Human milk lipids are the second largest component of human milk and provide the infant with energy, bioactive components and fatty acids, which vary in content and composition(Reference Innis1,Reference Koletzko2) . A sufficient intake of fatty acids in particular during the first 2 years of life is necessary for the growth and proper development of the brain and central nervous system(Reference Lauritzen, Brambilla and Mazzocchi3). The composition of human milk changes dynamically during a single feed, during the day, over lactation and varies between mothers and populations. For example, during a feed, some bioactive components in human milk increase and some decrease depending on the infants’ nutritional requirements(Reference Ballard and Morrow4). In addition, some fatty acids in human milk originate directly from the maternal diet; some can be synthesised in the maternal liver or mammary gland and others mobilised from maternal stores(Reference Bravi, Wiens and Decarli5).
In light of this, variations in human milk fatty acid composition between individuals and populations have been attributed to cultural differences (such as diet and other lifestyle factors)(Reference Miliku, Duan and Moraes6–Reference Morales, García-Esteban and Guxens11) and human genetic differences(Reference Lattka, Rzehak and Szabo12,Reference Barreiro, Díaz-Bao and Cepeda13) amongst others. Human milk fatty acid composition can therefore reflect both dietary sources and endogenous biological synthesis of fatty acids(Reference Picó, Serra and Rodríguez14–Reference Butts, Hedderley and Herath17). Thus, in order to ensure an adequate transfer of fatty acids through human milk, lactating women are advised to consume a well-balanced diet with adequate dietary sources of fatty acids(Reference Logan, Zittel and Striebel18). However, for infants who do not receive human milk, human milk composition is often used as an important gold standard to define the adequacy of replacement infant nutrition products(Reference Grote, Verduci and Scaglioni19).
Despite the large body of literature on human milk fatty acid composition, most studies have investigated variations in human milk fatty acid composition across countries and demographic groups. In addition, little is known about the changes in human milk fatty acid composition over time in otherwise similar populations. Repeat birth cohort studies conducted multiple years apart, in populations with some change in sociodemographic, dietary and lifestyle components, might be most informative in this respect. Therefore, we compared human milk fatty acid composition and investigated the changes thereof in two birth cohorts, which recruited lactating women from the general population in the city of Ulm, located in southern Germany, approximately a decade apart.
Methods
Study design and population
Data were obtained from the Ulm Birth Cohort Study (UBCS 2000) and the Ulm SPATZ Health Study (SPATZ 2012), two birth cohort studies, which employed very similar methodology. Both cohort studies included mothers and their live newborns recruited from the general population shortly after delivery at the University Medical Center in Ulm, southern Germany (the only maternity clinic in Ulm, a city of approximately 100 000 inhabitants), in 2000–2001 and 2012–2013, respectively(Reference Logan, Zittel and Striebel18). Mothers were excluded if they had outpatient birth, <18 years of age, postpartum transfer of either mother or child to intensive care, still birth or had inadequate German (UBCS and SPATZ), Turkish or Russian (both UBCS only) language skills. At baseline, a total of 1090 newborns of 1066 mothers (67 % of all 1593 eligible families) and 1006 newborns of 970 mothers (49 % of all 1999 eligible families) were included in UBCS and SPATZ, respectively. Ethical approval for both cohort studies was obtained from the ethics board of Ulm University (UBCS: no. 98/2000; SPATZ: no. 311/11) and for UBCS only from the Physicians’ boards of the states of Baden-Wuerttemberg and Bavaria. Participation in both studies was entirely voluntary, and written informed consent was obtained from all mothers participating in the studies.
Data collection and measurements
Demographic data, maternal lifestyle and birth-related data (child sex, delivery mode, birth season, birth weight, maternal age, education, parity, pre-pregnancy BMI (BMI calculated as (mass (kg)/height (m)2)) and history of smoking) were collected using self-administered questionnaires, hospital records and records from routine screening examinations during pregnancy. Maternal BMI at the time of human milk sampling was not available in UBCS; thus, pre-pregnancy BMI was used for both studies.
Human milk sample collection and analysis
Human milk samples were collected from consenting lactating mothers who were actively breast-feeding at approximately 6 weeks (UBCS, n 782; SPATZ, n 706). For the purpose of this analysis, the study sample sizes were restricted to generally healthy lactating mothers of German nationality with term (>37 weeks) singleton births for whom fatty acid data were available at approximately 6 weeks of lactation. Thus, a total of 567 and 458 human milk samples from UBCS and SPATZ, respectively, were included in this analysis (Fig. 1). Human milk samples were collected using the same protocol and processed at the same location thereby ruling out study bias and effect of different batches. Lactating mothers were instructed to wash their hands and clean the breast prior to sampling, then express or pump 10 ml of breast milk between 09.00 hours and noon, after breakfast and before lunch, but at least 1 h after the infant’s last human milk feed, to reduce possible variations resulting from times of expression and feed. In rare instances and where necessary, trained study nurses helped mothers with expression or pumping human milk into study-provided collection tubes. Mothers were also instructed to refrigerate their human milk samples until study nurses collect them. Upon collection, human milk samples were transported in cold insulated containers directly to the study centre where they were aliquoted and stored at −80°C until assayed.

Fig. 1. Flow diagram of human milk samples used for the current analysis. SPATZ, Ulm SPATZ Health Study; UBCS, Ulm Birth Cohort Study; GDM, gestational diabetes mellitus.
Fatty acids were measured by high-resolution capillary GC–LC in both studies as previously described for the UBCS(Reference Szabó, Boehm and Beermann20) and SPATZ(Reference Siziba, Lorenz and Stahl21) in 2004 and between 2015 and 2018, respectively. The fatty acids in UBCS were measured on a Finnigan 9001 GC with flame ionization detector and programmed temperature vaporizer injector. This GC was equivalent to the HP (Agilent) 5890 in terms of having the same configuration. The fatty acids in SPATZ were measured on an Agilent 6890N GC with flame ionization detector and Cool On-Column injector. The cleaning analytical gases in the laboratory were also changed from 4·6 to 5·0 by N2, H2 and analytical air. However, the capillary column was of the same type (Agilent/J&W DB-23, 60 m × 0·25 mm × 0·25 μm) in both studies. We could therefore detect more fatty acids in the more recent SPATZ study on a newer GC with cleaner analytical gases. A total of twenty-eight and forty-five individual fatty acids were measured in the UBCS and SPATZ studies, respectively. Fatty acid levels were recorded as % weight of total fatty acids in both studies. To be able to compare fatty acids between the two studies, the fatty acids measured in the SPATZ were recalculated and restricted to the twenty-eight fatty acids measured in the UBCS study.
Statistical analysis
χ 2 and Kruskal–Wallis tests were used to assess and identify differences between demographic and lifestyle characteristics across the two study cohorts (P < 0·05). Centred log ratio transformation was applied to fatty acid data to account for the mutual dependence of individual fatty acids(Reference Logan, Brandt and Wabitsch22). Wilcoxon rank sum test for two unpaired samples was used to compare individual fatty acid constituents between the two study cohorts. A single principal component analysis was used to determine study-dependent fatty acid profiles. The scree plot visual inspection was used to determine the number of principal components (PC) to be retained. Based on this, the first two PC were retained for subsequent analysis. The least square (LS) means for the two PC retained were then calculated to compare whether these were different between the two studies. A general linear model was used to assess the associations between the LS means with the two birth cohort studies. The period effect (or study effect) on the individual centred log ratio-transformed fatty acids was also assessed using a general linear model adjusting for maternal age, delivery mode, pre-pregnancy BMI, education, maternal history of smoking and duration of gestation. Collinearity of these maternal lifestyle variables was assessed using the variance inflation factor, and all variance inflation factor values were <2. These factors (age, delivery mode, pre-pregnancy BMI, education and smoking) were selected based on the observed differences in prevalence over time between the two studies(Reference Logan, Zittel and Striebel18) and their strong associations with human milk fatty acid composition that have been reported in previous studies(Reference Bravi, Wiens and Decarli5,Reference Bokor, Koletzko and Decsi23) . Furthermore, maternal age was previously closely associated with maternal education, less exposure to cigarette smoking and breast-feeding(Reference Logan, Zittel and Striebel18). Therefore, maternal age was regarded a potential indicator of aspects of lifestyle that could influence human milk fatty acid composition. Pre-pregnancy BMI was also included in the model because of its close association with breast-feeding(Reference Logan, Zittel and Striebel18), and education was used as proxy for socio-economic status. Using the above named variables as dichotomous and continuous variables in the adjusted model yielded similar results. Statistical level of significance was based on Bonferroni-corrected P values to account for multiple testing. Although applying the Bonferroni correction could reduce the probability of observing a statistically significant result, this also reduces the risk drawing conclusions based on overestimated assertions(Reference Lee and Lee24). All statistical analyses were performed using SAS version 9.4 (The SAS Institute) and R (version 3.5.1; R Foundation for Statistical Computing).
Results
Overall, human milk samples from 567 and 458 (53·2 and 47·2 % of women participating in the cohorts) collected at 6 weeks postpartum from lactating women in the UBCS and SPATZ were included in the current analysis (Table 1). Mean age (32·3 and 32·7 years, respectively) and age distribution were very similar in both studies. Lactating mothers in SPATZ (the more recent cohort) had higher mean pre-pregnancy BMI (SPATZ 23·5 (range 16·9–50·2) kg/m2; UBCS 22·2 (range 16·5–43·2) kg/m2) and were more often highly educated (Table 1) compared with lactating mothers in UBCS.
Table 1. Characteristics of lactating mothers who had fatty acid data available at 6 weeks in the Ulm Birth Cohort studies*
(Numbers and percentages; mean values)

SPATZ, Ulm SPATZ Health Study; UBCS, Ulm Birth Cohort Study.
* P values are χ 2 for association with categorical variables or Kruskal–Wallis for association with continuous variables. Sums may not add up to total because of missing values for some variables.
Oleic acid (C18 : 1n-9) was the most abundant fatty acid, and palmitic acid (C16 : 0) was the major SFA in both studies (Table 2). In comparison with UBCS, all individual fatty acid constituents in SPATZ differed significantly between the two studies (using a Bonferroni-corrected α threshold = 0·0018 to adjust for multiple testing), except capric (C10 : 0), lauric (C12 : 0), myristic (C14 : 0), stearic (C18 : 0) and arachidic (C20 : 0).
Table 2. Centred log ratio (CLR)-transformed fatty acid concentrations of human milk samples measured at 6 weeks in the Ulm Birth Cohort studies*
(Mean values and standard deviations)
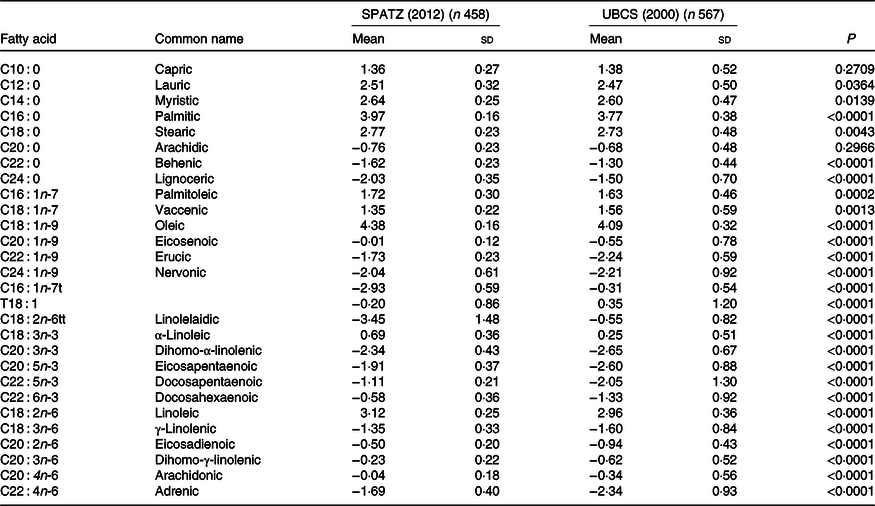
SPATZ, Ulm SPATZ Health Study; UBCS, Ulm Birth Cohort Study.
* P values derived from Wilcoxon rank sum test comparing fatty acid concentrations measured at a 6 weeks in the SPATZ and UBCS (SPATZ v. UBCS) cohort studies. Bonferroni-adjusted level of statistical significance is α = 0·05/28 = 0·0018.
In both unadjusted and adjusted models, and following Bonferroni correction, twenty individual fatty acid constituents were higher (including all measured n-3 and n-6 fatty acids), while only eight constituents were lower (including all trans-fatty acids; TFA) in the human milk of lactating mothers in SPATZ compared with their counterparts in UBCS (Table 3). Means and standard deviations of relative proportions (% weight of total fatty acids) of both studies are also shown in the online Supplementary Table S1.
Table 3. Study effects on human milk fatty acid composition in the Ulm Birth Cohort studies in unadjusted and adjusted models*
(β Values and 95 % confidence intervals)

SPATZ, Ulm SPATZ Health Study; UBCS, Ulm Birth Cohort Study.
* Adjustments made for maternal age, delivery mode, pre-pregnancy BMI, education, duration of gestation and smoking. P values derived from general linear models comparing fatty acid concentrations measured at 6 weeks in the SPATZ and UBCS cohort studies (SPATZ v. UBCS). Bonferroni-adjusted level of statistical significance is α = 0·05/28 = 0·0018.
Two PC (explaining 47 % cumulative variance of all twenty-eight fatty acids) were retained. The loading plot (Fig. 2) showed the presence of study-dependent human milk fatty acid profiles. The two studies clustered differently and could be easily distinguished based on the fatty acids predominantly contributing to the human milk fatty acid profile, thus displaying potential differences of the dietary habits in each study.

Fig. 2. Scores from the two principal components retained from the principal component analysis of centred log ratio-transformed fatty acid concentrations of human milk samples collected at 6 weeks in the Ulm Birth Cohort Study (UBCS) (n 567) and Ulm SPATZ Health Study (SPATZ) (n 458) studies. Colour key: purple – SFA; blue – trans-fatty acids; green – n-3 fatty acids; pink – MUFA; brown – n-6 fatty acids. ![]() , SPATZ;
, SPATZ; ![]() , UBCS.
, UBCS.
PC score loadings (i.e. >0·40 or <–0·40, online Supplementary Table S2) were used to define the two PC retained. The first PC (PC1) was characterised by positive scores of even-chain SFA (C10 : 0–C22 : 0) and TFA (C16 : 1n-7t, T18 : 1 and C18 : 2n-6tt) and negative scores of the MUFA erucic acid (C22 : 1n-9) and nervonic acid (C24 : 1n-9), n-3 (C20 : 3n-3, C20 : 5n-3, C22 : 5n-3, C22 : 6n-3) and n-6 (C20 : 2n-6, C20 : 3n-6, C20 : 4n-6, C22 : 4n-6) long-chain PUFA.
The second PC (PC2) was characterised by positive scores of the even-chain SFA (C16 : 0 and C18 : 0), α-linolenic acid (ALA, C18 : 3n-3), n-6 PUFA linoleic acid (LA; C18 : 2n-6; C20 : 2n-6 and C20 : 3n-6), MUFA (C16 : 1n-7, C18 : 1n-9, C20 : 1n-9) and negative scores of lignoceric acid (C24 : 0), C18 : 1n-7, TFA (C16 : 1n-7t, C18 : 2n-6tt).
PC1 was associated with UBCS, whilst PC2 was associated with the second more recent cohort, SPATZ. In both adjusted and unadjusted models, the LS means of PC1 were significantly lower in SPATZ (LS −1·08 (95 % CI −1·65, −0·52)) compared with UBCS (LS 1·29 (95 % CI 0·75, 1·83), P < 0·0001) (online Supplementary Fig. S1). The inverse was true for PC2 (UBCS; LS −1·47 (95 % CI −1·77, −1·17); SPATZ; LS 1·80 (95 % CI 1·49, 2·13), P < 0·0001).
Discussion
This is one of the first studies to investigate the trends in human milk fatty acid composition over a 10-year period in two cohorts recruited from the general population in the same setting using similar methodology. Two PC which potentially show the changes of maternal dietary practices over the past 10 years and possible differences in mammary gland function were identified. The PC1 was characterised by high contents of SFA and TFA, and lower contents of very long-chain MUFA, as well as n-3 and n-6 long-chain PUFA. The PC2 was characterised by high contents of the SFA C16 : 0 and C18 : 0, MUFA, n-3 and n-6 essential PUFA (ALA and LA, respectively) and low contents of TFA, C18 : 1n-7 and C24 : 0. PC1 was associated with UBCS (2000) and PC2 with the more recent cohort, SPATZ (2012). The differences in human milk fatty acid composition between the two studies were independent of differences in maternal age, education, pre-pregnancy BMI, delivery mode, maternal history of smoking and duration of gestation.
In the present study, a lower SFA profile was observed in SPATZ and we speculate that the differences between the two cohorts are driven by changes in total fat intake over the past decade. The SFA in human milk are related to the content of carbohydrates, fats in a daily diet, total energy intake and the mobilisation of adipose tissue(Reference Krešić, Dujmović and Mandić8,Reference Innis25) . High SFA intakes are typically accompanied by high total fat intake, and the inverse is true(26). In Europe, dairy products, added fats and oils including fats such as butter, lard, beef drip as well as meat and meat products are the main dietary sources that contribute to SFA intakes(Reference Harika, Eilander and Alssema27). In line with the results from this study, the European Health For All Database shows that fat intake per person (fat available per person per day (g) selected as a lifestyle indicator) has actually somewhat decreased over the past decade(Reference Antonakou, Skenderi and Chiou28). However, an inverse relationship between the concentration of endogenously synthesised fatty acids with palmitic acid (C16 : 0) in human milk has previously been reported(Reference Gardner, Rahman and Lai29,Reference Kumar, du Toit and Kulkarni30) . Thus, it is plausible that a decrease in the synthesis of de novo fatty acids in SPATZ may have been compensated for by an increased uptake of preformed fatty acids such as palmitic acid (C16 : 0). Thereby, explaining the observed higher proportion of palmitic acid in SPATZ compared with UBCS.
In contrast to previous studies(Reference Gao, Liu and Whitfield31–Reference Xie and Innis41) showing similar levels of palmitoleic acid (C16 : 1n-7) and oleic acid (C18 : 1n-9), the content of oleic acid was twice as much as the content of palmitoleic acid in SPATZ human milk samples. On one hand, levels of oleic acid have been strongly linked to the consumption and use of olive oil in different populations, although it is also present in other vegetable oils, nuts and in fruits like avocados, as well as in meat and cheese(Reference Minda, Kovács and Funke42). On the other hand, it has been shown that palmitoleic acid is an important metabolic product of endogenous lipogenesis under strong genetic control in humans(Reference Okada, Furuhashi and Kuromori40). Therefore, we speculate that the observational differences between palmitoleic and palmitic acids in this study could be an indication of a potential biosynthesis of palmitoleic acid from palmitic acid.
In addition, Xie & Innis(Reference Xie and Innis41) previously reported an association of fatty acid desaturase (FADS) polymorphisms with vaccenic acid (C18 : 1n-7), and similar associations with genotyped SNP were also tested amongst lactating women in UBCS(Reference Lattka, Rzehak and Szabo12). However, very marginal associations were observed, and significance was lost following correction for multiple testing in UBCS(Reference Lattka, Rzehak and Szabo12). Granted that the largest changes in human milk fatty acid composition occur during the first month of breast-feeding(Reference Minda, Kovács and Funke42), it is plausible that the genetic effect on human milk composition is also more pronounced and visible during this early stage, that is, 6 weeks of lactation. Although the sample size was restricted to lactating women of German nationality, we cannot rule out changes over the last decade, in the genetic background of these lactating women. Thus, we further speculate that the differences observed between these two demographically similar populations could also be attributed to subtle differences associated with an altered synthesis rate of fatty acids in the mammary gland. Nonetheless, whether these differences could be due to lower Δ-5-desaturase activity in other tissues such as the liver, and subsequently a reduction in the uptake into the mammary gland remains to be studied.
Similar to SFA, we observed lower TFA in SPATZ, which also likely reflect a decrease in TFA contents in a variety of foods and in dietary TFA intake over the years. However, the secretion of TFA in human milk is apparently largely unregulated, with the amount transferred into milk being dependent on the maternal intake of a specific fatty acid(Reference Innis1). In their systematic review published in 2017, Wanders et al.(Reference Wanders, Zock and Brouwer43) also observed a marked decrease of TFA intake over the past decade. Despite the lack of dietary information, researchers can easily compare differences in consumption of TFA of different populations and even countries based on human milk composition(Reference Craig-Schmidt44). Conversely, TFA have also been shown to influence n-6 and n-3 PUFA metabolism in infants and consequently having negative effects on health(Reference Innis and King45). Consequently, lactating women are advised to keep their intake of TFA as low as possible.
Furthermore, sources of dietary PUFA are mainly LA and ALA obtained from added fats and oils which include but are not limited to vegetable oils, margarines and mayonnaises(Reference Eilander, Harika and Zock46). As a result, the differences in PUFA intakes between countries and populations are most likely driven by differences in local food habits and types or amounts of cooking oils and fats used. It is therefore most likely that a part of the reported variations in human milk composition in this study reflects the true underlying differences in intakes and types of fats and oils consumed(Reference Harika, Eilander and Alssema27). Although, the consumption of foods of plant origin in Germany between 2005/2006 and 2012/2013 has remained low in comparison with the European region(Reference Gose, Krems and Heuer47). The consumption of fruit and vegetables (average amount of fruits and vegetables available per person per year (kg) selected as a lifestyle indicator) in Germany has increased slightly from 2000 to 2013(26). It is therefore likely that the use of vegetable oils and other dietary sources of LA and ALA has also increased over the past decade. However, further assessment of food consumption and nutrient intake of the lactating women in the south of Germany is necessary in order to evaluate whether the observed trends will continue over the next few years.
The period effect (study effect) on individual fatty acids was further assessed and none of the lifestyle-related factors (i.e. pre-pregnancy BMI, maternal age, education, duration of gestation and history of smoking) explained the time trend differences in the fatty acid profiles between these two studies. Our results suggest the influence of dietary habits, possibly other exposures, individual and circadian variations in fatty acid synthesis(Reference Gnocchi, Pedrelli and Hurt-Camejo48–Reference Buckley, Racimo and Allentoft50) and genetic factors that were not assessed in this study. Both the UBCS and SPATZ studies were done in populations in the south of Germany where the consumption of salt water fish is typically low. This is evidenced by both PC1 and PC2, in which scores of n-6 and n-3 PUFA were lower in the UBCS, and although positive in SPATZ, were not high enough to be included in defining PC2. Moreover, a detailed comparison of breast-feeding practices and other demographic lifestyle factors between the two complete cohort populations has been published previously(Reference Logan, Zittel and Striebel18). In brief, all lactating mothers who provided human milk samples at 6 weeks in both UBCS (n 782) and SPATZ (n 706) cohort studies were higher educated, less likely to have had a history of smoking and less likely to have been overweight or obese compared with their respective full cohort population(Reference Logan, Zittel and Striebel18). Therefore, granted that the lactating women in both studies were of high maternal age, highly educated and using these as proxies of socio-economic status, they presumably had a higher socio-economic status. It is most likely that, in addition to an increased use of vegetable oils and overall higher PUFA intake, lactating women in SPATZ consumed or took fish oil/n-3 fatty acid supplements.
The authors acknowledge that the lack of dietary information is a major limitation of this study, and given the observational and speculative nature of this study, this limits the possibility of drawing conclusions on causality between the observed human milk fatty acid profiles and diet. In addition, granted that human milk fatty acid composition is influenced by diet, the lack of dietary information in this study could have led to an underestimation of true dietary fatty acid intakes. Thus, these results should be interpreted with caution, as they do not fully describe the influence of diet, genetics and endogenous synthesis in the mammary gland. Despite these limitations, our results provide valuable information on the possible differences in subsequent dietary habits and quality of diet between the two study populations at a specific time period of lactation (6 weeks). Therefore, population-specific interventions are required to ensure that infants receive an adequate supply of fatty acids through human milk. Strengths of this study include the homogeneity of study populations with respect to design, methodology, defined geographic region, human milk sampling and similar fatty acid analysis methods. Strict and similar protocols in sampling of human milk were employed to take into account diurnal variations that exist between individuals. Although a newer GC and cleaner analytical gases were used in SPATZ, fatty acid analysis was done at the same location thereby possibly reducing study bias and batch effect. While the studies were conducted in one region of southern Germany, our results cannot be generalised to the broader population of all lactating women in that region.
In conclusion, over the past decade, human milk fatty acid composition has higher contents of LA, ALA, some MUFA and lower TFA and SFA. Our findings suggest that human milk remains an important source of essential fatty acids. However, infants may be at risk of inadequate n-3 and n-6 long-chain PUFA. Although the quality of diet as shown by a combination of fatty acids has seemingly improved, women should be given adequate support and information regarding the importance of meeting their nutritional needs during lactation. The implications of these differences in human milk fatty acid composition on maternal and infant health should be investigated further in other populations. Future studies are needed to validate these findings in other populations and identify other potential determinants of human milk fatty acid composition in a general population.
Acknowledgements
The authors would like to extend their utmost gratitude to the midwives, nurses and obstetricians of the Department of Gynaecology and Obstetrics, University Medical Centre Ulm and the caring paediatricians and mothers and their families for their study support and participation. The authors would also like to thank Mrs Gerlinde Trischler, Gisela Breitinger and Christa Johanna for providing excellent technical assistance.
The Ulm Birth Cohort Study was supported by grants of the German Research Council (BR 1704/3-1, BR 1704/3-2 and BR 1704/3-3). The Ulm SPATZ Health Study was funded through an unrestricted grant by the Medical Faculty of Ulm University. The current research study was funded by Danone Nutricia Research, Utrecht, The Netherlands. These funders had no role in the design, analysis or writing of this article.
H. B. and D. R. conceived and designed the Ulm Birth Cohort Study; D. R. and J. G. conceived and designed the Ulm SPATZ Health Study. E. S., T. M. and T. D. analysed the fatty acids in the laboratory. L. P. S. and J. G. conceived the study question. L. P. S. conducted the statistical analyses. L. L. contributed to the statistical analyses. L. P. S. and J. G. interpreted the data and wrote the manuscript. All authors (including B. S., M. M. and P. C.) critically reviewed the manuscript and contributed to its final version.
The research was funded by Danone Nutricia Research. However, the principal investigators (J. G. and D. R.) along with the first author (L. S.) made final decisions on the interpretation and dissemination of results. None of the other researchers has any conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004006








