There is a consensus among diabetes and heart associations in the recommendation to restrict the intake of fructose to avoid adverse metabolic effects(Reference Sievenpiper, Carleton and Chatha1, 2). Whether there is a level of fructose such as that provided by fruit below which there may be benefit is unclear. An emerging literature has shown that low-dose fructose ( ≤ 10 g/meal) may benefit glycaemic control. Fructose, through its metabolite fructose-1-P, has been shown to have catalytic effects on hepatic glucose metabolism by increasing glucokinase activity(Reference Hawkins, Gabriely and Wozniak3). This mechanism has been shown to relate to an approximately 30 % decrease in hepatic glucose production under hyperglycaemic conditions in type 2 diabetic subjects(Reference Hawkins, Gabriely and Wozniak3) and an approximately 3-fold increase in glycogen synthesis by 13C NMR spectroscopy under euglycaemic conditions in non-diabetic subjects(Reference Petersen, Laurent and Yu4). Translation of these findings in the acute clinical setting has shown that ‘catalytic’ fructose doses ( ≤ 10 g/meal) can decrease the postprandial glycaemic response to high-glycaemic index (GI) meals from about 15 to 30 %(Reference Moore, Cherrington and Mann5–Reference Heacock, Hertzler and Wolf7). To assess whether this benefit is sustainable without adverse effects on other aspects of metabolic control, we undertook a meta-analysis of controlled feeding trials of ‘catalytic’ fructose doses.
Methods
Study selection
We conducted separate searches of the effect of ‘catalytic’ doses of fructose on glycaemic endpoints (fasting blood glucose, fasting blood insulin and HbA1c), fasting TAG, body weight, blood pressure and uric acid in human subjects in MEDLINE, EMBASE, CINAHL and the Cochrane Central Register of Controlled Trials. We defined a ‘catalytic’ fructose intake in chronic feeding trials as ≤ 36 g/d based on doses of ≤ 10 g/bolus in acute trials(Reference Moore, Cherrington and Mann5–Reference Heacock, Hertzler and Wolf7), allowing for three meals (10 g/meal) and two snacks (3 g/snack) per d. Trials that were < 7 d follow-up, administered fructose intravenously, lacked an adequate control or did not provide suitable endpoint data were excluded. The study has been registered in the ClinicalTrials.gov registry (study identifier: NCT01363791).
Data extraction
At least two reviewers independently reviewed and extracted relevant data from each report on trial characteristics and outcomes. The quality of each study was assessed using the Heyland Methodological Quality Score (MQS)(Reference Heyland, Novak and Drover8). Disagreements were reconciled by consensus. Differences in mean values and standard deviations were extracted as the main endpoints. Trials that did not report either had these calculated from the available data. Missing standard deviation values were calculated from the available statistics or imputed using standard formulae(Reference Higgins and Green9).
Statistical analyses
Data were analysed using Review Manager (RevMan) 5.1.4 (The Nordic Cochrane Centre, The Cochrane Collaboration). Meta-analyses were conducted using the generic inverse variance method using random-effects models with data expressed as mean differences (MD) with 95 % CI. Between-treatment change-from-baseline differences were preferred over end differences as the primary endpoint. We approximated paired analyses for the one cross-over trial(Reference Grigoresco, Rizkalla and Halfon10–Reference Rizkalla, Baigts and Fumeron14) using a conservative correlation coefficient (0·5) with sensitivity analyses at 0·25 and 0·75. To mitigate a unit-of-analysis error in the trials by Blayo et al. (Reference Blayo, Fontveille and Rizkalla11) and Rizkalla et al. (Expt 1 and 2)(Reference Rizkalla, Baigts and Fumeron14), we used only the starch and glucose comparisons, respectively. This approach of using a single arm as opposed to combing arms was selected, so as to minimise the influence of heterogeneity. Inter-study heterogeneity was tested by the Q statistic with the significance level set at P < 0·10 and quantified by the I 2 statistic, where I 2 ≥ 50 % is the evidence of substantial heterogeneity(Reference Higgins and Green9). Sources of heterogeneity were investigated by subgroup analyses (diabetes status, comparator, fructose delta, fructose form, follow-up, MQS, randomisation and design). Publication bias was investigated by inspection of funnel plots.
Results
Search results
Fig. S1 of the supplementary material (available at http://www.journals.cambridge.org/bjn) shows the flow of the literature applying the systematic search and selection strategies. The search identified 7762 eligible reports. A total of five reports(Reference Grigoresco, Rizkalla and Halfon10–Reference Rizkalla, Baigts and Fumeron14) providing data for six trials were selected for analyses.
Trial characteristics
The trial characteristics are shown in Table 1. There were six trials in 118 subjects(Reference Grigoresco, Rizkalla and Halfon10–Reference Rizkalla, Baigts and Fumeron14). All six (100 %) trials were randomised. Of these trials, one (17 %) used a cross-over design. Starch (four comparisons), glucose (two comparisons), galactose (two comparisons) and sucrose (one comparison) were the comparators. Fructose was administered in liquid or mixed formats at a median dose of 32·5 (range 22·5–36) g/d. Of the trials, three (50 %) used metabolically controlled designs. Background diets in the trials consisted of 25–60 % energy as carbohydrate, 25–50 % energy as fat and 15–25 % energy as protein. The median follow-up was 6 (range 1–52) weeks. The Heyland MQS in the trials ranged from 6 to 10 with four trials (67 %) considered high quality (MQS ≥ 8).
Table 1 Characteristics of controlled feeding trials investigating the effect of ‘catalytic’ doses (≤36 g/d) of fructose on cardiometabolic endpoints

MQS, Methodological Quality Score; OW/OB, overweight/obese; OP, outpatient; P, parallel; Met, metabolic; DM2, type 2 diabetes mellitus; M, male; F, female; C, cross-over; S, supplement; DM1, type 1 diabetes mellitus; N, normal; IP, inpatient.
* Met feeding control represents the provision of all meals, snacks and study supplements (test sugars and foods) during the study. S feeding control represents the provision of study supplements.
† Doses preceded by ‘about’ represent average doses, where fructose was administered on a % energy or g/kg body-weight basis.
‡ Fructose was provided in one of two forms: (1) a liquid form, where all or most of the fructose was provided as beverages or crystalline fructose to be added to beverages, or (2) in a mixed form, where all or most of the fructose was provided as beverages, solid foods and/or crystalline fructose to be added to beverages and/or foods.
§ Comparator refers to the reference carbohydrate (glucose, galactose, starch or sucrose).
∥ Values are for the ratio of carbohydrate:fat:protein.
¶ The Heyland MQS assigns scores from 0 to 1 or 0 to 2 over nine categories of quality related to study design, sampling procedures and interventions for a total of thirteen points. Trials scored ≥ 8 were considered high quality(Reference Heyland, Novak and Drover8).
** Agency funding represents funding from government, university or not-for-profit health agency sources. None of the trialists declared any conflicts of interest.
Glycaemic effects of ‘catalytic’ fructose feeding
Fig. 1 shows the effect of ‘catalytic’ fructose doses on fasting glucose, fasting insulin and HbA1c. HbA1c was significantly reduced (MD − 0·40, 95 % CI − 0·72, − 0·08) without evidence of heterogeneity, while fasting glucose was significantly reduced (MD − 0·25, 95 % CI − 0·44, − 0·07) with significant inter-study heterogeneity (I 2 = 95 %, P < 0·001). There was no effect on fasting insulin, with the evidence of inter-study heterogeneity (I 2 = 100 %, P < 0·001) explained away by the removal of Rizkalla et al. (Expt 1)(Reference Rizkalla, Baigts and Fumeron14) (data not shown). A priori subgroup analyses (see Table S1 of the supplementary material, available at http://www.journals.cambridge.org/bjn) showed significant effect modification by diabetes status, comparator, follow-up, fructose form and MQS for fasting glucose; and comparator and fructose form for fasting insulin. None of the subgroup analyses explained away the heterogeneity for fasting glucose or insulin. The results of HbA1c and fasting glucose were sensitive to the removal of individual trials, and the results of HbA1c were sensitive to the correlation used (0·75) for paired analyses (data not shown).
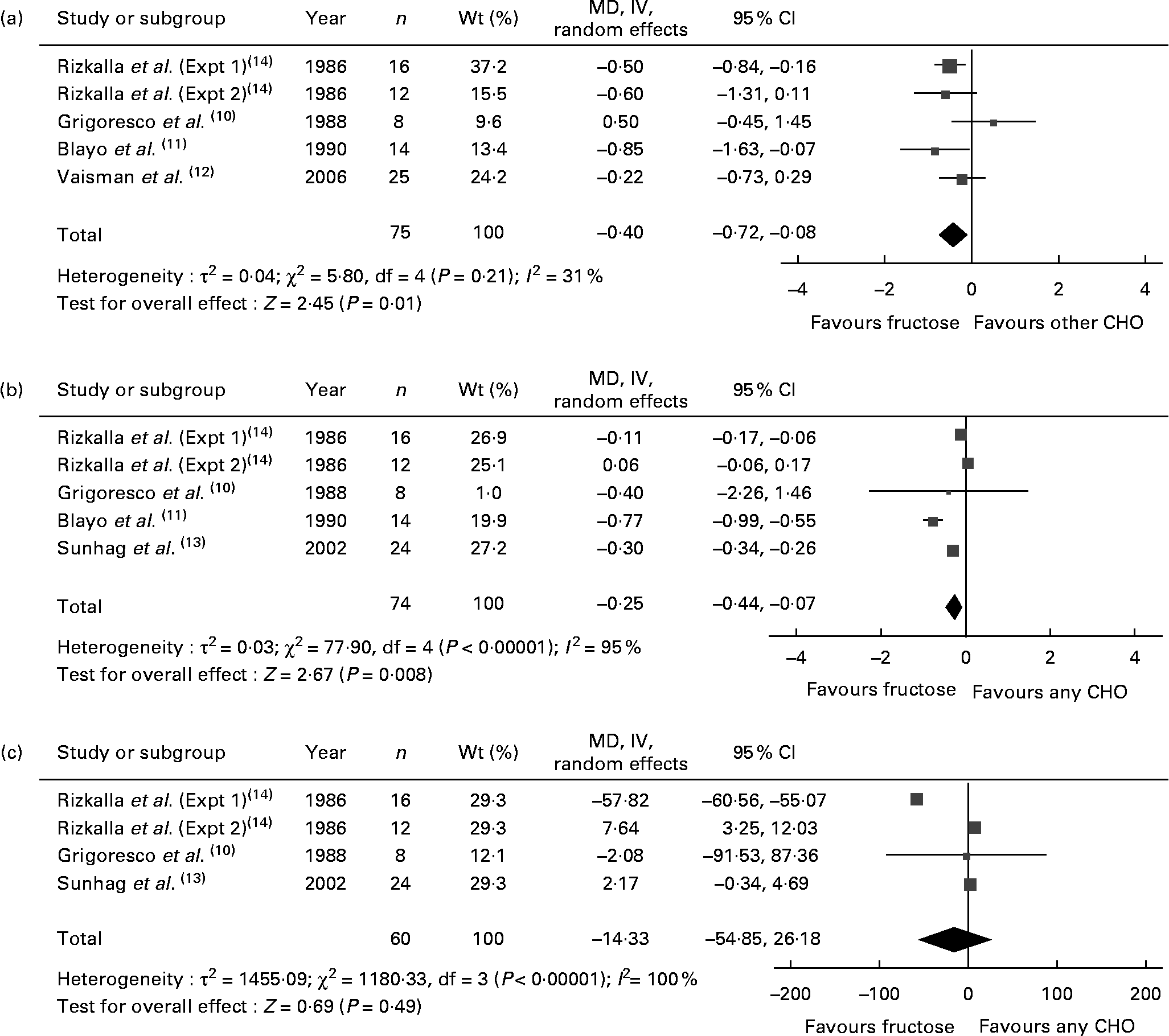
Fig. 1 Forest plots of controlled feeding trials investigating the effect of isoenergetic exchange of ‘catalytic’ fructose doses ( ≤ 36 g/d) for other carbohydrates on glycaemic endpoints: (a) HbA1c, (b) fasting blood glucose (FBG) and (c) fasting blood insulin (FBI). Paired analyses were applied to the one cross-over trial by Grigoresco et al. (Reference Grigoresco, Rizkalla and Halfon10). To mitigate a unit-of-analysis error, we used only the starch comparison for Blayo et al. (Reference Blayo, Fontveille and Rizkalla11) and the glucose comparison for Rizkalla et al. (Expt 1 and 2)(Reference Rizkalla, Baigts and Fumeron14). Values are between-treatment end differences for five of the six trials (Grigoresco et al. (Reference Grigoresco, Rizkalla and Halfon10), Blayo et al. (Reference Blayo, Fontveille and Rizkalla11), Vaisman et al. (Reference Vaisman, Niv and Izkhakov12), and Sunehag et al. (Reference Sunehag, Toffolo and Treuth13), Rizkalla et al. (Expt 1)(Reference Rizkalla, Baigts and Fumeron14)) in the HbA1c analysis and for all trials in the FBG and FBI analyses, as change-from-baseline data were not available. P values are for generic inverse variance (IV) random-effects models, with differences expressed as mean differences (MD) with 95 % CI(Reference Higgins and Green9). Inter-study heterogeneity was tested by Cochrane's Q statistic (χ2) at a significance level of P < 0·10 and quantified by I 2 (Reference Higgins and Green9). CHO, carbohydrate.
Other metabolic effects of ‘catalytic’ fructose feeding
There was no effect of ‘catalytic’ fructose on body weight, TAG or uric acid without evidence of significant heterogeneity (see Fig. S2 of the supplementary material, available at http://www.journals.cambridge.org/bjn). None of the subgroup analyses was significant (see Table S2 of the supplementary material, available at http://www.journals.cambridge.org/bjn). Sensitivity analyses did not alter the results (data not shown).
Publication bias
Funnel plots for each of the analyses were inspected for the presence of publication bias (see Fig. S3 of the supplementary material, available at http://www.journals.cambridge.org/bjn). There was no evidence of funnel plot asymmetry, although the small number of trials made assessment difficult.
Discussion
This small meta-analysis of six controlled feeding trials in 118 subjects over a median follow-up of 6 weeks showed that ‘catalytic’ doses (22·5–36 g/d) of fructose in isoenergetic exchange for other carbohydrates may improve glycaemic control without adversely affecting other cardiometabolic risk factors. The reduction in HbA1c of 0·4 % was clinically significant, lying at the lower limit of efficacy expected for oral hypoglycaemic agents(2, 15). These results support an earlier meta-analysis by Livesey & Taylor(Reference Livesey and Taylor19), which did not show a dose threshold for HbA1c reductions across a wider dose range of fructose.
A benefit of ‘catalytic’ doses of fructose has implications for the benefit of low-GI fruit. In a secondary analysis of a randomised controlled trial investigating the effect of a 6-month low-GI diet compared with a high-cereal fibre diet in 152 participants with type 2 diabetes(Reference Jenkins, Srichaikul and Kendall16), we showed that low-GI fruit intake was the strongest independent predictor of HbA1c. The HbA1c decrease of 0·5 % (highest v. lowest quartile of low-GI fruit intake) was similar to that seen in the present analysis, despite none of the trials in the meta-analysis using fruit. Although it is unclear whether this reduction in HbA1c was attributable to a ‘catalytic’ effect of fructose, its ability to lower the GI of the diet, or both, the low-GI fruit increase (2·2 servings/d) was equivalent to a ‘catalytic’ increase in fructose, which expressed as the most commonly consumed low-GI fruit in the study, apples, represents approximately 24 g/d of fructose.
A dose threshold for harm, however, remains an important consideration for fructose(Reference Sievenpiper, de Souza and Kendall17). The ‘catalytic’ mechanism through which fructose is thought to operate, up-regulation of glucokinase, may under certain circumstances contribute to increased de novo lipogenesis with downstream metabolic sequelae(Reference Peter, Stefan and Cegan18). Despite showing an improvement in HbA1c, the meta-analysis by Livesey & Taylor(Reference Livesey and Taylor19) showed a consistent TAG-raising effect of fructose at doses >100 g/d (>95th percentile total US fructose intake(Reference Marriott, Cole and Lee20)). In another meta-analysis of controlled feeding trials, we also showed that fructose in excess of the Canadian Diabetes Association threshold of >60 g/d(2) increased TAG in type 2 diabetes(Reference Sievenpiper, Carleton and Chatha1). Body-weight-raising effects have otherwise been restricted to more extreme doses in hyperenergetic feeding trials (+18 to 50 % energy)(Reference Sievenpiper, de Souza and Kendall17), making it difficult to disentangle the relative contributions of excess fructose and energy. Sugar-sweetened beverage intakes as low as one or two servings/d, nevertheless, have been associated with overweight/obesity, the metabolic syndrome and diabetes in meta-analyses of prospective cohort studies(Reference Malik, Popkin and Bray21). The present findings confirm that fructose at intakes below these thresholds does not appear to have adverse effects on related cardiometabolic risk factors: fasting insulin, body weight, TAG or uric acid.
Limitations of the present analysis need to be considered. First, only two to six trials were included in the meta-analyses for each endpoint. It meant that effects were sensitive to the removal of individual trials, there may have been too little power to detect some differences and publication bias was difficult to assess reliably. Second, the trials were of short duration with only three of the six trials ≥ 8 weeks. It is possible that shorter trials may have underestimated the true effect on HbA1c, given the evidence of a t 1/2 for HbA1c reductions of 35·2 d(Reference Rech22). Subgroup analyses, however, did not show effect modification by follow-up. Third, inter-study heterogeneity complicated the analyses of fasting glucose and insulin. Although the removal of Rizkalla et al. (Expt 1)(Reference Rizkalla, Baigts and Fumeron14) explained the heterogeneity for fasting insulin, sensitivity and subgroup analyses did not explain the heterogeneity for fasting glucose. Random-effects models, however, were used to address residual heterogeneity in all analyses. Finally, although there was a preference for change-from-baseline differences, end differences were used almost exclusively owing to the data reported. There was, however, no evidence of baseline differences between the trials (data not shown).
In conclusion, this small meta-analysis of controlled feeding trials supports earlier 13C NMR spectroscopy investigations(Reference Hawkins, Gabriely and Wozniak3, Reference Petersen, Laurent and Yu4) and acute feeding studies(Reference Moore, Cherrington and Mann5–Reference Heacock, Hertzler and Wolf7) showing that ‘catalytic’ doses ( ≤ 36 g/d) of fructose may improve glycaemic control. This benefit is seen without the adverse cardiometabolic effects reported when fructose is fed at high doses or as excess energy. The strength of these conclusions is limited by the small number of trials and their relatively short duration, especially in relation to HbA1c measurements. That such a small number of eligible trials were identified despite our broad inclusion criteria reinforces that there is a lack of adequate data. To clarify the effect of ‘catalytic’ doses of fructose and low-GI fruit as sources of ‘catalytic’ doses of fructose on glycaemic control, larger and longer-term ( ≥ 6 months) feeding trials are required.
Acknowledgements
This study was funded by a Canadian Institutes of Health Research (CIHR) Knowledge Synthesis grant to J.L.S., R.J.D., A.M., A.J.C., J.B., M.D., A.L.J., L.A.L., T.M.S.W., C.W.C.K. and D.J.A.J. and an unrestricted grant from the Calorie Control Council to J.L.S., R.J.D., J.B., C.W.C.K. and D.J.A.J. J. L. S. was supported by a Province of Ontario Postdoctoral Fellowship, the Edie Steinberg Scholarship Fund and the Edward Christie Stevens Fellowship in Medicine. R.J.D. was funded by a CIHR Postdoctoral Fellowship Award and A.M. was funded by a CIHR Canada Graduate Scholarship Master's award. D. J. A. J. was funded by the Government of Canada through the Canada Research Chair Endowment. None of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. J. L. S. has received several unrestricted travel grants to present research at meetings from The Coca-Cola Company and is a co-investigator on an unrestricted research grant from The Coca-Cola Company. J. L. S. has also received travel funding and honoraria from the Archer Daniels Midland, International Life Sciences Institute (ILSI) North America and Abbott Laboratories; and research support, consultant fees and travel funding from Pulse Canada. R. J. d. S, J. B. and C. W. C. K. are co-investigators on an unrestricted grant from The Coca-Cola Company. C. W. C. K. has served on the scientific advisory board, received research support, travel funding, consultant fees, or honoraria from Pulse Canada, Barilla, Solae, Unilever, Hain Celestial, Loblaws, Inc., Oldways Preservation Trust, the Almond Board of California, the International Nut Council, Paramount Farms, the California Strawberry Commission, the Canola and Flax Councils of Canada, and Saskatchewan Pulse Growers. C. W. C. K. also receives partial salary funding from research grants provided by Unilever, Loblaw's and the Almond Board of California. D. J. A. J. holds an unrestricted grant from The Coca-Cola Company and has served on the scientific advisory board for or received research support, consultant fees or honoraria from Barilla, Solae, Unilever, Hain Celestial, Loblaws Supermarkets, Inc., Sanitarium Company, Herbalife International, Pacific Health Laboratories, Inc., Metagenics/MetaProteomics, Bayer Consumer Care, Oldways Preservation Trust, The International Tree Nut Council Nutrition Research & Education, The Peanut Institute, Procter and Gamble Technical Centre Limited, Griffin Hospital for the development of the NuVal System, Soy Advisory Board of Dean Foods, Alpro Soy Foundation, Nutritional Fundamentals for Health, Pacific Health Laboratories, Kellogg's, Quaker Oats, The Coca-Cola Sugar Advisory Board, Pepsi Company, Agrifoods and Agriculture Canada (AAFC), Canadian Agriculture Policy Institute (CAPI), The Almond Board of California, The California Strawberry Commission, Orafti, the Canola and Flax Councils of Canada, Pulse Canada, the Saskatchewan Pulse Growers, and Abbott Laboratories. D. J. A. J. also holds additional grant support from the CIHR, Canadian Foundation for Innovation (CFI), Ontario Research Fund (ORF), and Advanced Foods and Material Network (AFMNet). T. M. S. W. is the President, A. L. J. a Vice-President and Director of Research and L. C. a casual Clinical Research Coordinator at GI Laboratories, Toronto, Canada. A. I. C., A. M., A. J. C., V. H., D. D. W., M. E. Y., M. D. B. and L. A. L. have no declared conflicts of interest related to this paper.




