Adherence of bacteria to the intestinal epithelium is known to be a prerequisite step for the colonisation and infection of the gastrointestinal tract by many pathogens. Some strains of pathogenic Escherichia coli have developed mechanisms of adhesion to intestinal(Reference Fairbrother, Nadeau and Gyles1) or renal cells(Reference Korhonen, Parkkinen and Hacker2). In particular, enterotoxigenic E. coli (ETEC) strains adhere to receptors on the intestinal epithelium by proteinaceous surface appendages called fimbriae(Reference Grange, Mouricout and Levery3). ETEC expressing the K88 fimbrial antigen is the most common pathogroup in young pigs(Reference Fairbrother, Nadeau and Gyles1), and glycoproteins, sialoglycoproteins or glycosphingolipids are considered the main receptors for different K88 (ab, ac or ad) fimbrial variants(Reference Jin and Zhao4–Reference Coddens, Valis and Benktander7).
The potential of certain compounds to inhibit the adherence of micro-organisms, specifically E. coli, to the intestinal epithelium has been studied in vitro by various authors. Schwertmann et al. (Reference Schwertmann, Shroten and Hacker8) and Shahriar et al. (Reference Shahriar, Ngeleka and Gordon9) described the potential of different milk glycoproteins to bind the fimbriae of E. coli and to inhibit the F4ac-positive E. coli attachment to intestinal villi in vitro. Naughton et al. (Reference Naughton, Mikkelsen and Jensen10) also described different types of prebiotics (non-digestible oligosaccharides) capable of reducing the numbers of E. coli in jejunal organ cultures of pigs. Some reports suggest that milk contains glycoconjugates that have structural homology to the glycan moieties of the intestinal mucosal cell surface and may act as competitive inhibitors of pathogen binding to their glycoconjugate receptors. Examples include oligosaccharides containing α1,2-linked fucosylated oligosaccharides(Reference Newburg, Ruiz-Palacios and Altaye11) or glycoproteins containing sialic compounds(Reference Schwertmann, Shroten and Hacker8).
Caseins are the most abundant bovine milk proteins. The four major types are αs1-, αs2-, β- and κ-casein(Reference Swaisgood and Fox12). Casein glycomacropeptide (CGMP) is a glycoprotein originating from the C-terminal portion of κ-casein during cheese manufacture. Chymosin, an enzyme added to milk, hydrolyses κ-casein into para-casein (residues 1–105), which remains with the curd, and CGMP (residues 106–169), which is removed, becoming the most abundant protein/peptide in whey proteins (20–25 %). CGMP is entirely free of aromatic amino acids(Reference Thomä-Worringer, Sørensen and López-Fandiño13), which has permitted its use in human phenylketonuria diets(Reference LaClair, Ney and MacLeod14). Furthermore, threonine constitutes 18 % of the total amino acid content and a large proportion is glycosylated, resulting in a sialic acid content of about 4·2 %(Reference Nakano, Ikawa and Ozimek15).
Several reviews describe the biological activities of CGMP(Reference Brody16–Reference Krissansen18). Probably one of the most studied effects has been its interaction with the microbiota through the activity of carbohydrate moieties present in the molecule. Some authors have reported that CGMP binds the cholera toxin of Vibrio cholera (Reference Kawasaki, Isoda and Tanimoto19) and promotes the growth of bifidobacteria(Reference Idota, Kawakami and Nakajima20) and Lactococcus species(Reference Bouhallab, Favrot and Maubois21)in vitro, but inhibits the growth of Bacillus subtilis, Salmonella enterica serovars Typhimurium and Enteriditis in Luria-Bertani medium(Reference Wong, Nakamura and Kitts22). Moreover, other studies have shown that milk oligosaccharides(Reference Newburg23) and CGMP(Reference Rhoades, Gibson and Formentin24) inhibited the adhesion of pathogenic E. coli to the mucosal surface or E. coli growth in vitro (Reference Malkoski, Dashper and O'Brien-Simpson25). In a previous study, we also reported that CGMP prevented the attachment of ETEC K88 and reduced the associated inflammatory gene expression using porcine intestinal cell culture(Reference Hermes, Manzanilla and Martín-Orúe26). This anti-inflammatory activity was also reported in rats with induced colitis(Reference Daddaoua, Puerta and Zarzuelo27).
The activity of CGMP in vivo, especially in the distal segment of the small intestine, has not been well explored. Peptides derived from CGMP were detected in the intestinal lumen and blood after ingestion of milk products in human subjects(Reference Chabance, Marteau and Rambaud28, Reference Ledoux, Mahé and Dubarry29) and animals(Reference Fosset, Fromentin and Gietzen30), which suggests that some CGMP fragments may resist protein digestion, probably due to its O-glycosylation(Reference Boutrou, Jardin and Blais31), and reach the distal segment of the gastrointestinal tract.
The objective of the present study was to confirm the ability of CGMP to bind purified K88ac fimbriae and block the attachment of ETEC K88 to the ileal mucosa in vitro (Trial 1), and to assess if a dietary inclusion of CGMP may modify the ileum and hindgut intestinal microbiota and prevent the intestinal dysbiosis and disturbances provoked by an ETEC challenge in weanling piglets (Trial 2).
Materials and methods
The experiments with animals were performed at the Experimental Unit of the Universitat Autònoma de Barcelona and received prior approval (permit no. CEAAH 746) from the Animal and Human Experimental Ethical Committee of this institution. The treatment, management, housing, husbandry and slaughtering conditions conformed to European Union Guidelines(32).
Trial 1. In vitro inhibition assay
Bacterial strain and culture conditions
Two E. coli strains were used in the present experiment to elucidate the effect of CGMP on bacterial adhesion to the intestinal epithelium of weaned piglets. A wild-type ETEC E. coli K88 (F4+, LT1+, ST1+, ST2 +) strain associated with post-weaning diarrhoea in pigs was kindly donated by Dr Ignasi Badiola (CReSA, Barcelona, Spain). The second strain was a non-fimbriated E. coli (F4 − , F6 − , F18 − , LT1 − , ST1 − , ST2+, Stx2e − ) isolated from the faeces of post-weaning piglets and kindly donated by Dr Enric Mateu (Department de Sanitat i Anatomia Animal from the Universitat Autònoma de Barcelona). The strains are named here as Bc-1 and Bc-2, respectively. Bacteria were cultured overnight at 37°C on Luria agar.
Tissue samples
Two 25-d-old piglets were fed a commercial diet and treated with colistin (5 mg/kg body weight per d; Nipoxyme®, Andersen S.A.) over 3 d to reduce the microbial load in the gastrointestinal tract of the animals. After the antibiotic treatment, piglets were euthanised with an intravenous injection of sodium pentobarbital (200 mg/kg body weight). Sections (2 cm long) from the proximal duodenum, proximal jejunum, middle ileum and proximal colon were taken. The intestinal tissue samples were aseptically removed, washed in PBS (pH 7·1), covered with Tissue-Tek® OCT™ (Sakura Finetek Europe B.V.) and immediately snap frozen in liquid N2, as described previously(Reference Nowicki, Holthöfer and Saraneva33). Frozen sections of 5 μm thickness were cut in a Leica cryostat (Leica Instruments GmbH), mounted on SuperFrost Plus glass slides (KeboLab) and stored at − 20°C until use. For the adhesion inhibition assay, tissue sections were fixed for 10 min at room temperature with cold 3·5 % paraformaldehyde in PBS and then washed three times with 50 ml PBS.
Inhibition assay
For the adhesion inhibition studies, bacteria were conjugated with fluorescein isothiocyanate (FITC, Sigma), as described earlier(Reference Nowicki, Holthöfer and Saraneva33–Reference Edelman, Leskelä and Ron35). To localise and characterise adhesion sites, tissue sections were double stained, first with FITC-labelled bacteria and then using an indirect immunofluoresence method with tissue-specific primary antibodies (anti-laminin or anti-villin) and tetramethylrhodamine-conjugated secondary antibodies (tetramethylrhodamine-anti-rabbit or tetramethylrhodamine-anti-mouse immunoglobulins, DakoCytomation)(Reference Korhonen, Parkkinen and Hacker2, Reference Edelman, Westerlund-Wikström and Leskelä34). Briefly, the cell densities of FITC-labelled bacteria were first determined in a Petroff-Hausser chamber and four different concentrations of the bacteria (5 × 107 to 109 cells/ml) were tested in the adhesion assays. Bacteria were diluted in PBS containing 0·01 % (v/v) Tween 20 and 1 % (w/v) bovine serum albumin (BSA) and incubated with tissue sections for 1 h at room temperature. After washing, tissue sections were stained either with anti-laminin serum (diluted 1:100 in PBS) to identify the extracellular tissue domains or with anti-villin to identify the apical surface of the epithelium. Mouse laminin (Sigma) was used as an immunogen to obtain polyclonal antiserum(Reference Virkola, Parkkinen and Hacker36). Commercial anti-villin antibody was used (2 μg/ml in PBS, Chemicon International Inc.). Tissue sections were analysed in an Olympus BX50 fluorescence microscope equipped with filters for FITC and tetramethylrhodamine. The images were digitally recorded using the Image-Pro® Plus program, version 4.0 (Media Cybernetics, Inc.).
The adhesion properties of the Bc-1 and Bc-2 strains were initially analysed in the tissue sections of post-weaning piglets. We use a commercial product (LACPRODAN® CGMP-10, Arla Foods) as a source of CGMP with the following declared composition: protein (N × 6·38) 83–87 %, lactose maximum 2·0 %, fat maximum 0·5 %, ash approximately 6·5 %, moisture maximum 5 %, CGMP content (of protein) 75–85 % and sialic acids content approximately 4·2 %. For the inhibition studies, the FITC-labelled bacteria (1 × 108 bacteria/ml) were first incubated with CGMP on crushed ice for 30 min and then overlaid on the tissue sections and incubated for 1 h at room temperature. Different concentrations of CGMP (0, 0·5, 1·5 and 2·5 mg/ml in PBS) were used to evaluate the inhibition property of CGMP to E. coli adhesion on the post-weaning ileum.
K88ac fimbrial binding to casein glycomacropeptide
Binding of purified K88ac fimbriae to CGMP was tested in a dot blot assay(Reference Virkola, Parkkinen and Hacker36) using K88ac fimbriae previously purified from ETEC E. coli strain 5/95 (O149:F4ac:LT+, ST+)(Reference Joensuu, Kotiaho and Teeri37). To compare K88ac fimbrial binding to other well-characterised glycoproteins, we included laminin (mouse, Sigma), fetuin (fetal calf serum, Sigma), α-casein (bovine milk, Sigma) and mucin type III (porcine stomach, Sigma) as well as BSA, a non-glycosylated serum protein (a negative control), in the assay. CGMP (4 μg and 8 μg/dot) and the test proteins (4 μg/dot) were immobilised on nitrocellulose membranes. After blocking for 1 h at 37°C in 2 % (w/v) BSA/PBS, the membranes were washed three times with PBS containing 0·05 % Tween 20 (PBS-Tween) and incubated with purified K88ac fimbriae (50 μg/ml in 1 % BSA/PBS-Tween) overnight at 4°C with gentle shaking. Membranes were washed three times with cold PBS-Tween and incubated with anti-FaeG polyclonal serum (diluted 1:1000 in 1 % BSA/PBS-Tween(Reference Joensuu, Kotiaho and Teeri37)) for 2 h at 4°C. After washing and incubation with alkaline phosphatase-conjugated anti-rabbit IgG (1:1000; DakoCytomation) for 2 h at 4°C, the bound proteins were visualised by bromochloroindolylphosphatenitrobluetetrazolium (Sigma). Terminal carbohydrates of CGMP, mucin type III, laminin, fetuin and BSA were analysed by a DIG Glycan Differentiation Kit (Roche Diagnostics Corporation), as recommended by the manufacturer(Reference Virkola, Parkkinen and Hacker36).
Effect of casein glycomacropeptide on growth of enterotoxigenic Escherichia coli K88
Minimal mineral medium(Reference Stumpe and Bakker38) was used to test the effect of CGMP on the growth of ETEC K88 strain Bc-1. Modified minimal mineral media contained 46 mm-Na2HPO4, 23 mm-NaH2PO4, 8 mm-(NH4)2SO4, 1 mm-sodium citrate, 1 mm-MgSO4, 6 μm-FeSO4, 1 mg/l thiamin and 20 mm-KCl. Bacteria were inoculated to this basic media or basic media supplemented with either glucose (65 μl per 10 ml of 20 % glucose) or CGMP (130 μl per 10 ml of 10 % CGMP). Bacteria were grown aerobically for 18–20 h at 37°C. Viable counts of bacterial suspensions and optical densities at 600 nm were measured at the beginning and at the end of incubation.
Trial 2. In vivo assay: inclusion of casein glycomacropeptide in the diet of enterotoxic Escherichia coli-challenged weaning piglets
Bacterial strain
The bacterial strain used in the present study (serotype O149:K91:H10 [K-88]/LT-I/STb) was isolated from a colibacillosis outbreak in Spain(Reference Blanco, Blanco and Gonzalez39). It was provided by the E. coli Reference Laboratory, Veterinary Faculty of Santiago de Compostela, Lugo (reference FV12048). The infection inoculum was prepared by 16 h incubation at 37°C in Luria broth (Sigma) with slow agitation (1 g) in an orbital incubator (WY-100, Comecta S.A.).
Animals and housing
The trial was conducted as a Level 2–High Risk Biosecurity Procedure, with appropriate training of the personnel involved. A total of seventy-two piglets ((Large White × Landrace) × Pietrain) from a commercial farm (CollSuri) were weaned at 24 (sem 3) d of age, with an average body weight of 6·9 (sem 0·46) kg. Piglets were transported to the Universitat Autònoma de Barcelona facilities and placed in three rooms of eight pens each (twenty-four pens, three animals per pen). Each pen (3 m2) had a feeder and a water nipple to provide food and water for ad libitum consumption. The weaning rooms were equipped with automatic heating and forced ventilation. The experiment was conducted during the winter season (February), with an average room temperature of 30 ± 2°C.
The experiment was conceived as a 2 × 2 factorial design that included two diets (control v. CGMP) and challenged or not with ETEC K88 (yes v. no). Two rooms were used for the microbial challenge and one room for the non-challenged animals. The two experimental diets were randomly distributed between the pens of each room.
Diets (Table 1) were isoenergetic and isonitrogenous and formulated to satisfy the nutrient requirement standards for pigs(40). In the CGMP diets, LACPRODAN® CGMP-10 (Arla Foods) was added at 2 % (w/w), representing about 1·5 % of CGMP. This dose was based on the previous in vitro assay results wherein 0·25 % presented the best inhibition of ETEC adhesion to the ileum epithelium samples. For the translation of this dose to the diet, we assumed that the CGMP would be partially digested (about 40 %) and diluted in the ileal digesta (approximately 20 % DM).
Table 1 Composition and chemical analysis of the diets*
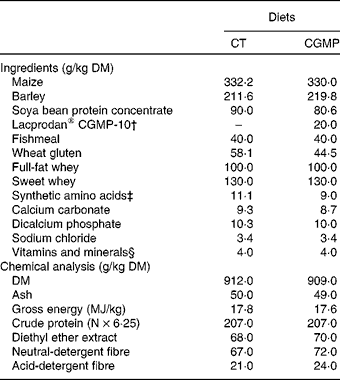
CT, control; CGMP, casein glycomacropeptide.
* Trial 2: in vivo experiment.
† Lacprodan® CGMP-10: 74 % of purity (Arla Foods).
‡ l-Lys 0·99, dl-Met 0·99, l-Try 0·10 and l-Thr 0·98.
§ Supplied per kg of feed: 13 000 IU (3900 μg) vitamin A, 1800 IU (45 μg) vitamin D3, 60·0 mg vitamin E, 3·0 mg vitamin K1, 2·0 mg vitamin B1, 6·0 mg vitamin B2, 3·0 mg vitamin B6, 0·02 mg vitamin B12, 35·0 mg niacin, 15·0 mg calcium pantothenate, 0·12 mg biotin, 1 mg folic acid, 20·0 mg Fe, 120·0 mg Cu, 110 mg Zn, 45·0 mg Mn, 0·30 mg Se, 0·10 mg Co, 1 mg I and 2·5 mg ethoxyquin as an antioxidant (Capsoquin; Itpsa, Barcelona, Spain).
Experimental procedures
Animals received the experimental diets over a period of 15 d. After 1 week of adaptation, a single 2 ml oral dose (1 × 109–1010 colony-forming units (CFU)/ml) of the ETEC K88 strain was administered to the challenged animals or a single 2 ml oral dose of sterile Luria broth (Sigma) to the non-challenged animals. Individual body weight and pen feed consumption were registered weekly. Animals were checked daily to evaluate their status after the E. coli challenge. Briefly, the rectal temperature was measured every 2 d and clinical signs (i.e. dehydration, apathy and diarrhoea) were monitored daily. Diarrhoea incidence was measured as the percentage of animals in each pen that presented inconsistent to liquid faeces. The mortality rate was also recorded.
At 4 d after the ETEC challenge (day 11) and on the final day of the experiment (day 15 after weaning), one animal from each pen was euthanised. On day 11, from each pen the pig closest to the medium weight was selected; on day 15, the heavier of the two remaining pigs was taken. Animals were sequentially sampled during the morning (between 09.00 and 12.00 hours). At first blood samples were taken from the jugular vein and then the animals were euthanised with an intravenous sodium pentobarbital overdose (200 mg/kg body weight). The abdomen was immediately opened and digesta samples of ileum and proximal colon were collected for bacterial counts. Four other sub-samples were maintained at − 20°C for further analyses including: quantification of microbial groups by quantitative PCR and determination of volatile fatty acids, ammonia and protein concentrations in the digesta. Moreover, for analysing enterobacteria attached to the ileal mucosa, 5-cm-long sections of ileum were collected from each animal, washed thoroughly three times with sterile PBS, opened longitudinally and scraped with a microscopy glass slide to obtain the mucosa scraping contents. For the histological study, 3-cm-long sections were removed from the middle ileum, opened longitudinally and fixed by immersion in Carnoy solution, as described by Swidsinski et al. (Reference Swidsinski, Weber and Loening-Baucke41). Another ileal section of 3 cm was removed, opened longitudinally, placed in cassettes recovered with Tissue-Tek® OCT™ cryoprotective solution (Sakura Finetek Europe B.V.), frozen in liquid N2-cooled isopentane and maintained at − 80°C for further analyses using fluorescence in situ hybridisation (FISH).
Analytical procedures
Chemical analyses of the diets including, DM, ash, gross energy, crude protein, diethyl ether extract, neutral-detergent fibre and acid-detergent fibre were performed according to Association of Official Agricultural Chemists (AOAC) standard procedures(42).
For bacterial counts, the content of ileal mucosa scrapings was seeded in eosin methylene blue agar (Scharlab, S.L.). The plates were incubated for 24 h at 37°C and the manufacturer's instructions for the quantification of the colonies were followed.
The DNA from ileal and colon digesta was extracted and purified using commercial QIAamp DNA Stool Mini Kit (Qiagen). Lactobacilli and enterobacteria were quantified by real-time PCR using SYBR Green dye following the procedure described by Castillo et al. (Reference Castillo, Martín-Orúe and Manzanilla43). For E. coli (K88) real-time PCR quantification, a new procedure was implemented. For this, the selected target gene was that coding the F4 fimbria of E. coli K88. The PCR products (439 bp) obtained using the primers 5′-GCACATGCCTGGATGA-CTGGTG-3′ and 5′-CGTCCGCAGAAGTAACCCCACCT-3′(44) and the DNA obtained from pure cultures of the challenge strain (QIAamp DNA Mini Kit, Qiagen) were used for the construction of the standard curves. The PCR product was purified using the commercial kit DNA purification system (Promega Biotech Ibérica) and the concentration measured using a Qubit™ Fluorometer (Invitrogen). The products obtained were also sequenced (ABI 3100 Genetic Analyzer, PE Biosystems) to confirm it and the number of copies calculated. Serial dilutions were performed and 104, 105, 106, 107 and 108 copies of the gene per reaction were used for calibration.
To quantify E. coli K88, the following pair of primers was designed using Primer Express Software (Applied Biosystems): 5′-CAGAAATGGGAATGGAA-AGTTG-3′ and 5′-CCATTGGTCAGGTCATTCAATACA-3′(44). Real-time PCR was performed with the ABI 7900 HT Sequence Detection System (PE Biosystems) using optical-grade ninety-six-well plates. The PCR was performed on a total volume of 25 μl using the SYBR-Green PCR Core Reagents Kit (PE Biosystems). Each reaction included 2·5 μl of 10 × SYBR Green buffer, 3 μl MgCl2 (25 mm), 2 μl deoxynucleotide triphosphates (2·5 mm), 0·25 μl AmpErase UNG (uracil-N-glycosylase) (1 U/μl), 0·125 μl AmpliTaq Gold (5 U/μl), 1 μl of each primer (12·5 μm) and 2 μl of DNA samples. The reaction conditions for amplification of DNA were 95°C for 10 min, forty cycles of 95°C for 15 s and 60°C for 1 min. To determine the specificity of amplification, an analysis of the product melting curve was performed after the last cycle of each amplification. The minimum level of detection of the method, considering the amount of DNA included in each reaction, was established in 3·249 (sem 0·419) log of 16S ribosomal RNA gene copies/g of fresh matter sample, compared to a non-template control dissociation curve.
Volatile fatty acids were determined by GC after submitting the samples to an acid–base treatment followed by an diethyl ether extraction and derivatisation(Reference Jensen and Jorgensen45). Ammonia was determined with the aid of a gas sensitive electrode (Crison ISE- 9665, Crison Instruments, S.A.). A measure of 3 g of digesta was diluted (1:2) with 0·16 m-NaOH, and after homogenisation, samples were centrifuged (1500 g) for 10 min. Subsequently, the ammonia released from the samples was measured in the supernatants using a digital voltmeter (Crison GLP 22, Crison Instruments, S.A.)(Reference Jensen and Jorgensen45). The crude protein measurement on colon digesta was performed in a combustion analyser (TruSpec CN, LECO Corporation).
Tissue samples for morphological measures were dehydrated and embedded in paraffin wax, sectioned at 4 μm thickness and stained with haematoxylin and eosin. Morphological measurements were performed with a light microscope (BHS, Olympus)(Reference Nofrarias, Manzanilla and Pujols46).
Serum was obtained from 10 ml blood drawn into the tubes (without anticoagulant) and by centrifugation of blood at 3000 g, for 15 min at 4°C. Concentrations of TNF-α and interferon-γ were determined by Quantikine® Porcine TNF-α and interferon-γ kits, respectively (R&D Systems). Pig major acute-phase protein (Pig-MAP) concentration was determined by a sandwich-type ELISA (Pig MAP Kit ELISA, Pig CHAMP Pro Europe S.A.), according to the manufacturer's instructions.
The FISH technique was performed by modifying the protocol described by Swidsinski et al. (Reference Swidsinski, Weber and Loening-Baucke41). Briefly, triplicate samples of frozen ileum were sliced (5 μm thick) on a Leica CM 1900® (Leica Microsystems GmbH) cryostat. The tissue samples were placed in Superfrost Gold Plus® (Thermo Fisher Scientific) and fixed with 4 % (w/v) paraformaldehyde solution for 30 min. Oligonucleotide probes were synthesised by TIB Molbiol GmbH, using carbocyanite-3 and FITC dyes, added at the 5′ end to the EC1531 probe, and the EUB338, NON338 probes, respectively. EC1531 probe was used to identify E. coli bacteria on samples, EUB338 to all kind of bacteria, whereas NON338 was used to distinguish non-specific hybridisation sites. A hybridisation buffer (0·9 m-NaCl, 20 mm-Tris–HCl (pH 7·4), 2 % formamide and 0·1 % SDS) was used at 50°C for 45 min. Furthermore, 4,6-diamidino-2-phenylindole staining was used to mark all the eukaryotic cells' nuclei. The in situ quantification of mucosal bacteria was visualised with a Confocal Laser Microscope (Fluoview FV1000, Olympus GmbH) and photo documented with an Olympus camera and software (FV-ASW, version 1.7c; Olympus GmbH). Quantification was performed when the hybridisation signals were clear and morphologically distinguishable as bacterial cells by at least triple-colour identification with universal and group-specific FISH probes and 4,6-diamidino-2-phenylindole staining and by the absence of cross-hybridisation or hybridisation using the NON338 nonsense probe. From each triplicate sample, the percentage of villi with adhered bacteria was determined by the same person who was blind to the treatments, and using twenty microscopical fields, the total number of villi and the number of them with the presence of adherent E. coli were counted.
Statistical analyses
The results from Trial 2 (in vivo trial) are expressed as means with their standard errors unless otherwise stated. A two-way ANOVA was used to examine the effect of experimental infection and experimental diet, as well as the interaction between the two (only included when significant). All statistical analyses were performed using the mixed procedure of the Statistical Analysis Package, SAS version 9.1 (SAS Institute Inc.)(47). When treatment effects were established (P< 0·05), treatment least squares means were separated using the probability of differences function adjusted by Tukey–Kramer(47). Random effects were used to account for variation between pens. The α level used for the determination of significance for all the analysis was 0·05. The statistical trend was also considered for 0·05 < P< 0·10.
Results
Trial 1. In vitro inhibition assay
Escherichia coli intestinal adhesion and inhibition assay with casein glycomacropeptide
The ETEC K88 strain Bc-1 showed a strong adherence to the apical pole of villus enterocytes in the ileum (Fig. 1(a)), a lower adherence to the duodenum (Fig. 2(a)) and jejunum (Fig. 2(b)) and a negative adherence to the caecum and colon (data not shown). A weak bacterial adhesion was also seen in the lamina propria beneath the villus epithelium in the ileum that was identified with anti-laminin antibodies (Fig. 1(e)). No adhesion to the intestinal sections was found with the non-fimbriated E. coli strain Bc-2 (Fig. 2(c, d)). After these results, ileum sections were taken as models to continue with the inhibition assays.

Fig. 1 Inhibition of enterotoxigenic Escherichia coli K88 Escherichia coli strain Bc-1 adherence to the piglet ileum epithelium with casein glycomacropeptide (CGMP). The tissue sections were double stained with fluorescein isothiocyanate-labelled bacteria (panels a, b, c, d) and laminin (panels e, f, g, h). Tissue by phase contrast microscopy (panels i, j, k, l). CGMP was tested in the following concentrations: (a, e, i) 0; (b, f, j) 0·5; (c, g, k) 1·5; (d, h, l) 2·5 mg/ml. Arrows indicate the epithelial surface. e, Epithelium; LP, lamina propria. Size bars 100 μm. Trial 1: in vitro experiment.

Fig. 2 Adhesion of enterotoxigenic Escherichia coli K88 strain Bc-1 to the (a, e, i) duodenum and (b, f, j) jejunum and E. coli strain Bc-2 to (c, g, k) the jejunum and (d, h, l) the ileum epithelium of weaned piglets. The tissue sections were double stained with fluorescein isothiocyanate-labelled bacteria (panels a, b, c, d) and laminin (panels e, f, g, h). Tissue by phase contrast microscopy (panels i, j, k, l). Arrows indicate the epithelial surface. e, Epithelium; LP, lamina propria. Size bars 100 μm. Trial 1: in vitro experiment.
In the adhesion inhibition studies with CGMP, adherence of the Bc-1 strain was reduced on the apical epithelium (Fig. 1(b–d)). Increase in the inhibitor concentration resulted in a gradual decrease in the number of E. coli attached to the epithelial surface (Fig. 1(b–d)). The highest concentration of CGMP (2·5 mg/ml; Fig. 1(d)) was needed for E. coli Bc-1 inhibition on the apical epithelium of the ileum. However, this concentration did not inhibit bacterial adherence to lamina propria regions of the ileum (Fig. 1(b–d)).
K88ac fimbrial binding to casein glycomacropeptide
The K88ac fimbriae bound strongly to the highly glycosylated fetuin and laminin, but did not bind to BSA, a non-glycosylated protein (Fig. 3). Fetuin and laminin contain terminal Galβ(1-4)-GlcNAc structures, which are potential target structures for K88ac fimbrial binding(Reference Melt, Kloetzlen and Vosbeck48, Reference Arumugam, Hsieh and Tanzer49). The K88ac fimbria also bound to CGMP and α-casein, as well as mucin type III from porcine stomach (Fig. 3). DIG Glycan Differentiation Kit (Roche Diagnostics Corporation) analysis of the CGMP and the glycoproteins showed the presence of terminal Galβ(1-3)GalNAc- and/or NeuAc(2-6)Gal- in all K88ac-positive target proteins.

Fig. 3 Dot blot analysis with purified K88ac fimbriae. (a) Binding of purified K88ac fimbriae of enterotoxigenic Escherichia coli strain 5/95 to immobilised casein glycomacropeptide (CGMP), laminin, bovine serum albumin (BSA), fetuin, α-casein and mucin type III. (b) Binding of anti-FaeG serum. Trial 1: in vitro experiment.
Effect of casein glycomacropeptide on the growth of enterotoxigenic Escherichia coli K88
Minimal mineral medium was used to test the effect of CGMP on the growth of ETEC K88 E. coli strain Bc-1. In the presence of glucose, the bacteria grew from 2·19 × 104 to 1·96 × 109 CFU/ml (time 0 and 20 h incubation, respectively). In the minimal mineral media and in the minimal mineral media supplemented with CGMP, bacteria were growing weakly reaching about 103 times lower cell densities (2·19 × 104 to 1·17 × 106 CFU/ml at time 0 and 20 h incubation, respectively). Very minimal growth stimulation effect with CGMP was seen, as compared to media without added substrate (2·19 × 104 to 4·75 × 105 CFU/ml at time 0 and 20 h incubation, respectively).
Trial 2. In vivo assay: inclusion of casein glycomacropeptide in the diet of enterotoxigenic Escherichia coli-challenged weaning piglets
Animal performance, acute immune response and colon microbial activity
Neither experimental diet nor ETEC challenge affected feed intake and growth (data not shown). The average final body weight on day 15 after weaning was 9·6 (sem 1·44) kg. No significant differences were observed in mortality rate (experimental average = 8 %) between diets. Furthermore, no significant increase was observed in the body temperature recorded (data not shown).
Table 2 shows the Pig-MAP and TNF-α serum concentrations. The administration of CGMP did not affect the Pig-MAP and TNF-α concentrations. On the other hand, the ETEC challenge tended to increase Pig-MAP concentration 4 d after challenge (P= 0·082). ETEC challenge also increased TNF-α concentration (P =0·036) 4 d after the challenge. The interferon-γ results were below ( < 39 pg/ml) the sensitivity used by the kit assay (data not shown).
Table 2 Serum concentration of Pig-major acute phase protein (Pig-MAP; μg/ml) and TNF-α (pg/ml) and concentrations of protein (mg/g of DM), ammonia and volatile fatty acids (VFA; mmol/g of FM) and VFA profile (butyric acid and branched-chain fatty acids (isoacids); % of VFA) in colonic digesta at 4 and 8 d post-challenge in weaned piglets challenged (Yes) or not (No) with enterotoxigenic Escherichia coli (ETEC) K88 (Trial 2) (Mean values with their standard errors)

CT, control; CGMP, casein glycomacropeptide.
* Mean values were not significantly different for diet × challenge interaction (P>0·05).
† Diet × challenge, effect of diet and challenge with ETEC in weaning piglets.
Regarding microbial activity, Table 2 also presents volatile fatty acid concentrations, the relative concentrations of butyric and isoacids, the ammonia concentration and the protein content in the proximal colon digesta 8 d post-challenge. Experimental factors did not cause significant differences in the total volatile fatty acid concentration. However, a diet effect was observed with the CGMP diet, increasing butyric acid (P =0·047) and isoacid relative amounts (P =0·007) and the crude protein content (P =0·050) of colonic digesta, and it tended to increase the ammonia concentration (P =0·059) compared to the control diet. The ETEC challenge also tended (P =0·083) to increase ammonia concentration compared to non-challenged treatments. No significant differences were observed in the colon fermentation parameters of piglets 4 d post-challenge (data not shown).
Changes in the intestinal morphology and lumen microbial population
Table 3 presents the results of ileum morphology (4 d post-challenge). The ETEC challenge reduced (P= 0·043) the villus:crypt ratio, increased intraepithelial lymphocyte numbers (P= 0·046) and tended to increase crypt depth (P= 0·054). On the other hand, CGMP inclusion increased crypt depth (P= 0·016). No significant differences were observed in ileum histology of piglets 8 d post-challenge (data not shown).
Table 3 Villus height (VH; μm), crypt depth (CD; μm), villi:crypt ratio (VCR), intraepithelial lymphocytes (IEL; cells/100 μm) of the ileum of weaned piglets 4 d post-challenge; enterobacteria counts on ileum mucosa scrapes (log of colony forming units/g of content), Escherichia coli in situ villi adherence (percentage of ileal villi with E. coli adhered) and diarrhoea incidence (percentage of animals in each pen that presented inconsistent to liquid faeces) of weaned piglets (whole experimental period) challenged (Yes) or not (No) with enterotoxigenic E. coli (ETEC) K88 (Trial 2) (Mean values with their standard errors)

CT, control; CGMP, casein glycomacropeptide.
* Mean values were not significantly different for diet × challenge interactions (P>0·05).
† Diet × challenge, interaction between diet and ETEC challenge.
Counts of lactobacilli, enterobacteria and E. coli K88 in ileum and proximal colon digesta, measured by real-time PCR, are presented in Table 4. A significant effect was observed in the lactobacilli counts for the ETEC challenge and the dietary treatment. The ETEC challenge promoted a decrease (P= 0·020) in lactobacilli numbers in ileum digesta 4 d after challenge, while the administration of CGMP increased lactobacilli numbers 8 d post-challenge in ileum (P= 0·038) and proximal colon (P= 0·015) digesta. No significant interactions were observed between treatments for the lactobacilli counts. A significant interaction was observed between the ETEC challenge and the dietary treatment on the enterobacteria numbers in the digesta of the ileum (P= 0·006) and the proximal colon (P= 0·005) 4 d after challenge. The ETEC challenge promoted and increased enterobacteria counts in the animals fed on the control diet but not in those fed on the CGMP diet. No significant differences were observed for enterobacteria counts in the ileum or in the colon 8 d after challenge.
Table 4 Counts of lactobacilli, enterobacteria and Escherichia coli K88 at 4 and 8 d post-challenge (log of 16S ribosomal RNA gene copies/g of fresh matter sample) in digesta of weaned piglets challenged (Yes) or not (No) with enterotoxigenic E. coli (ETEC) K88 (Trial 2) (Mean values with their standard errors)

CT, control; CGMP, casein glycomacropeptide, ND, not detected.
a,bMean values within a row with unlike superscript letters were significantly different (P <0·05).
* Interaction between diet and challenge with ETEC.
† Minimum detection level of the method: 3·249 (sem 0·419) log of 16 S ribosomal RNA gene copies/g of fresh matter sample.
Regarding the E. coli K88 counts, we only detected measurable numbers in the challenged groups, but no significant differences were observed between diets.
Ileal bacterial adhesion and diarrhoea incidence
Table 3 shows the number of enterobacteria adhered to the ileal mucosa, the percentage of ileal villi with adherent E. coli and the incidence of diarrhoea in animals from the whole experimental period.
The CGMP diet tended to reduce (P= 0·074) the enterobacteria number of mucosa scrape contents by up to 6 log units and also the percentage of villi with adherent E. coli (P =0·062), but did not reduce the incidence of diarrhoea. In any case, ETEC challenge promoted diarrhoea (P= 0·015) and increased E. coli attachment to ileal mucosa (P= 0·008). However, diarrhoea was not severe enough for antibiotic intervention during the experimental period.
To better illustrate the ileal in situ monitoring of E. coli adhesion, Fig. 4 shows a positive sample stained with carbocyanite-3 E. coli-specific probe (Fig. 4(a)), where E. coli bacteria adhered to the ileal mucosa, another positive sample stained with FITC universal bacteria-specific probe (Fig. 4(b)) and a negative sample (Fig. 4(c)) without adherent bacteria.

Fig. 4 Escherichia coli adhesion to the ileal mucosa measured by the fluorescence in situ hybridisation technique. (a) Positive, merge picture with 4,6-diamidino-2-phenylindole (DAPI) staining (fluorescent blue, intestinal cells nucleus), carbocyanite-3 staining (EC 1531 probe, fluorescent red, E. coli cells) and transmitted light (intestinal villi). (b) Positive, merge picture with DAPI staining (fluorescent blue, intestinal cells nucleus), fluorescein isothiocyanate staining (EUB 338 universal bacterial probe, fluorescent green, E. coli cells) and transmitted light (intestinal villi). (c) Negative, merge picture with DAPI staining (fluorescent blue, intestinal cells nucleus) and transmitted light (intestinal villi). An Olympus Laser Confocal Microscope (Fluoview FV1000, Olympus GmbH) was used. Size bars 50 μm. Trial 2: ileal adhesion.
Discussion
Casein glycomacropeptide as an inhibitor of the Escherichia coli attachment to the intestinal mucosa
In the present study, we first used in vitro methods to elucidate the effect of CGMP in host–pathogen interactions. Using immunohistology with frozen intestinal tissue sections, we localised the receptor-active tissue sites for ETEC expressing K88 fimbriae in the ileum of weaned piglets. Less adhesion was found in the jejunum mucosa and poor adhesion was found in duodenal and colonic mucosa. We observed ETEC K88-specific adherence to the apical surface of the villus enterocytes, whereas no adhesion was seen with a non-fimbriated porcine E. coli isolate. This K88 fimbrial-specific adhesion to the apical pole of the ileum enterocytes confirmed that pigs in the present study had K88 receptors. Anderson et al. (Reference Anderson, Whitehead and Kim50) first studied K88 fimbrial binding to purified brush-border membranes of porcine small intestine and since then several studies have shown that glycoproteins, sialoglycoproteins or glycosphingolipids are recognised by different K88 fimbrial variants(Reference Grange, Mouricout and Levery3, Reference Van den Broeck, Cox and Oudega6, Reference Coddens, Valis and Benktander7, Reference Shahriar, Ngeleka and Gordon9). The fimbrial K88ac variant is the most prevalent and clinically important variant of ETEC, which tends to colonise the jejunum and ileum in neonatal and weaned piglets(Reference Fairbrother, Nadeau and Gyles1, Reference Nagy and Fekete5). Because adhesion to host cells is the first key step in causing microbial infections, it should be possible to prevent them by blocking the adhesion sites. In human subjects, milk oligosaccharides have been reported to act as soluble receptors for bacterial adhesins(Reference Fairbrother, Nadeau and Gyles1), which block their binding sites and prevent bacteria adhesion to intestinal epithelial cells. These oligosaccharides generally have a lactose moiety at the reducing end of the molecule and often contain a fucose and/or sialic acid moiety at the non-reducing end, which confers the biological activities of human milk in breastfeeding infants(Reference Newburg, Ruiz-Palacios and Altaye11, Reference Brody16). In the present study, CGMP (4·2 % of sialic acid) showed an effective blocking activity of the ETEC K88 attachment to the ileal mucosa at a CGMP concentration of 2·5 mg/ml. The dot blot analysis with K88 purified fimbriae further confirmed the ability of this fimbriae to bind CGMP, where terminal carbohydrates, such as Galβ(1-3)GalNAc- and NeuAc(2-6)Gal-, may act as potential receptor analogues(Reference Grange, Mouricout and Levery3).
A previous study(Reference Hermes, Manzanilla and Martín-Orúe26) of our research group and other authors have also described the ability of CGMP(Reference Brück, Graverholt and Gibson51, Reference Strömqvist, Falk and Lennart Hansson52) and several sialic acid ingredients(Reference Korhonen, Parkkinen and Hacker2, Reference Schroten, Plogmann and Hanisch53) to inhibit the adhesion of different pathogenic bacteria to the intestinal epithelium in vitro. A striking example of the successful application of receptor analogues was obtained by Mouricout et al. (Reference Mouricout, Petit and Carias54) who protected colostrum-deprived newborn calves from a lethal dose of ETEC K99 by oral administration of sialylated glycoproteins. In the results obtained in Trial 2, the CGMP diet was also able to reduce the counts of enterobacteria attached to the ileal mucosa scrapings, as well as the percentage of villi with adherent E. coli. To our knowledge, this is the first report using this approach to measure the adhesion of E. coli in situ.
Digestive effects of the casein glycomacropeptide in early weaned piglets
The CGMP diets increased the crude protein, ammonia and isoacid concentrations in colonic digesta, probably suggesting a higher amount of protein reaching the hindgut and being fermented by the bacteria(Reference Blachier, Mariotti and Huneau55). These results could reflect a lower digestion of the total dietary N compounds and specifically that of CGMP in the small intestine(Reference Boutrou, Jardin and Blais31) or an increased endogenous protein secretion(Reference Ledoux, Mahé and Dubarry29) into the intestinal lumen. However, the present study was not intended to identify or explain the origin of this increase of crude protein concentration in the hindgut digesta. We cannot discard that likely effects of the CGMP on the intestinal microbiota may modulate the end product of fermentation towards an increase in proteolytic activity. Regarding the digestion of CGMP, it has been previously observed that glycoproteins are less digested along the gastrointestinal tract than their unglycosylated homologs, probably due to their large number of O-glycosidic linkages which confer a unique conformation that reduces digestion by endopeptidases(Reference Boutrou, Jardin and Blais31). Moreover, CGMP amino acid sequence, which is free from aromatic amino acids and other essential amino acids (arginine, cysteine, histidine, tryptophan and tyrosine)(Reference Brody16), confers to CGMP a structure more difficult for endogenous proteases such as trypsin, chymotrypsin and pepsin that preferably cleave peptidic bonds near these amino acids(Reference Erickson and Kim56). A likely arrival of intact CGMP or related smaller peptides to the distal small intestine should not be discarded and could help to explain how CGMP in the diet may reduce the enterobacteria counts in the ileal mucosa and the number of villi with adhered bacteria.
The effect of casein glycomacropeptide on intestinal health and microbiota
The challenge with ETEC K88 promoted mild diarrhoea during the 1st day after infection. However, the animals recovered quickly without antibiotic treatment. Most of the histological parameters were significantly affected on day 4 but not on day 8 after ETEC challenge, confirming an early (1–5 d) acute enteric disease(Reference Fairbrother, Nadeau and Gyles1). We also observed an increase in serum TNF-α and Pig-MAP acute protein, which indicates an acute immune response to the pathogen(Reference Piñeiro, Piñeiro and Morales57), but these responses were not modified by the experimental diet. Furthermore, ETEC challenge caused a reduction in the villus height:crypt depth ratio and an increase in the number of intraepithelial lymphocytes, representing typical histological findings of an acute enteric disease. Although we did not measure fermentation products in ileum digesta, we observed increases in butyric acid concentrations in colonic digesta of the CGMP piglets. Some authors have described the cell proliferation effect of butyrate on the intestinal tract(Reference Scheppach58, Reference Guilloteau, Martin and Eeckhaut59).
Regarding microbiology results, it is interesting to remark the significant interaction found for enterobacteria counts at day 4 post-challenge, both in ileum and proximal colon digesta. In the ileum, where the receptors for E. coli are localised, the ETEC challenge did not increase the numbers of enterobacteria in the animals fed on CGMP, but it did so in those receiving the control diet. Therefore, these results suggest the ability of CGMP to somehow prevent ileal colonisation by enterobacteria after an E. coli challenge. These effects could have been mediated by interference in the adhesion mechanisms of ETEC K88 to the intestine, as it has been observed in the in vitro study, and also by a possible prebiotic effect of the CGMP that could have modulated the microbiota, promoting the growth of other bacteria groups. In that sense, it is fair to remark how CGMP stimulated lactobacilli in ileum and proximal colon digesta 8 d after challenge, in both challenged and non-challenged animals. Some authors have also reported a growth-promoting effect of CGMP on both Bifidobacterium (Reference Naughton, Mikkelsen and Jensen10, Reference Janer, Peláez and Requena60) and Lactococcus species(Reference Bouhallab, Favrot and Maubois21)in vitro and also in children(Reference Janer, Peláez and Requena60). Brück et al. (Reference Brück, Redgrave and Tuohy61) suggested CGMP inclusion in infant formulae to stimulate the beneficial bacteriological effect of breast milk. Furthermore, Nakajima et al. (Reference Nakajima, Tamura and Kobayashi-Hattori62) suggested CGMP potential to prevent intestinal infection caused by Salmonella enteritidis and enterohaemorrhagic E. coli. Regarding these data, we cannot discard the facts that the changes observed in the amount of enterobacteria on the ileal mucosa and the number of villi with adhered E. coli could have been mediated by a competitive effect of the lactobacilli population together with the blockade of F4 fimbria demonstrated in vitro in this and other studies(Reference Rhoades, Gibson and Formentin24, Reference Hermes, Manzanilla and Martín-Orúe26).
Conclusions
The present results confirm the ability of CGMP to prevent the adhesion of ETEC K88 to the pig intestine. Moreover, its inclusion in the diet of early weaning pigs can prevent the overgrowth of intestinal enterobacteria after an ETEC K88 challenge. The possible prebiotic effect of this milk derivative is also suggested by the reported increase of lactobacilli population in both ileum and proximal colon and in significant changes in the colonic fermentation profile.
Acknowledgements
The present study was supported by the Ministerio de Educación y Ciercia, Spain (project no. AGL2007-60851). We thank Dr Alexander Swidsinski (Charité-Universitätsmedizin, Berlin, Germany) for his help in applying the FISH technique; Dr Ignasi Badiola (CReSA, Barcelona, Spain) for the ETEC strain used in the first trial and Dr Kirk C. Klasing (University of California, Davis, USA) for his careful reading of the present paper. We are grateful to Arla Foods (Viby J, Denmark) for providing the CGMP and the Servei de Granges i Camps Experimentals de la UAB for its service and assistance during the experiment. We thank Dr Sinikka Pelkonen (Veterinary Bacteriology Research Unit, Kuopio, Finland) for typing the strains Bc-1 and Bc-2. We thank Dr Jussi Joensuu for the ETEC E. coli strain 5/95, the purified K88ac fimbriae and the anti-FaeG serum. We thank Mireia Cases for her collaboration in the preparation of the manuscript figures. There are no conflicts of interest statements between the authors. All the co-authors participated in the research and the writing of the present manuscript equally.










