Enteral feeding is the best method of nutrition in critically ill children not only because of its safety and cost(Reference Major, Lefor and Wilson1), but also because it is more physiological and stimulates intestinal trophism. Furthermore, enteral nutrition reduces bacterial translocation and the incidence of sepsis and multiorgan failure, and has few adverse effects(Reference Galbán, Montejo and Mesejo2). The early initiation of enteral nutrition is therefore recommended(Reference Chellis, Sanders and Webster3).
However, the reduction of gastric motility that frequently occurs in the critically ill patient often leads to a poor tolerance to oral or nasogastric feeding, particularly in patients on mechanical ventilation(Reference Dunham, Frankenfield and Belzberg4). Duodeno-jejunal enteral nutrition has been shown to be a good alternative in these patients(Reference Galbán, Montejo and Mesejo2, Reference Panadero, López-Herce and Caro5). One of the major difficulties at the time of starting enteral nutrition is to be able to predict whether the patient will tolerate the nutrition and whether digestive tract complications might occur.
Several systems have been developed for evaluating the severity of illness of critically ill children and for predicting the risk of mortality at the time of admission. The most widely used are the paediatric risk of mortality (PRISM)(Reference Pollack, Ruttimann and Getson6), the paediatric index of mortality (PIM)(Reference Shann, Pearson and Slater7, Reference Slater, Shann and Pearson8) and the paediatric logistic organ dysfunction index (PELOD)(Reference Leteurtre, Martinot and Duhamel9).
There are no clinical studies that have analysed the utility of the severity of illness scores to predict tolerance to enteral nutrition. This was the objective of the present study.
Patients and methods
A prospective, observational study was performed including all critically ill children admitted to the paediatric intensive care unit between May 2005 and December 2007 and who received transpyloric enteral nutrition (TEN). The indications for TEN were the need for mechanical ventilation (198 patients) and the presence of acute respiratory failure with a risk of aspiration without mechanical ventilation (eleven patients). Tube placement was performed by blind insertion or by placing the patient in the lateral decubitus position with air insufflation(Reference Chellis, Sanders and Dean10). Nasoduodenal tubes with a guidewire were used, with a calibre of 6 to 10 French gauge. All the tubes were situated in the duodenum. The position of the tube was initially confirmed by aspiration and measurement of the pH (equal to or higher than 6) and this was subsequently confirmed radiologically A second tube was inserted through the same nasal orifice for drainage and measurement of the gastric residue every 3–4 h. The type of nutrition administered depended on the age of the patient: infant formula was administered to children less than the age of 2–3 years, and this was substituted by protein hydrolysate in patients previously diagnosed of milk-protein intolerance or with risk of severe intestinal damage (severe shock and/or hypoxia). Energy supplements in the form of dextrin-maltose, medium-chain TAG or cereals were added in some patients. Isoenergetic or hyperenergetic paediatric liquid formulae were administered to children over the age of 2–3 years. The feeding was started at a rate of 0·5–1 ml/kg per h and was increased by 0·5–1 ml/kg every 3–4 h if the gastric residue was less than 25 % of the volume administered. The general nutritional end point was 420 kJ (100 kcal) per kg metabolised per d. However, individual adaptation was performed according to clinical state, sedation and muscle relaxed administration.
PIM-2, PRISM and PELOD scores were performed at the same time, by the same investigator in all patients, at the time of starting TEN. The following data were gathered prospectively: age, sex, weight, diagnosis, surgery, indication for TEN, time from admission to the initiation of TEN, time to reach maximum energy delivery, maximum energy delivery and duration of the nutrition.
The SPSS statistical package (version 15; SPSS, Inc., Chicago, IL, USA) was used to perform the statistical study. The χ2 test and Fisher's exact test were used for the analysis of qualitative variables. Student's t test was used to compare quantitative variables between independent groups. The non-parametric tests used were the Mann–Whitney U test and the Wilcoxon W test. The Spearman test was used to study correlations. The study of the ability of the severity scores to predict digestive tract complications was performed using receiver operating characteristic (ROC) curves. A P value less than 0·05 was considered significant.
Results
During the study period, TEN was administered to 209 patients, 122 (58·4 %) boys and eighty-seven (41·6 %) girls, with a median age of 5 months (25th percentile, 3 months; 75th percentile, 17 months) and a median weight of 5·5 kg (25th percentile, 4·0 kg; 75th percentile, 9·4 kg). The diagnoses of patients were: cardiac surgery, 53 %; other surgeries, 8·2 %; respiratory insufficiency, 3 %; other medical causes (sepsis, trauma, neurological alterations, cardiac insufficiency), 35·8 %.
Sedative drugs (midazolam, fentanyl and/or propofol) were administered to 189 children (90 %) during the TEN and fifty-nine children (28 %) also received muscle relaxants (vecuronium) by continuous infusion. Inotropic drugs were required by 147 (70 %) of the patients (dopamine, 70 %; adrenaline, 28·6 %; milrinone, 53·4 %; prostaglandins, 2·2 %).
The PRISM and PELOD scores were calculated in all 209 patients whereas, due to methodological difficulties, the PIM-2 score could only be obtained in 166 patients The mean score and the risk of mortality were: PRIMS, 9·4 (sd 5) and 7 (sd 7·3) %; PELOD, 6·2 (sd 7·5) and 3 (sd 9·7) %; PIM-2, 8·2 (sd 12·4) %. There was a moderate statistically significant correlation between the severity scores: PRISM–PELOD, r 0·61 (P < 0·001); PRISM–PIM-2, r 0·44 (P < 0·001); PELOD–PIM-2, r 0·425 (P < 0·001).
The correlations found between the PIM-2, PRISM and PELOD indices and the characteristics of the enteral nutrition are summarised in Table 1. The mean time from the patients' admission until the initiation of enteral nutrition was 1·5 (sd 4·5) d; it was started within the first 24 h of admission in 151 (72 %) patients and within the first 48 h in 193 (92·3 %). There was a low but statistically significant correlation between the time from admission and the initiation of TEN with the three indices (Table 1). The maximum energy delivery in the first 24 h was 234 (sd 100) kJ/kg body weight per d (56 (sd 24) kcal/kg body weight per d) (range 22–619 kJ (5·2–148 kcal)/kg body weight per d). There was no correlation between the maximum energy delivery in the first 24 h and the clinical severity of the patients. The time to reach the maximum energy delivery was 1·4 (sd 4·7) d, and this was significantly longer in the patients with a higher PELOD score (P = 0·001). The mean duration of TEN was 13·3 (sd 16) d (range 1–96 d). There was a statistically significant correlation between the severity scores and the duration of enteral nutrition (Table 1). There was no correlation between the maximum energy delivery and the severity scores.
Table 1 Correlation between the characteristics of the nutrition and the severity scores at the time of starting nutrition

PRISM, paediatric risk of mortality; PELOD, paediatric logistic organ dysfunction index; PIM-2, paediatric index of mortality; TEN, transpyloric enteral nutrition.
Abdominal distension and/or excessive gastric residues were observed in ten (4·7 %) children; the severity scores were higher in these patients than in the other children, although the differences did not reach statistical significance (Table 2). Diarrhoea occurred in nine (4·3 %) patients. The children who developed diarrhoea presented significantly higher PRISM scores than the other children, but significant differences were not found with the PIM-2 or PELOD scores (Table 2). Digestive tract complications were not related to the type of diet.
Table 2 Relationship between the severity scores and digestive tract complications of nutrition and mortality
(Mean values and standard deviations)
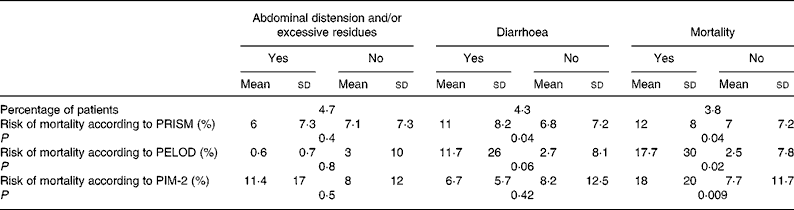
PRISM, paediatric risk of mortality; PELOD, paediatric logistic organ dysfunction index; PIM, paediatric index of mortality.
Receiver operating characteristic (ROC) curve analysis was used to assess the ability of the severity scores to predict the appearance of digestive tract complications. These indices were only able to predict the onset of diarrhoea. The PRISM score had an area under the curve of 0·67 (95 % CI 0·52, 0·82); the PELOD score had an area under the curve of 0·63 (95 % CI 0·44, 0·83) and the PIM-2 score had an area under the curve of 0·60 (95 % CI 0·47, 0·74). A PRISM score of 7·5 had a sensitivity of 78 % and a specificity of 42 %, a PELOD score of 1·5 had a sensitivity of 67 % and a specificity of the 52 %, and a PIM-2 score of 3·5 had a sensitivity of 80 % and a specificity of 48 %.
Eight patients (3·8 %) died during the study. The severity scores in these patients were significantly higher than in the survivors, although the differences in the PRISM and PELOD were not statistically significant (Table 2). Children with digestive tract complications did not have a significantly higher mortality (5·3 %) than the rest of the patients (3·7 %) (P = 0·540).
Discussion
TEN is an important method of nutrition in the critically ill patient. In our experience, 10·3 % of critically ill children receive TEN, and there is a progressive increase in its use and decrease in the use of parenteral nutrition(Reference Pettignano, Heard and Davis11, Reference De Lucas, Moreno and López-Herce12).
There are no specific scores in the critically ill patient that relate clinical severity with tolerance to the nutrition. Furthermore, although nutritional status affects prognosis in critically ill patients, and the failure of enteral nutrition is a marker of the risk of mortality, neither the nutritional status nor enteral tolerance is included among the parameters used to assess clinical severity in critically ill children.
We calculated three of the prognostic scores most widely used in critically ill children(Reference Martha, Ramos García and Piva13, Reference Leclerc, Leteutre and Duhamel14). The PRISM has been the most widely used. It has been shown to have excellent discrimination and predictive ability(Reference Pollack, Ruttimann and Getson6). PRISM analyses fourteen physiological variables in the child, taking the worst score in the first 24 h after admission. The PIM-2 only requires the measurement of eight variables and is performed within 1 h after admission. It requires the use of complex mathematical formulae, although a number of programs perform the calculation automatically. The PELOD was developed because multiorgan dysfunction syndrome is one of the most common causes of death in paediatric intensive care units(Reference Proulx, Fayon and Farell15). This index analyses a number of variables of the function of six organs or systems: cardiovascular, neurological, renal, respiratory, haematological and hepatic. This index is valid and reproducible during the first days of admission and throughout the clinical course and shows a good correlation with mortality(Reference De Lucas, Moreno and López-Herce12). In the present study we determined the three indices at the same time, at the initiation of enteral nutrition, in order to evaluate their predictive ability with regard to enteral tolerance. All the measurements of the severity scores were performed by a single observer in order to avoid interobserver variability(Reference Polderman, Thijs and Girbes16). The three indices showed a relatively good predictive ability for mortality and there was an acceptable statistically significant correlation between the indices, confirming their validity.
Several studies have shown that the early initiation of enteral nutrition reduces the incidence of septic complications and improves prognosis(Reference Briassoulis, Filippou and Kanariou17). The early initiation of enteral nutrition improves malnutrition and reduces secondary complications(Reference Briassoulis, Zavras and Hatsis18). In the present study, TEN was started in the majority of patients within the first 24 h of admission, with good tolerance. However, in the most seriously ill patients with the highest scores, the nutrition was started somewhat later and the rate of increase of delivery was slower. This could suggest that more seriously ill patients have a poorer tolerance to the rapid increase in nutrition. However, we believe that the most likely reason for this finding is that the doctors responsible for these patients considered it wiser to delay the initiation of nutrition and to reduce the rate of increase in delivery.
Even so, the early initiation of TEN in the most seriously ill patients was not associated with a higher incidence of complications(Reference Watters, Kirkpatrick and Norris19, Reference Sánchez, López-Herce and Carrillo20). Nor were there differences in the maximum energy delivery in relation to the severity scores. Thus, although the initiation and rate of increase of TEN in the more seriously ill patients was somewhat later and slower, the maximum energy delivery was independent of the degree of clinical severity of the patients(Reference De Lucas, Moreno and López-Herce12, Reference Taylor, Preedy and Baker21, Reference Briassoulis, Venkataraman and Thompson22).
In the present study the incidence of digestive tract complications was low(Reference Mentec, Dupont and Bochetti23). Only 4·7 % of the patients presented abdominal distension and/or excessive gastric residues. This low incidence may have been because the administration of the nutrition via the transpyloric route bypassed problems of gastric emptying(Reference Pupelis, Selga and Austrums24). The incidence of diarrhoea was also low (4·3 %). The patients who developed diarrhoea presented higher scores, although the differences were only significant for PRISM.
Earlier studies in adults and children have found that digestive tract intolerance is associated with a higher mortality(Reference Dunham, Frankenfield and Belzberg4). We previously found that the patients with digestive tract complications presented a higher mortality than the other patients(Reference López-Herce, Santiago and Sánchez25), although this was not found in the present study.
The present study has several limitations. PRISM and PIM were designed to be determined at the time of admission of the child to the paediatric intensive care unit. However, we calculated the scores at the time of starting nutrition in order to assess the clinical severity of the patients at that moment. In addition, the decision for increasing the rate of delivery of enteral nutrition depended on the doctor responsible for each patient and the rate of increase in the nutrition may therefore be biased by individual variations. Furthermore, the small percentage of digestive tract complications may mean that the differences did not reach statistical significance.
In conclusion, there was a greater delay in starting nutrition in the patients with higher severity scores at the time of starting nutrition; these patients also took longer to reach the maximum energy delivery and the duration of nutrition was longer. However, there were no differences in the maximum energy delivery achieved. The children with digestive tract complications presented slightly higher severity scores than the other patients, but significant differences were only observed in the PRISM scores in the patients who presented diarrhoea. The clinical severity scores do not therefore appear to be good predictors of digestive tract complications in critically ill children receiving TEN.
Acknowledgements
C. S. participated in the design of the study, analysis of the data and writing of the manuscript. J. L.-H. participated in the design and coordination of the study, collection and analysis of the data, and review of the manuscript. S. M., J. U. and A. C. participated in the collection of the data and review of the manuscript. J. M. B. contributed to the analysis of the data.
The authors declare no conflict of interests and no funding.




