteine levels at 200 d after the start of the feeding study.
In human subjects, decreased levels of DHA are associated with cognitive decline in both normal elderly subjects and subjects with dementia and Alzheimer's disease(Reference Conquer, Tierney and Zecevic47, Reference Heude, Ducimetière and Berr48). In addition, fish oil supplementation has been reported to improve aspects of cognitive function in people(Reference Yurko-Mauro, McCarthy and Rom49). Positive effects of fish oil administration have also been reported in aged rodents(Reference Jiang, Shi and Wang50). The optimal levels of DHA and EPA in cats for maximal brain benefits have not been determined yet. In human subjects, it was proposed that maximal cardiovascular protection of DHA and EPA is achieved with a concentration of 8 % erythrocyte fatty acids as EPA+DHA(Reference Harris51). Coincidentally, the levels of DHA and EPA in the nutrient blend resulted in 8 % of erythrocyte fatty acids as EPA and DHA in the cats. More studies are needed to determine the optimal levels of erythrocyte fatty acids as EPA+DHA for maximal protection against brain ageing and CDS in cats.
High blood pressure is a risk factor for brain ageing and high risk of Alzheimer's disease in people(Reference Duron and Hanon17, Reference Qiu, Winblad and Fratiglioni22). Another mechanism that could contribute to the beneficial effects is the role of l-arginine in the production of NO, which acts both directly on the central nervous system as a neuroregulator and also peripherally. NO has frequently been linked to cognition through a variety of pathways, and administration of NO donors in rats is reported to protect against the development of cognitive disorders(Reference Manukhina, Pshennikova and Goryacheva52). Peripherally, NO is known to be important in controlling blood pressure. In rats, for example, induced hypertension can be counteracted by the administration of l-arginine(Reference Rajapakse, De Miguel and Das53). Dietary l-arginine supplementation has been shown to decrease both systolic and diastolic blood pressure in subjects with mild hypertension(Reference Ast, Jablecka and Bogdanski54).
Oxidative stress and inflammation are important contributors to brain ageing and dementia(Reference Markesbery21, Reference Weninger and Yankner23); the antioxidants and EPA in the blend may help to reduce oxidative stress-induced damage and low-grade inflammation in the whole body including the brain. Both Se and vitamin E came from the diet ingredients and exceeded the daily requirements of adult cats; there was no significant difference in the total blood antioxidant capacity between control and the test diet.
It is worth noting that all of the nutrients included in the blend, except vitamin C, DHA and EPA, were also present in the control diets in amounts higher than daily requirements of cats. Therefore, the control cats were not deficient in any of the essential nutrients. However, all the nutrients included in the BPB were presented in the test diets at levels higher than those in the control diet. For instance, the levels of B vitamins were at least 3·5 times that of the daily requirements in the nutrient blend, and arginine was two times that of the daily requirement for adult cats. In addition to enhanced n-3 fatty acids, the blood level of folic acid was significantly increased in the cats fed the BPB diet. Enhanced brain function in the cats fed the BPB diet suggests that cats need to consume nutrients at levels higher than their daily requirement to benefit more from the effects of nutrients on brain health.
In summary, the present study shows that the BPB that we selected to reduce or eliminate the known risk factors for brain ageing and dementia can significantly improve cognitive function and may retard age-related decline in cognitive function in normal middle-aged and old cats. More studies are required to determine the lowest level needed for each nutrient to have maximal beneficial effects on brain health and function, and confirm any inhibitory effects of the BPB on neuron loss and brain atrophy in cats.
Acknowledgements
All authors declare no conflicts of interests associated with the present study. The study was entirely funded by Nestlé Purina Research. Y. P., J. A. A., C. d. R. and N. W. M. designed the study, interpreted the results and prepared the manuscript; J. B. organised and supported the feeding trial; A. G. and S. B. contributed to the formulation and production of the diets, respectively. The authors wish to thank Wendell Kerr at Nestlé Purina Research for his statistical analysis of the blood chemical data, Drs Bruce A. Watkins and Yong Li at the University of Connecticut for erythrocyte fatty acid profile analysis, Ms Shanon L. Byous at Nestlé Purina Petcare PTC for the analysis of blood B vitamins and total antioxidant status and Ms Rachel Anderson at Nestlé Purina Petcare PTC for homocysteine analysis.