Vitamin D is important for bone health because of the actions of its hormonal form, 1,25-dihydroxyvitamin D, elevating serum Ca and P levels necessary for bone mineralisation.
Cod liver oil is a traditional source of vitamin D in Iceland. As the country is located at 62–67°N, little or no vitamin D can be synthesised in the skin from approximately October to April( Reference Gunnarsson, Indridason and Franzson 1 ). Dietary sources are also limited, and milk and dairy products are generally not fortified with vitamin D. Supplements are therefore especially important, and the intake of cod liver oil or other vitamin D-containing supplements is recommended for people of all ages( 2 ).
However, when and to what extent, cod liver oil intake at different periods of life becomes significant to variations in bone mineral density (BMD) in old age is not clear, as there are few studies focusing on this relationship. Findings from a Norwegian follow-up study suggested that childhood cod liver oil intake may be associated with adverse effects on BMD in elderly women, supposedly due to the high vitamin A (in the form of retinol) content of cod liver oil consumed early in life( Reference Forsmo, Fjeldbo and Langhammer 3 ). However, as studies are few, it is still not clear what effect cod liver oil intake can have on bone health, due to its consumption during childhood, supplying high amounts of both retinol and vitamin D.
The association between cod liver oil intake in adulthood or old age and bone density has not been studied either in detail. Two studies on intakes of elderly women have found no adverse effects on BMD( Reference Sigurdsson, Franzson and Steingrimsdottir 4 , Reference Macdonald, Mavroeidi and Barr 5 ), while one study found an association with lower overall fracture risk( Reference Barker, McCloskey and Saha 6 ). However, these studies did not involve intakes in childhood or adolescence, and it is possible that bone density may be differently affected in different stages of life. Furthermore, studies on cod liver oil intake and BMD of elderly men have not been undertaken at all.
It is of public health importance to assess possible effects of cod liver oil intake on bone at different periods of life, particularly as bone health is the primary justification for recommending and taking cod liver oil.
The aim of the present study — the Age, Gene/Environment Susceptibility (AGES) –Reykjavik Study — was to assess whether retrospective self-reports of cod liver oil intake during adolescence and midlife are associated with hip BMD in old age. Further, the investigation examined the association between intake of cod liver oil in old age and hip BMD, as well as the association between cod liver oil intake in old age and serum 25-hydroxyvitamin D (25(OH)D) concentration. The AGES–Reykjavik Study is a large epidemiological study of 5764 elderly participants with extensive health related data for all participants, including that on bone health and serum 25(OH)D concentrations, as well as data on dietary intake in adolescence, midlife and old age.
Methods
Subjects
The AGES–Reykjavik Study originates from the Reykjavik Study, a large population-based cohort study which was launched in 1967. All men and women born in 1907–35 (n 30 795) and residing in Reykjavik and nearby communities in 1967 were selected, of them 27 281 were invited to participate and a total of 19 381 attended( Reference Olafsdottir 7 – Reference Sigurdsson, Thorgeirsson and Sigvaldason 10 ). Of the 11 549 previously examined Reykjavik Study cohort members still alive in 2002, when the AGES–Reykjavik examinations began, 8030 individuals were randomly chosen and invited to participate. In 2006, when the AGES–Reykjavik examinations concluded, 5764 individuals (72 %) had been enrolled and examined (42 % male). Participants were 66–96 years old at time of the examinations, average age being 76 years.
The AGES–Reykjavik examination was completed in three clinic visits within a 4- to 6-week time window. Extensive data were collected during clinical examinations, e.g. data on physical and cognitive function, anthropometry, health history, and food history during adolescence, midlife and in old age. Participants also underwent quantitative computed tomography-scans and were asked to bring to the clinic all medications and supplements used in the previous 2 weeks, representing current usage( Reference Harris, Launer and Eiriksdottir 8 , Reference Sigurdsson, Aspelund and Forsberg 11 ).
Of the 5764 participants, 933 individuals did not undergo the quantitative computed tomography scanning, and additional thirty-three individuals did not give adequate dietary information. Therefore, excluding those 966, data from 4798 individuals (44 % male) were used in the present study.
The AGES–Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the MedStar IRB for the Intramural Research Program, Baltimore, MD.
Measurement of serum 25-hydroxyvitamin D
Blood samples were drawn at recruitment into the AGES Study, i.e. old age. Measurement of 25(OH)D was conducted by means of a direct, competitive chemiluminescence immunoassay using the Liaison ‘Flash’ Chemiluminescence Immunoassay from DiaSorin 25(OH)D TOTAL assay (DiaSorin, Inc.). The interassay CV was < 6·5 % when calculated data are from measurements using a frozen serum pool as the control sample and < 12·7 % when calculated data are from measurements using Liaison quality controls.
Bone mineral density/bone variables
Quantitative computed tomography measurements, providing true volumetric density, were performed on the left hip using a 4-detector CT system (Sensation; Siemens Medical Solutions). Scans were acquired using a standardised protocol, and encompassed the proximal femur from a level 1 cm above the acetabulum to a level 5 mm inferior to the lesser trochanter with a 1 mm slice thickness. Further procedures and quality assessments are described in detail elsewhere( Reference Harris, Launer and Eiriksdottir 8 , Reference Sigurdsson, Aspelund and Chang 12 ).
The variables used in the present study are volumetric integral BMD (g/cc), reflecting both trabecular and cortical bone mass, of femoral neck and trochanteric region, encompassing both trochanters. Reasons for exclusions from the quantitative computed tomography were the inability of some participants to lie supine or their weight being over 150 kg. Furthermore, hip scans were not performed on individuals who had undergone hip replacement surgery.
Dietary information
Dietary data were gathered using a short FFQ (AGES-FFQ) designed for the AGES–Reykjavik Study. The questionnaire is divided into three parts, including sixteen questions on adolescent intake (14–19 years), seventeen questions on midlife intake (40–50 years) and thirty questions on current intake. Foods and food groups were selected for the questionnaire on the basis of their contribution to the absolute food intake of elderly Icelanders according to former National Nutrition Surveys( Reference Steingrimsdottir, Thorsgeirsdottir and Aegisdottir 13 ), as well as unique nutritional qualities and possible connection to the development of various diseases in later life. The questionnaire has been described previously( Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 14 , Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 15 ). Frequency of cod liver oil intake was measured by asking a question in each part of the AGES-FFQ. The response categories were: (1) never; (2) less than once a week; (3) one to two time(s) a week; (4) three to four times a week, (5) five to six times a week; (6) daily.
Validity of the parts of the AGES-FFQ relating to midlife and present intake have been assessed in previous papers( Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 14 , Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 15 ). Cod liver oil was among the items showing the highest validity in the questionnaire. When assessing validity of questions on midlife diet, frequency of intake reported in the AGES-FFQ by 56–72-year old individuals was compared with detailed dietary data, gathered from the same individuals 18–19 years previously, i.e. in midlife, as a part of a national dietary survey( Reference Steingrimsdottir, Thorsgeirsdottir and Aegisdottir 13 ). Correlation using Spearman's ρ was r 0·53, P≤ 0·001; r 0·56, P≤ 0·001 for men and women respectively( Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 14 ). Validity of questions on old age intake was assessed among elderly individuals (65 years and older) by comparing answers of the AGES-FFQ to the 3-d weighed food records completed by the same individuals. Correlation using Spearman's ρ was r 0·51, P< 0·001; r 0·42, P< 0·001 for men and women respectively( Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 15 ).
Covariates
For examining the association between intake of cod liver oil through different periods of life and hip BMD, we selected a priori the following set of covariates: physical activity both in current (old age) and midlife, current (old age) alcohol intake, current (old age) and previous smoking, education, oral oestrogen intake (current (old age) and previous) for women, age, BMI, and milk consumption at the same period of life. Midlife BMI was chosen as a covariate for the retrospective data, and current (old age) BMI for current data.
Participants were asked about the level of moderate or vigorous physical activity, both present and past activities, and split into categories (never, rarely, occasionally, moderate or high). Education was categorised into primary school, secondary school, college or university. Old age consumption of alcohol was converted into g/week using 14 g of alcohol as a standard drink and was divided into < 25, 25–50 and >50 g/w. Midlife data on BMI had been collected in the Reykjavik-Study( Reference Olafsdottir 7 ).
For early life most of these covariates can only be considered surrogate measures of corresponding early life characteristics. On the other hand, for midlife and old age, the covariates selected are potential predictors of both bone health and dietary habits.
Statistical analysis
Characteristics of the study participants are described using mean and standard deviation of normal variables, median and interquartile range for skewed variables and percentages for dichotomous variables.
Due to the approximate normal distribution of the source BMD variables in our population, they were transformed into sex-specific z-scores, reflecting the number of standard deviations from the mean BMD in our population of 66–96 years of age. Univariate and multivariate linear regression were then used for examining the association between intake of cod liver oil and BMD variables.
Intake of cod liver oil in the AGES-FFQ was categorised into three groups; never or < once/week, one to six time(s) a week, and daily. The lowest intake group (never or < once/week) was in all cases used as the referent, and results represented as difference in z-scores (Δ) with higher frequencies of consumption compared to the referent. Student's t test was used to test whether BMD was linearly related to cod liver oil intake (ordinal values). Visual inspection of model residual suggested that use of z-scores was justifiable.
Data are presented unadjusted and adjusted for age, midlife or current (old age) BMI, past and present physical activity, alcohol consumption, milk consumption at the same period of life.
For stability analyses individuals taking medication known to affect bone health at the time of AGES examinations, 435 men (21 %) and 992 women (37 %), were excluded. The list of medications that resulted in exclusion for this secondary analysis was antiepileptic medication, Ca supplements, oral oestrogens, glucocorticoids, osteoporosis drugs, prostate disease drugs, proton pump inhibitors, oral steroids and thyroid agonists. Statistical analyses were conducted using SAS (version 9.2; SAS Institute, Inc.).
Results
Potential confounding factors in relation to cod liver oil intake at different periods of life are shown in Table 1. Intake of cod liver oil was fairly common, with the proportion of participants reporting daily intake increasing with age, from about 30 % in adolescence to midlife, and about 60 % at old age.
Table 1 Possible confounding factors in relation to cod liver oil intake in adolescence (14–19 years), midlife (40–50 years) and current old age (66–96 years) (Mean values and standard deviations; median values and interquartile ranges (IQR); proportions and percentages)

* F-test (type III) for continuous variables, χ2 test for dichotomous variables.
† Medications: reported intake of medication known to affect bone density: antiepileptic medication, Ca supplements, oral oestrogens, glucocorticoids, osteoporosis drugs, prostate disease drugs, proton pump inhibitors, oral steroids and thyroid agonists.
For both sexes the correlation between intake of cod liver oil in adolescence and at older age was relatively weak (Spearman r 0·20, P< 0·0001), while the correlation between intake in midlife and old age was stronger (Spearman r 0·49, P< 0·0001). A total of 16 % of participants reported no intake of cod liver oil from adolescence to older age, while 21 % of subjects reported daily intake at all three time points.
The association between retrospective intake of cod liver oil and difference in z-scores, calculated from hip BMD in old age (using < once/week as a referent) is shown in Table 2. Data are shown separately for men and women and both unadjusted and adjusted for confounders. Individuals taking cod liver oil more frequently in adolescence and/or midlife did not have significantly different hip BMD in old age, compared with those with the lowest frequency of intake. This was seen for both men and women, and for femoral neck and trochanter.
Table 2 Difference in hip bone mineral density (BMD) in old age, presented as z-scores, between individuals with retrospective cod liver oil intake of 1–6 time(s)/week or daily intake, compared to <once/week (z Scores and 95 % confidence intervals)

* Adjusted for age, past and present physical activity, midlife BMI, alcohol consumption, current and previous smoking, education, current or previous use of oral oestrogens (among women) and milk consumption at the same period.
The association between cod liver oil intake in current old age and hip BMD was also assessed (Table 3), excluding supplement users from the analysis, that is, individuals taking vitamin and/or mineral supplements (other than cod liver oil). There was no significant difference in hip BMD in relation to cod liver oil intake for men, while women with daily intake had significantly higher z-scores on average (0·10 for femoral neck and 0·09 for trochanter), compared to those with the lowest frequency of intake ( < once/week). Analysing the association between lifetime cod liver oil consumption (three time periods amalgamated through an overall score) and old age BMD showed similar results, as for BMD and current consumption (data not shown).
Table 3 Difference in hip BMD, presented as z-scores, in relation to current cod liver oil intake (dietary supplement users excluded) (z Scores and 95 % confidence intervals)
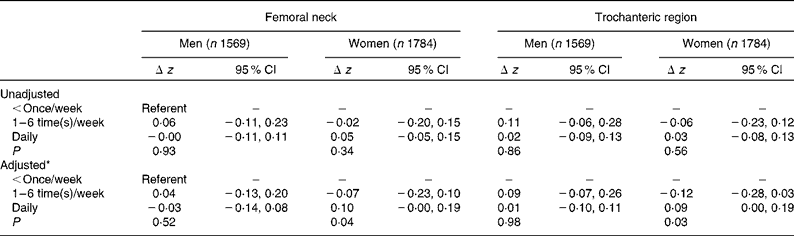
* Adjusted for age, past and present physical activity, current BMI, alcohol consumption, current and previous smoking, education, current or previous use of oral oestrogens (among women) and current milk consumption.
There was a clear association between current intake of cod liver oil and serum 25(OH)D concentrations (Table 4). Median concentrations for men and women with the lowest frequency of intake being 40·2 and 37·8 nmol/l respectively, compared to 61·9 and 56·4 nmol/l for those with daily intake. When excluding supplement users from the analysis, median serum 25(OH)D concentrations for those with the lowest cod liver oil intake were 37·2 and 31·9 nmol/l for men and women respectively, compared with 60·6 and 55·2 nmol/l for those with daily intake of cod liver oil. Both men and women with intake of one to six time(s)/week or daily intake of cod liver oil had significantly higher serum levels than those with the lowest frequency of intake.
Table 4 Serum 25-hydroxyvitamin D concentration in nmol/l in relation to current cod liver oil intake (Number of participants and median values; 10th percentile (P10) and 90th percentile (P90))
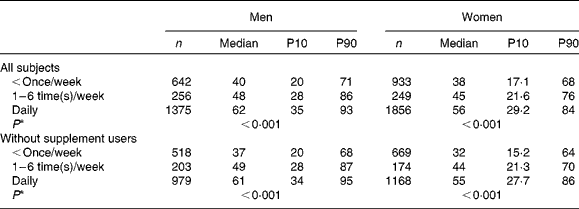
* Test for trend.
Almost one-third of the participants were taking medications known to be able to affect bone health. Proton pump inhibitors were most common (13 %), followed by thyroid agonists, osteoporosis related drugs, oral oestrogens for women, and prostate disease drugs for men. We therefore performed analysis without these individuals, reaching the same conclusions as in our primary analysis where these subjects were included.
Discussion
No significant association was found between retrospective intake of cod liver oil in adolescence or midlife and hip BMD among participants of the AGES–Reykjavik Study. Current intake was also not associated with hip BMD in men. Women with daily intake had slightly higher z-scores of both femoral neck and trochanteric region, compared to those with the lowest frequency of consumption. Current cod liver oil intake of both sexes was positively associated with serum 25(OH)D concentration.
Adequate vitamin D is important in adolescence for bone accretion associated with rapid growth( 16 ). Still, intervention studies are limited in number, with some studies showing that vitamin D supplementation can increase bone mineral content and BMD of young adolescent girls( Reference Viljakainen, Natri and Kärkkäinen 17 , Reference Mølgaard, Larnkjaer and Cashman 18 ), while other studies show no significant effect( Reference Winzenberg, Powell and Shaw 19 ).
In the mid twentieth century, when our participants were in adolescence, cod liver oil was given to children in most schools. At that time the concentrations of both retinol and vitamin D, the two major vitamins in the oil, probably reflected that of cod liver, 83:1( 20 ). Until 2002, cod liver oil in Iceland still contained high amounts of retinol (30·000 μg/100 g), and the recommended daily spoonful (8 g) supplied approximately 2400 μg retinol and 20 μg vitamin D. Today an 8 g spoonful contains 400 μg retinol and 16 μg vitamin D. Average intake of vitamin A was approximately three times the recommended daily intake in 1990( Reference Steingrimsdottir, Thorsgeirsdottir and Aegisdottir 13 ), but has since decreased, both as a result of decreased concentration of retinol in cod liver oil, but also changes in food intake, including decreased intake of whole milk, margarine, and other vitamin A rich foods( Reference Steingrimsdottir, Thorsgeirsdottir and Olafsdottir 21 , Reference Thorgeirsdottir, Valgeirsdottir and Gunnarsdottir 22 ). Fish intake has also decreased considerably, which has resulted in minimal changes in vitamin A and D intake, as lean fish containing minimal amounts of these vitamins constituted about 80 % of total fish intake( Reference Thorgeirsdottir, Valgeirsdottir and Gunnarsdottir 22 , Reference Gunnarsdottir, Gustavsdottir and Steingrimsdottir 23 ). Changes in milk intake are mirrored in our AGES-FFQ data( Reference Eysteinsdottir, Halldorsson and Thorsdottir 24 ).
Cod liver oil is a traditional source of vitamin D in Iceland as it is in other Nordic countries. In a follow-up study of 50–70 year old women in the Norwegian Nord-Trøndelag Health Study, elderly women reporting any intake of cod liver oil in childhood had significantly lower old age forearm BMD than those with no intake( Reference Forsmo, Fjeldbo and Langhammer 3 ). The researchers concluded that the previously high concentration of vitamin A in cod liver oil, when added to an already vitamin A rich diet, may have led to total intake reaching harmful levels, as high intakes of retinol have been linked to adverse effects on bone health and even increased risk of osteoporosis and osteoporotic fractures( Reference Melhus, Michaelsson and Kindmark 25 – Reference Opotowsky and Bilezikian 29 ).
In the light of the results from the Nord-Trøndelag Health Study, we set out to explore whether those findings could be replicated using Icelandic data. In short, we did not find any indication that the intake of cod liver oil during adolescence or midlife was associated with adverse effects on BMD of either femoral neck or trochanter. It should be noted that different methods were used for measuring BMD in the two studies. Also, the Nord-Trøndelag Health Study measured forearm BMD, while we measured hip BMD, and it is possible that these bones respond differently to cod liver oil intake. Finally, we cannot rule out the possibility that misclassification of previous intake or lack of some relevant confounder adjustment might have contributed to our findings.
Intervention studies among elderly individuals have shown that increased intake of vitamin D can be associated with increased BMD( Reference Ooms, Roos and Bezemer 30 , Reference Nakamura and Iki 31 ), decreased bone loss( Reference Nakamura and Iki 31 – Reference Dawson-Hughes, Harris and Krall 33 ), and lower risk of osteoporotic fractures( Reference Nakamura and Iki 31 – Reference Trivedi, Doll and Khaw 35 ). However, many of the intervention studies include Ca supplements parallel to the vitamin D ones, and according to recent reviews the effect of vitamin D supplementation alone on BMD and fracture risk is minimal, while vitamin D given alongside Ca can have significant effect( Reference Reid, Bolland and Grey 36 , Reference Avenell, Mak and O'Connell 37 ).
Previous studies of elderly women have not shown any association between old age cod liver oil intake and BMD( Reference Macdonald, Mavroeidi and Barr 5 , Reference Sigurdsson, Franzson, Thorgeirsdottir, Burckhardt, Dawson-Hughes and Heaney 38 ). While the present results showed no such association for men, there was a slight positive association for women. It has been estimated that a 1 sd decrease in hip BMD is associated with an approximately 2·5-fold increased risk of hip fracture( Reference Marshall, Johnell and Wedel 39 , Reference Cummings, Black and Nevitt 40 ). In the present study the difference between women with daily intake v. < once/week was 0·1 z-scores for femoral neck and 0·09 z-scores for the trochanteric region (equal to 0·1 and 0·09 sd respectively). The clinical relevance of our finding is therefore most likely minimal and may even be a case of a chance finding.
The weak or insignificant association between old age cod liver oil intake and hip BMD in the present study may possibly be explained by the relatively adequate serum 25(OH)D levels in our population. Even in those individuals with no intake of cod liver oil or < once/week, median serum levels were approximately 40 nmol/l, which is close to the 50 nmol/l, considered adequate for bone health by the Institute of Medicine( 16 ). In previous Icelandic studies, serum parathyroid hormone levels stabilised at approximately 45–50 nmol/l in healthy adults and elderly individuals, suggesting vitamin D sufficiency with respect to bone health( Reference Sigurdsson, Franzson and Thorgeirsdottir 41 , Reference Steingrimsdottir, Gunnarsson and Indridason 42 ).
Steingrimsdottir et al ( Reference Steingrimsdottir, Halldorsson and Siggeirsdottir 43 ) found that only levels below 30 nmol/l were associated with significantly lower BMD of the femoral neck in this same population, and more than double the risk of hip fracture compared with the referent (50–75 nmol/l). Intake of cod liver oil may therefore mostly benefit individuals with the lowest serum 25(OH)D concentrations.
The association between old age frequency of intake and serum 25(OH)D concentrations may be considered as further validation of the question on cod liver oil in the AGES-FFQ. Participants with intake of < once a week, one to six time(s) a week and daily intake had serum levels of approximately 40, 50 and 60 nmol/l respectively.
The AGES–Reykjavik study, with its large number of participants, provided a unique opportunity to assess the association between cod liver oil intake in different periods of life and bone health in old age of both sexes. Also, extensive data gathered in the AGES-Study and midlife data received from the Reykjavik-Study allowed for adjustments of various confounding factors.
The main limitation of the study is that we relied partly upon retrospective data with 60 years of temporal separation on average, which is inherently imprecise and subjective and is likely to mask any potential modest or weak association. However, according to Dwyer & Coleman( Reference Dwyer and Coleman 44 ), foods with special characteristics (such as cod liver oil), can be recalled particularly well, and the question on midlife cod liver oil intake showed the highest validity of any food item in our FFQ. Also, midlife milk intake, an important covariate in the present study, was among the foods showing the highest correlation in validation studies of the FFQ( Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 14 , Reference Eysteinsdottir, Gunnarsdottir and Thorsdottir 15 , Reference Eysteinsdottir, Halldorsson and Thorsdottir 24 ). Another limitation is that, although the most common portion of cod liver oil is the recommended spoonful, we did not have access to the absolute amount consumed.
As we did not have any information on supplements used during midlife, we do not know if an analysis excluding midlife supplement users might have yielded different results.
Our observational associations between old age cod liver oil intake and serum 25(OH)D concentrations are cross-sectional, and thus we have to remain cautious in our interpretation as influence of other unmeasured confounders, e.g. outdoor activities and the amount of sunlight exposure, cannot be excluded. However, the seasonal variation observed in the present study was 3·9 nmol, suggesting that sun exposure is a relatively small confounding factor here.
Also, we do not have accurate enough information in order to calculate total vitamin D intake. The AGES-FFQ only includes simple global questions, such as frequency of fish intake, without specifying different types of fish, e.g. fatty fish and lean fish. However, fish intake in Iceland during the whole study period was characterised by lean fish species, containing minimal amounts of vitamin D. Also, milk intake was not fortified with vitamin D during the study period. In a recent Icelandic National Dietary Survey (INDS), the portion of the oldest age group (61–80 years), not taking cod liver oil, had an average intake of 5·3 μg/d of vitamin D from foods( Reference Thorgeirsdottir, Valgeirsdottir and Gunnarsdottir 22 ). Assuming comparable intake of our participants, it indicates that 5–6 μg/d of vitamin D might be sufficient to keep an average serum level of approximately 40 nmol/l. This is however, lower than the previously reported approximate figure of 9 μg/d of vitamin D, required to achieve an average serum 25(OH)D concentration of 50 nmol/l( Reference Cashman, Fitzgerald and Kiely 45 ).
Conclusion
In conclusion, we found no evidence that cod liver oil intake at any age might be harmful to hip BMD in old age. Old age intake of cod liver oil showed a slight positive association with hip BMD among women. Possibly, the relatively high median serum 25(OH)D concentration in the present study population, even among those not taking cod liver oil or other vitamin D-containing supplements, may mask any putative, more profound relationship between old age intake and hip BMD. Old age daily intake of cod liver oil was associated with an increase of approximately 20 nmol/l in 25(OH)D concentration, compared to no intake or less than once a week.
The significance of cod liver oil intake at various ages for the ultimate old age bone health warrants further studies, especially on intakes during childhood and adolescence, as cod liver oil is supplied in several schools and child care centres in Iceland for public health purposes.
Acknowledgements
We thank all the participants of the AGES–Reykjavik Study and the clinical staff at the Icelandic Heart Association for their invaluable contribution. We also thank Gudmundur Gudmundsson at Lysi hf for sharing his knowledge on Icelandic fish oil production in Iceland in the past.
T. E. received funding from the Icelandic Research Fund for Graduate Students (RAN090310-0796) and the Nordforsk NCoE Program HELGA.
The AGES–Reykjavik Study was funded by NIH (contract no. N01-AG-12100), the Intramural Research Program of the National Institute on Aging, and by the Icelandic Heart Association and the Icelandic Parliament.
Authors' contributions are as follows: T. E. drafted the manuscript and handled basic statistical analysis; T. I. H. handled more complex statistical analysis and interpretation of data; I. T., I. G. and L. S. participated in the conception and design of the study and revision of the manuscript; other authors contributed to material supply and conduct of the study; all authors read and approved the final manuscript.
The authors have no conflicts of interest.







