Obesity is an important public health problem(Reference Carnethon, Loria and Hill1). In 2008, 1·5 billion adults, 20 years and older, were overweight. Of these, over 200 million men and nearly 300 million women were obese. It is important to emphasise that 65 % of the world's population live in countries where overweight and obesity kill more people than underweight(2).
Some studies have shown benefits of breast-feeding in reducing and preventing obesity(Reference Arenz, Rückerl and Koletzko3, Reference Owen, Martin and Whincup4). The protective effect of breast-feeding against obesity is proportional to the duration and exclusivity of breast-feeding(Reference Arenz, Rückerl and Koletzko3, Reference Harder, Bergmann and Kallischnigg5). Exclusive breast-feeding is defined by the WHO as the consumption of breast milk until 6 months without any other type of food intake, juice or even water(6). However, < 35 % of children worldwide are exclusively breast-fed during the first postnatal 4 months(7). Weaning is defined as the introduction of any food in the diet of a child who had a regimen of exclusive breast-feeding(Reference Araújo, Gonçalves and Cornetta Mda8). Thus, early weaning would be the cessation of natural breast-feeding before the child is 6 months old(Reference Candeias9).
It is well known that an adequate nutrient supply during early life is essential to establish the future endocrine and metabolic status. In fact, epidemiological and experimental data suggest that intra-uterine undernutrition is closely associated with adulthood obesity related to detrimental metabolic functions(Reference Godfrey and Barker10, Reference Breier, Vickers and Ikenasio11), giving rise to the concept of ‘developmental origins of health and disease’. This association has been denominated programming, which is defined as a biological phenomenon that determines the relationship between physical and chemical stimuli in critical periods of early life (gestation and lactation) with the future functional status(Reference de Moura, Lisboa and Passos12). In addition, malnutrition only during lactation can programme for metabolic and endocrine disorders of the progeny in adulthood(Reference Vicente, de Moura and Lisboa13–Reference Fagundes, Moura and Passos17). In two different models of precocious weaning, our group evidenced the programming for metabolic syndrome parameters, such as obesity, type 2 diabetes and dyslipidaemia. In the first model, the suppression of lactation through maternal treatment with bromocriptine (a prolactin inhibitor) for 3 d caused milk yield inhibition and programmed the offspring for higher total and central fat, hyperleptinaemia, resistance to the anorexigenic action of leptin(Reference Bonomo, Lisboa and Pereira18), insulin resistance, lower HDL-cholesterol, higher serum TAG and cholesterol concentrations in adulthood(Reference Moura, Bonomo and Nogueira-Neto19) as well as hypothyroidism(Reference Bonomo, Lisboa and Passos20). Recently, in the second model of early weaning caused by lactation interruption with breast banding, the adult progeny showed higher adiposity, higher TAG and insulin resistance(Reference Lima, de Moura and Passos21). In addition, these animals also displayed lower hypothalamic Janus tyrosine kinase 2 (JAK2), phosphorylated signal transducer and activator of transcription 3 (pSTAT3) and higher suppressor of cytokine signalling 3 (SOCS3) levels, a feature that indicates leptin resistance. This second programming model is advantageous since lactation interruption occurs without the use of pharmacological substances or maternal separation.
The correction of metabolic disturbance observed in obesity is important for reducing cardiometabolic risk. Nutritional bioactive compounds have been used in the prevention of chronic diseases associated with obesity, acting as an adjunct of weight loss, because they are able to elevate energy expenditure and promote satiety(Reference St-Onge22). Several studies have investigated the relationship of dietary Ca with energy balance management. Some studies have shown that a diet rich in Ca reduced body weight (BW)(Reference Gonzalez, White and Kristal23) and adiposity(Reference Zemel24–Reference Lin, Lyle and McCabe26), and improved both insulin sensitivity(Reference Choi, Willett and Stampfer27–Reference Pittas, Harris and Stark29) and lipid profile(Reference Jacqmain, Doucet and Despres30, Reference Reid, Ames and Mason31).
One hypothesis for the explanation of the beneficial effect of Ca supplementation is its ability to modulate energy metabolism through calciotropic hormone concentrations: calcitriol and parathyroid hormone(Reference Zemel32), which seem to be the main mechanism for the anti-obesity effect of Ca supplementation. Calcitriol rapidly increases Ca uptake by the adipocyte, decreasing uncoupling protein activity, lipolysis and apoptosis. Higher Ca intake, decreasing parathyroid hormone and calcitriol levels, induce opposite effects in adipocytes. A diet poor in Ca increasing these hormones leads to lipogenesis, and inhibits lipolysis and lipid oxidation. Thus, a Ca-rich diet could cause opposite effects, decreasing lipid storage(Reference Zemel32, Reference Xiaoyu, Payal and Melissa33). Another hypothesis is that Ca has the ability to form insoluble complexes with lipids (soaps) in the intestine, increasing faecal excretion and decreasing their absorption, which reduces the available energy to the body, contributing to its anti-obesity effect(Reference Teegarden, White and Lyle34, Reference Zemel35). There is strong evidence that Ca has a specific action on appetite control in rats(Reference Tordoff and Rabusa36) and overweight women(Reference Major, Alarie and Doré37). Moreover, recently Gilbert et al. (Reference Gilbert, Joanisse and Chaput38) have shown that milk supplementation attenuates the known increased motivation to eat, which occurs during BW loss.
Thus, considering that dietary Ca therapy could have an anti-obesity action, in the present study, we evaluated the possible beneficial effects of Ca supplementation in preventing some endocrine–metabolic alterations, as higher adiposity, dyslipidaemia, peripheral insulin resistance and central leptin resistance, which have been previously found in the experimental model of obesity programmed by the interruption of lactation in the last 3 d(Reference Lima, de Moura and Passos21).
Materials and methods
The use of the animals according to our experimental design was approved by the Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro (CEA/017/2009), which based its analysis on the principles adapted and promulgated by Brazilian Law no. 11.794/2008. Wistar rats were kept in a room with controlled temperature (25 ± 1°C) and artificial light–dark cycles (lights on 07.00 hours, lights off 19.00 hours). Virgin female rats, 3 months old, were caged with male rats (3:1), and after mating, each female was placed in an individual cage with free access to food and water until delivery. We only used dams whose litter size was ten pups in order to avoid the influence of the litter size in the programming effect. At birth, to maximise the lactation performance(Reference Passos, Ramos and Moura39), the litters were adjusted to six male pups per dam.
Experimental model of programming by early weaning
After birth, twenty lactating rats were randomly divided into two groups:
(1) Early weaning (EW, n 10) – dams were lightly anaesthetised with thiopental (0·06 mg/ml per 100 g) and wrapped with a bandage to interrupt lactation in the last 3 d of lactation.
(2) Control (C, n 10) – dams whose pups had a standard lactation period, i.e. weaning at 21 d of lactation.
EW and C groups received food directly into the cage and pups had easy access to drinking-water. From postnatal day 21 until postnatal day 180, BW and food intake (g) of offspring were monitored every 4 d, and feed efficiency was calculated (BW gain/g food intake). We used two offspring from each dam, which were randomly chosen to be followed throughout the experiments, one of them to be treated with Ca.
Dietary calcium supplementation for 2 months
At 120 d, EW and C offspring were subdivided into four groups (n 10/group): (1) C, received standard rat chow; (2) control Ca (CCa), received standard chow supplemented with calcium carbonate; (3) EW, received standard rat chow; (4) early weaning Ca (EWCa), received standard rat chow supplemented with calcium carbonate.
Calcium carbonate was added in the standard chow. The Ca-enriched diet provided twice the amount of Ca (in the form of calcium carbonate) that is recommended for rodents, which is 5 g Ca/kg of chow(Reference Reeves40). This amount is based on the recommendation of supplementation for human subjects, where values of up to two times the recommended amount have no toxic effect. Ca was supplemented from postnatal day 120 until postnatal day 180, at which time all rats were killed by quick decapitation, with no prior anaesthesia since it affects hormone and lipid metabolism(Reference Chen, Dohi and Tan41). Blood, hypothalamus, liver, carcass and visceral fat mass (VFM) were excised and kept frozen ( − 80°C). Calcaemia and phosphataemia were measured using colourimetric BioSystems commercial kits (BioSystems, Barcelona, Spain). The metabolite 25-hydroxyvitamin D3 was measured using monoclonal antibody immunoassay (Elecsys and Cobas immunoassay analysers; Roche Diagnostics GmbH, Mannheim, Germany), with a range of detection from 4·0 to 100 ng/ml. This hormone is generally measured to determine the overall vitamin D status.
Body composition evaluation
After killing, VFM was quickly excised and weighed for evaluation of the central adiposity – mesenteric, epididymal and retroperitoneal(Reference Fagundes, Moura and Passos15), and data were expressed as g/100 g BW. Total fat was determined by carcass analysis(Reference Fagundes, Moura and Passos17). All rats were eviscerated; carcasses were weighed, autoclaved for 1 h and homogenised in distilled water (1:1). Homogenates were stored at 4°C for analysis. Homogenates (3 g) were used to determine the fat content gravimetrically. Samples were hydrolysed in a shaking water-bath at 70°C for 2 h with 30 % KOH and ethanol. Total fatty acids and non-esterified cholesterol were removed with three successive washings with petroleum diethyl ether. After drying overnight in vacuum, all tubes were weighed and data were expressed as g fat/100 g carcass. The estimate of the subcutaneous fat was calculated by subtracting the visceral fat from the total fat. Data were expressed as g of fat/100 g carcass.
Serum hormones measurement by RIA
Blood samples were centrifuged (1500 g/20 min per 4°C) to obtain serum, which was kept at − 20°C until assay. All determinations were performed in one assay and samples were analysed in duplicate. Leptin was measured with a specific RIA kit (Linco Research, St Charles, MO, USA) with a range of detection from 0·5 to 50 ng/ml; the intra-assay variation was 2·9 %. Insulin was determined using a RIA kit (ICN Pharmaceuticals, Inc., Orangeburg, NY, USA) with an assay sensitivity of 0·1 ng/ml; the intra-assay variation was 4·1 %. Adiponectin was measured with a specific RIA kit (Linco Research) with an assay sensitivity of 0·5 ng/ml; the intra-assay variation was 7·1 %. Free and total thyroid hormone levels were determined with a commercial RIA kit (ICN Pharmaceuticals, Inc.) with assay sensitivities of 0·45 ng/l (free thyroxine), 0·06 pg/ml (total triiodothyronine), 7·6 μg/l (total thyroxine), 0·06 pg/ml (free triiodothyronine). The intra-assay variations were 2·8 % (free thyroxine) and 3·6 % (total triiodothyronine), 3·8 % (total thyroxine) and 4 % (free triiodothyronine).
Glucose homeostasis evaluation
Fasting blood glucose was determined from the tail vein of fasting rats using a glucometer (ACCU-CHEK Advantage; Roche Diagnostics, Mannhein, Germany). Insulin sensitivity was assessed by
where HOMA-IR is homeostasis model assessment of insulin resistance.
As hypertrophic adipocytes release less adiponectin, both adiponectinaemia:total fat ratio and adiponectinaemia:VFM ratio were used to evaluate insulin resistance(Reference de Oliveira, Moura and Santos-Silva42).
Lipid profile determination
Serum total cholesterol, TAG and HDL were analysed using Biosystem commercial test kits. LDL-cholesterol and VLDL-cholesterol were obtained using Friedewald calculations:
(1) LDL-cholesterol (mg/l) = (total cholesterol − HDL-cholesterol − TAG)/5.
(2) VLDL-cholesterol (mg/l) = TAG/5.
Western blot analysis
To obtain cell extracts, the hypothalamus was homogenised in ice-cold lysis buffer (50 mm-HEPES, 1 mm-MgCl2, 10 mm-EDTA, Triton X-100 1 %, pH 6·4) containing the following protease inhibitors: 10 μg/μl aprotinin, 10 μg/μl leupeptin, 2 μg/μl pepstatin and 1 mm-phenylmethylsulfonyl fluoride (Sigma-Aldrich, St Louis, MO, USA). After centrifugation (7500 g for 5 min), homogenates were stored at − 20°C. OB-R (leptin receptor), JAK2, pJAK2, pSTAT3 and SOCS3 contents were analysed by Western blot using actin as an internal control.
Briefly, protein concentrations were determined by the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Samples (30 μg total protein) were separated by 10 % SDS-PAGE according to the molecular weight of each protein and transferred onto nitrocellulose membranes (Hybond ECL; Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK). Rainbow standard markers (Amersham Biosciences, Uppsala, Sweden) were run in parallel to estimate the molecular weights. Membranes were blocked with 5 % non-fat milk in Tween–Tris-buffered saline (20 mm-Tris–HCl, pH 7·5, 500 mm-NaCl, 0·1 % Tween-20) for 1 h. Specific primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) used were anti-OB-R, anti-JAK2, anti-pJAK2, anti-pSTAT3, anti-SOCS3 and anti-actin. Membranes were incubated with primary antibodies at a 1:500 dilution in Tween–Tris-buffered saline for 1 h, with the appropriate secondary antibody (1:10 000; peroxidase-conjugated IgG; Santa Cruz Biotechnology, Inc.) for 1 h and then with streptavidin (1:10 000; Zymed, San Francisco, CA, USA) for 1 h. Targeted proteins were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and then exposed to X-ray film for 10 s to 30 min. Images were scanned and bands were quantified by densitometry using Image J 1.34 s software (Wayne Rasband National Institute of Health, Bethesda, MA, USA).
Statistical analysis
Results are reported as means with their standard errors. GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analyses and graphics. Experimental data were analysed by two-way ANOVA and the Newman–Keuls multiple comparison tests. The significance level was set at P < 0·05.
Results
Before Ca treatment (at 4 months), C and EW offspring did not present any change in BW or food intake. After 2 months of dietary Ca treatment, calcaemia and phosphataemia were not significantly different among the groups (Fig. 1(a) and (b)). As expected, the 180-d-old EW offspring (EW non-supplemented with Ca) showed higher BW, hyperphagia and high feed efficiency (9, 28 and 45 %, respectively, P < 0·05; Fig. 1 (a–c)), higher visceral and total body fat content (77 and 55 %, respectively, P < 0·05; Figs. 2 and (b) and 3) and hyperleptinaemia (twofold increase, P < 0·05; Fig. 4(a)); however, there was no difference in subcutaneous fat (Fig. 2(c)). These changes were normalised by dietary Ca supplementation for 2 months. Protein levels of the leptin signalling pathway in the hypothalamus are shown in Fig. 4. Adult EW offspring showed lower hypothalamic JAK2 ( − 36 %, Fig. 4(b); P < 0·05), pSTAT3 ( − 34 %, Fig. 4(d); P < 0·05) and higher SOCS3 (twofold increase, Fig. 4(e); P < 0·05) expressions, and Ca supplementation normalised JAK2 and pSTAT3. Ca treatment in C offspring caused lower hypothalamic JAK2 content ( − 31 %, Fig. 4(b); P < 0·05). OB-R content was not significantly altered by programming or Ca supplementation (data not shown).

Fig. 1 (a) Body weight, (b) food intake and (c) feed efficiency at 120 d (first day of calcium (Ca) supplementation) and 180 d (kill) of control (C, ■) and early weaning (EW, ![]() ) offspring treated with dietary Ca supplementation for 2 months (CCa (□) and EWCa (
) offspring treated with dietary Ca supplementation for 2 months (CCa (□) and EWCa (![]() )). Values are means, with their standard errors represented by vertical bars, n 10. * Mean values were significantly different from those of C (P < 0·05). † Mean values were significantly different from those of EW (P < 0·05).
)). Values are means, with their standard errors represented by vertical bars, n 10. * Mean values were significantly different from those of C (P < 0·05). † Mean values were significantly different from those of EW (P < 0·05).

Fig. 2 (a) Visceral fat, (b) body total fat and (c) subcutaneous fat masses of adult control (C) and early weaning (EW) offspring treated with dietary calcium (Ca) supplementation for 2 months (CCa and EWCa). Values are means, with their standard errors represented by vertical bars, n 10. * Mean values were significantly different from those of C (P < 0·05). † Mean values were significantly different from those of EW (P < 0·05).

Fig. 3 (a) Serum calcium (Ca), (b) serum phosphate and (c) 25-hydroxyvitamin D3 of adult control (C), CCa, early weaning (EW) and EWCa rats. Values are means, with their standard errors represented by vertical bars, n 10. * Mean values were significantly different from those of C (P < 0·05). † Mean values were significantly different from those of EW (P < 0·05).

Fig. 4 (a) Serum leptin and the effect of dietary calcium (Ca) supplementation for 2 months on protein content of leptin signalling pathway – (b) Janus tyrosine kinase 2 (JAK2), (c) phosphorylated JAK2 (pJAK2), (d) phosphorylated signal transducer and activator of transcription 3 (pSTAT3), (e) suppressor of cytokine signalling 3 (SOCS3) – in the hypothalamus of adult control (C), CCa, early weaning (EW) and EWCa rats. Detections were performed by Western blotting and protein contents were quantified by scanning densitometry of the bands. Actin content was used as control loading. Values are means, with their standard errors represented by vertical bars, n 10. * Mean values were significantly different from those of C (P < 0·05). † Mean values were significantly different from those of EW (P < 0·05). ‡ Mean values were significantly different from those of CCa (P < 0·05).
With regard to glucose homeostasis, adult EW rats showed hyperglycaemia (16 %, P < 0·05; Fig. 5(a)), higher HOMA-IR (38 %, P < 0·05; Fig. 5(c)), lower adiponectin:VFM ratio and adiponectin:total fat ratio ( − 44 %, Fig. 5(d) and − 45 %, Fig. 5(e), respectively, P < 0·05). These parameters were normalised by dietary Ca supplementation. Serum insulin (Fig. 5(b)) and adiponectin (C, 7·07 (sem 0·63); CCa, 7·45 (sem 0·55); EW, 7·45 (sem 0·40); EWCa, 7·43 (sem 0·50)) levels were not changed with or without Ca therapy.

Fig. 5 (a) Glycaemia, (b) insulinaemia, (c) homeostasis model assessment of insulin resistance (HOMA-IR), (d) adiponectin:visceral fat mass (VFM) ratio and (e) adiponectin:total fat ratio of adult control (C) and early weaning (EW) offspring treated with dietary calcium (Ca) supplementation for 2 months (CCa and EWCa). Values are means, with their standard errors represented by vertical bars, n 10. * Mean values were significantly different from those of C (P < 0·05). † Mean value was significantly different from that of EW (P < 0·05).
With regard to lipid profile (Table 1), EW offspring presented hypertriacylglycerolaemia (64 %, P < 0·05). EWCa offspring showed higher serum HDL-cholesterol (11 %, P < 0·05) and normal serum TAG. Other lipoproteins or total cholesterol were not affected by programming or Ca treatment.
Table 1 Lipid profile of adult control (C) and early weaning (EW) offspring treated with dietary calcium (Ca) supplementation for 2 months (CCa and EWCa)
(Mean values with their standard errors, n 10)
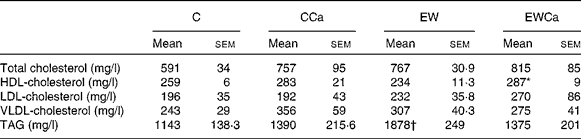
* Mean values were significantly different from those of EW.
† Mean values were significantly different from those of C (P < 0·05).
Serum-free or total thyroid hormones levels were not significantly different among the groups (Table 2).
Table 2 Serum thyroid hormones of adult control (C) and early weaning (EW) offspring treated with dietary calcium (Ca) supplementation for 2 months (CCa and EWCa)
(Mean values with their standard errors, n 10)
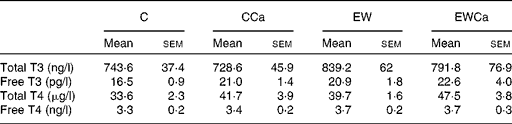
T3, triiodothyronine; T4, thyroxine.
Discussion
In humans, a significant decrease in abdominal obesity was shown with Ca supplementation or a diet rich in dairy products, and some studies have found that higher Ca intake is inversely associated with the prevalence of the metabolic syndrome(Reference Gonzalez, White and Kristal23, Reference Zemel35, Reference Major, Alarie and Doré37, Reference Chen, Dohi and Tan41, Reference Liu, Song and Ford43–Reference Potts46). More recently, our group evidenced in an experimental model of early weaning a programming effect for higher visceral, total body fat mass, insulin resistance, hypertriacylglycerolaemia and central leptin resistance in adulthood(Reference Lima, de Moura and Passos21). The present data showed that these disturbances were prevented by 60 d of dietary Ca supplementation when started in young adult rats (120 d old). These rats either presented higher feed efficiency that was normalised with a Ca-rich diet.
Because dairy products have other substances, such as Mg and leucine, which could affect the interpretation of the present findings, we chose calcium carbonate instead of dairy products. Ca treatment caused no significant increase in serum Ca and phosphate levels. The absence of substantial changes in calcaemia and phosphataemia after 60 d of treatment in CCa and EWCa offspring could be expected due to the very tight homeostatic mechanisms for the maintenance of serum Ca, which are mainly performed by parathyroid hormones and calcitriol(Reference Heaney47, Reference Christensen, Lorenzen and Svith48). Also, these results point to the fact that the Ca dose used in the present study was not toxic to the animals.
It is well known that leptin levels decrease during weight and fat losses(Reference Holm, Gamborg and Kaas-Ibsen49, Reference Desbriere, Vuaroqueaux and Achard50). In the present study, Ca supplementation was capable of decreasing total and VFM in EW offspring, which reflects in normoleptinaemia.
It is possible that calcitriol can play a role in energy metabolism by regulating the deposition and expansion of local fat in adipose tissue. The higher deposition of central fat in obesity may be due to the greater capacity for regeneration of glucocorticoids in the visceral fat depot(Reference Desbriere, Vuaroqueaux and Achard50). In the abdominal adipocyte, the availability of intracellular glucocorticoid is controlled by 11β-hydroxysteroid dehydrogenase-1 (11β-HSD-1) activity, which generates local active cortisol (or corticosterone, in rats) from cortisone. Obese individuals have higher mRNA of this enzyme in both subcutaneous and visceral fat tissues(Reference Holm, Gamborg and Kaas-Ibsen49). Calcitriol directly regulates the local 11β-HSD-1 expression and cortisol release, indicating a potential role of calcitriol in visceral adiposity(Reference Zemel51, Reference Monzillo, Hamdy and Horton52). In the present study, 25-hydroxyvitamin D3 had a huge increase in the EW group that is normalised by Ca supplementation. Thus, it is possible that Ca-rich diets inhibiting the calcitriol result in the inhibition of 11β-HSD-1 expression. Further studies are necessary to confirm this hypothesis.
In obesity, hyperleptinaemia does not produce the expected satiety or increase in energetic expenditure because of leptin resistance. This process is caused by the down-regulation of hypothalamic leptin receptors(Reference Jéquier53, Reference Martin, Perez and He54), by the reduced blood–brain barrier transport(Reference Martin, Perez and He54, Reference Burguera, Couce and Curran55) or by the impairment of the intracellular transduction pathway(Reference Jéquier53, Reference Banks56, Reference Bouret, Draper and Simerly57). As EW offspring are hyperphagic and present lower JAK2, lower pSTAT and higher SOCS3 expression, suggesting central leptin resistance(Reference Lima, de Moura and Passos21), we analysed the effect of Ca supplementation on the hypothalamic leptin pathway. As expected, the leptin receptor was not affected by programming and also Ca supplementation did not alter the OB-R content. Ca supplementation normalised JAK2 and pSTAT3 and increased pJAK2, suggesting the prevention of the central leptin resistance development, although the higher SOCS3 was not prevented by a Ca-rich diet. Thus, both lower body mass and fat corrected the leptinaemia and normalised the hypothalamic leptin signalling. Interestingly, CCa offspring showed lower hypothalamic JAK2 expression but no change in pJAK2. Wang et al. (Reference Wang, Wang and Yang58), in a whole-cell patch-clamp study of neuropeptide Y and proopiomelanocortin neurons, showed that leptin differently regulates the high voltage-activated Ca channels in neuropeptide Y and proopiomelanocortin neurons, decreasing in neuropeptide Y and increasing in proopiomelanocortin. It was also suggested that intracellular Ca could differently regulate leptin action in these two neuronal subpopulations. As in nutritional or hormonal imprinting, the development of these neuronal subpopulations can be affected(Reference Wang, Wang and Yang58), different responses for Ca and leptin interplay in the hypothalamic neurons can be obtained. The amount of our data may help to explain the inhibitory appetite effect previously reported by others(Reference Major, Alarie and Doré37). Hyperglycaemia, higher HOMA-IR, lower adiponectin:VFM and lower adiponectin:total fat ratio, despite normal insulin or adiponectin levels, confirm the insulin resistance previously observed in EW offspring(Reference Lima, de Moura and Passos21). Ca therapy was able to reverse this profile. The mechanism by which Ca improves insulin sensitivity is still elusive. Epidemiological studies showed a negative association between Ca intake and glycaemia, insulinaemia or insulin resistance(Reference Pittas, Harris and Stark29, Reference Villegas, Gao and Dai59). In rodent and human adipocytes, high concentrations of intracellular Ca reduce insulin-mediated glucose transport(Reference Villegas, Gao and Dai59–Reference Zemel61). Normal concentration of intracellular Ca is essential for insulin secretion by pancreatic β-cells as well as for insulin-mediated intracellular processes in tissues such as skeletal muscle and adipose tissue. Calcitriol can increase intracellular Ca on insulin target tissues, and the higher Ca intake can block the Ca influx by the inhibition of calcitriol. Thus, Ca intake results in higher insulin sensitivity, leading to a more efficient glucose uptake(Reference Tremblay and Gilbert62–Reference Reusch, Begum and Sussman65).
Hypertriacylglycerolaemia detected in EW offspring can suggest higher risk for atherogenesis development. Ca supplementation in EW offspring corrected serum TAG and increased HDL-cholesterol, shown to be an adjuvant factor for reducing the risk for CVD. Studies showed the relationship between Ca supplementation and lipid profile improvement, such as hypocholesterolaemia, hypotriacylglycerolaemia, higher HDL-cholesterol(Reference Major, Alarie and Dore66) and higher serum apo A-I, which is the main protein of HDL-cholesterol(Reference Groot, Grose and Dijkhuis-Stoffelsma67).
Although a hypometabolic status can be easily associated with a thyroid hypofunction, adult offspring programmed by early weaning did not have changes in serum thyroid hormone concentrations. Besides, dietary Ca supplementation did not change the thyroid function of these adult obese rats. Also, to our knowledge, there are no reports associating Ca supplementation with thyroid functional changes.
It is important to consider that early weaning can impair the hormonal regulation of Ca homeostasis, and this explains the different effects of Ca supplementation between the controls and EW-programmed groups.
In summary, our data reinforce the evidence that early weaning programmes for late development of the metabolic syndrome as well as for central leptin resistance. Maybe, both reduction in fat mass and normalisation of leptin resistance induced by 2 months of Ca-rich diet are mechanisms that lead to increased insulin and leptin action. The most remarkable finding of the present study is that dietary Ca supplementation plays a protective role in reducing the risk of some components of the metabolic syndrome. Thus, Ca supplementation seems to be a strategic approach (therapeutical/nutritional) to the treatment of endocrine–metabolic changes in obesity.
Acknowledgements
The present study was supported by the ‘National Council for Scientific and Technological Development’ (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq), the ‘Carlos Chagas Filho Research Foundation of the State of Rio de Janeiro’ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ), Coordination for the Enhancement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES). J. L. N., E. O. and N. S. L. were recipients of CAPES fellowships. All the authors are grateful to Monica Moura, Ulisses Risso Siqueira, Carlos Alberto Sampaio Guimarães and Marcos Borges for their technical assistance. E. d. O., P. C. L., E. G. d. M. designed the study and wrote the protocol and manuscript. J. L. N., N. d. S. L., J. G. F. and J. F. N. N. were responsible for the animal programming, biochemical and molecular procedures. All the authors contributed to and approved the final manuscript. The authors declare no conflict of interest.









