Bronchopulmonary dysplasia (BPD) is a chronic lung disease of the premature newborn. It is characterised by abnormal lung development provoked by factors such as mechanical ventilation, oxygen therapy or poor nutrition(Reference Thiess, Lauer and Woesler1,Reference Morag, Barkai and Wazana2) . Advances in the care of premature infants and the increased survival of the most immature ones have resulted in an increased incidence of BPD, which now affects about 50 % of those born with less than 30 weeks of gestational age, according to the Vermont Oxford Network(Reference Jensen, Edwards and Greenberg3,Reference Gianni, Roggero and Colnaghi4) . Various definitions for BPD have been proposed(Reference Jobe and Bancalari5,Reference Abman, Collaco and Shepherd6) . The most recent of these do not include the criteria that oxygen therapy be required at 28 d of life and consider only that of increased oxygen needs at 36 weeks postmenstrual age (PMA)(Reference Jensen, Dysart and Gantz7).
Nutrition is known to modulate lung development and maturation, and studies based on experimental models have shown that malnutrition heightens the alveolar damage caused by hyperoxia(Reference Mataloun, Leone and Mascaretti8). In this regard, early enteral nutrition, especially nutrition in the early neonatal period, may be decisive in conditioning neurological and pulmonary development(Reference Wemhoner, Ortner and Tschirch9,Reference Uberos, Jimenez-Montilla and Molina-Oya10) . Furthermore, although newborns with BPD receive less protein and energy in the first weeks of life(Reference Wemhoner, Ortner and Tschirch9,Reference Uberos, Jimenez-Montilla and Molina-Oya10) , nutritional interventions to increase their intake can improve short- and medium-term respiratory outcomes(Reference Natarajan, Johnson and Brozanski11).
The short-term benefits of high-energy nutritional intake for somatic growth and psychomotor development are reasonably well established(Reference Uberos, Jimenez-Montilla and Machado-Casas12); thus, a high level of protein intake with an appropriate balance of carbohydrates, proteins and lipids is known to reduce the risk of sequelae after preterm birth(Reference Brown, Embleton and Harding13,Reference Sjostrom, Ohlund and Ahlsson14) . There is evidence that suboptimal nutrition and growth, in neonates with BPD, can aggravate apnoea and desaturation in pulse oximetry compared with infants with equivalent enteral nutrition in the absence of BPD(Reference Wang, Luo and Hsieh15).
Insufficient nutritional intake in the first weeks of life can produce long-lasting damage to lung development, maturation and repair in extremely preterm infants, in whom alveolisation of the lung occurs mostly or entirely after birth(Reference Lin, Xiong and Lu16). The latter authors have also reported that a higher ratio of enteral volume received to total fluid intake in the second week of life is associated with a lower risk of BPD in extremely preterm infants. Breast milk is considered the best nutrition for all newborns during the first 6 months of life, including those who are very low birth weight (VLBW) and premature, who often require protein and energy supplements(Reference Agostoni, Buonocore and Carnielli17,Reference Embleton and van den Akker18) . Donor human milk is the preferred alternative for the nutrition of VLBW preterm infants when breast milk is unavailable or of insufficient volume(Reference Committee On, Section On and Committee On19). The composition of human milk is influenced by many parameters, which can be classified as maternal, neonatal and methodological(Reference Hsu, Chen and Lin20). In most neonatal units, donor milk is collected from mothers of full-term infants at a mature stage of lactation, when milk production is greater and protein content may be lower than in milk from mothers of preterm newborns(Reference Fomon, Ziegler and Vazquez21). This explains the need for protein and energy supplementation in such cases(Reference Arslanoglu, Boquien and King22).
BPD is the most common of the late complications related to extreme prematurity(Reference Laughon, Brian Smith and Bose23), and optimal nutritional support is fundamental to its prevention and treatment(Reference McEvoy, Jain and Schmidt24). Some authors have reported that exclusively formula-fed VLBW neonates are at increased risk of developing BPD, necrotising enterocolitis (NEC) or retinopathy of prematurity(Reference Spiegler, Preuss and Gebauer25).
The aim of our study is to analyse a cohort of VLBW newborns to determine whether breast milk nutrition during the first weeks of life is associated with the prevalence and severity of BPD.
Subjects and methods
This retrospective cohort study was conducted of neonates born at the San Cecilio Clinical Hospital (Granada, Spain) between January 2008 and December 2021. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the appropriate research ethics authorities (CEI Granada, 6hWMS694PFIRMALEIDs7SR/TmO/IwD, 28 April 2023). Written informed consent was obtained from the parents/guardians.
Inclusion and exclusion criteria
All newborns weighing < 1500 g admitted to the neonatal intensive care unit during the study period were included in our analysis. Each child was followed in pulmonology, neonatology and neurology consultations. Infants who died in the first 28 d of life and those transferred to other hospitals are excluded from the analysis, as in these cases it was not possible to establish the degree of BPD. Also excluded are newborns for whom data were not available on total nutritional intake during the first weeks of life. Figure 1 shows the flow diagram representing the process applied for patient recruitment and inclusion.
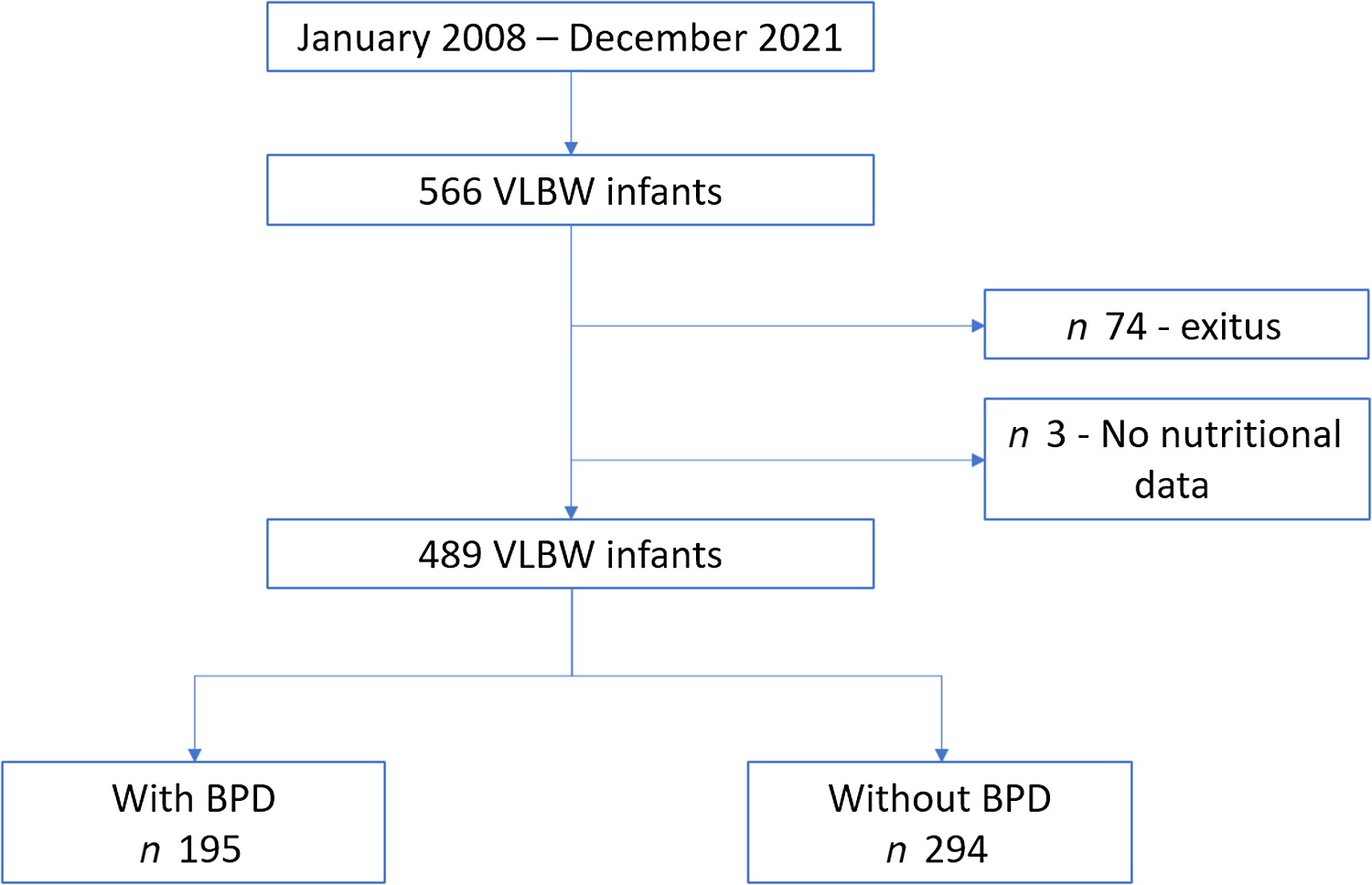
Fig. 1. Flow chart for the inclusion of patients in the study.
Anthropometry
Table 1 shows the maternal and anthropometric variables and z-scores obtained at birth and at week PMA. Fenton tables were used to calculate the z-scores(Reference Fenton and Sauve26). Newborn small for gestational age is defined as birth weight below the 10th percentile for gestational age(Reference Levine, Grunau and McAuliffe27).
Table 1. Maternal and infant characteristics: total study cohort, with BPD and BPD free
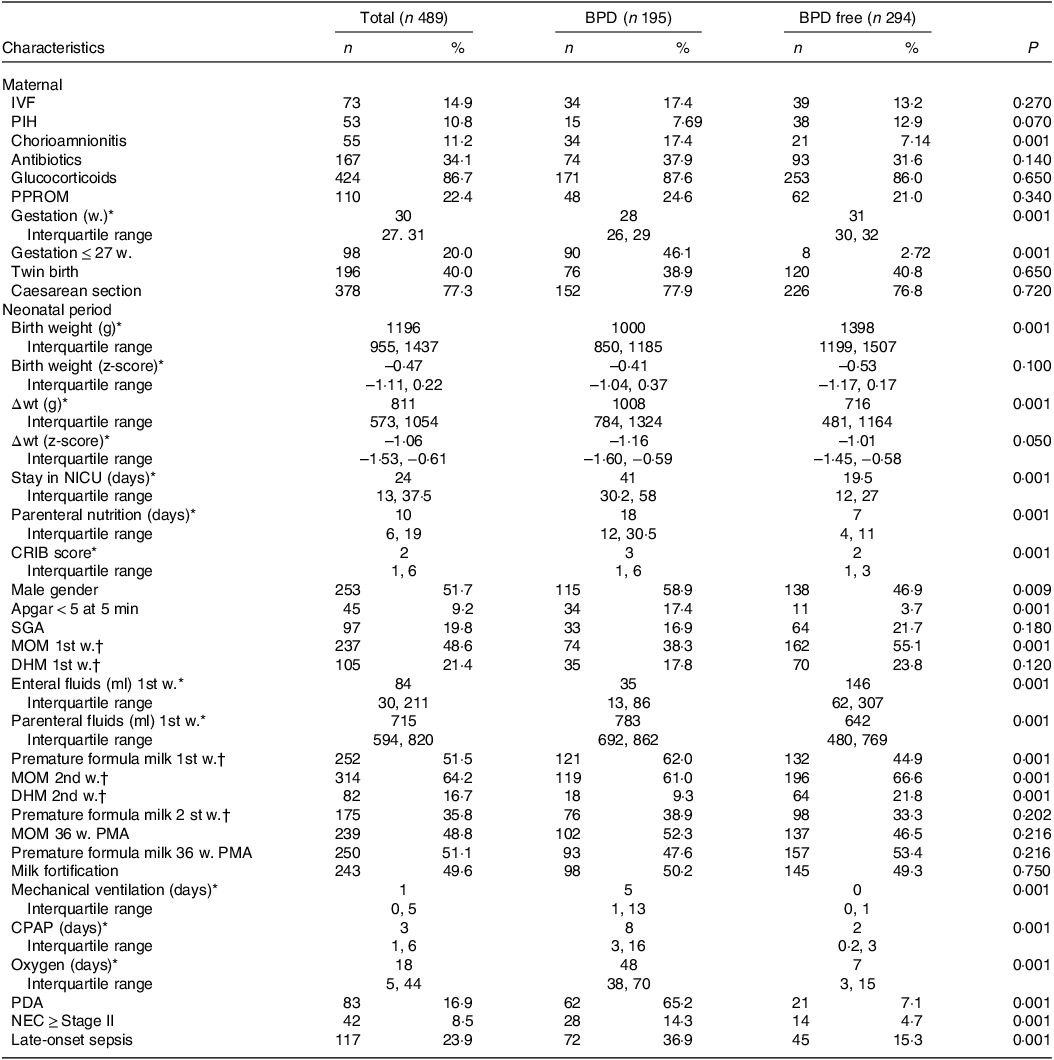
BPD, bronchopulmonary dysplasia; IVF, in vitro fertilisation; PIH, pregnancy-induced hypertension; PPROM, preterm pre-labour rupture of membranes; CRIB, clinical risk index for babies; SGA, small for gestational age; 1st w., first week; MOM, mother’s own milk; 1st w., second week; DHM, pasteurised donor human milk; PMA, postmenstrual age; CPAP, continuous positive airway pressure; PDA, patent ductus arteriosus; NEC, necrotising enterocolitis; Δwt, change in weight, from birth to 36 postmenstrual weeks.
* Interquartile range.
† More than 50 % of the weekly volume.
Nutritional management
In the neonatal unit, the nutritional strategy employed and the liquid intake supplied are in accordance with the unit’s standard protocol and with the recommendations of the Nutrition and Metabolism Group of the Spanish Neonatology Society(Reference Uberos, Narbona and Gormaz28).
For these infants, the normal procedure during the first days of life is to complement enteral nutrition with parenteral nutrition when complete enteral nutrition cannot be established. When enteral nutrition can be established, initial volume was 2 ml. According to digestive tolerance, progressively increasing the volume by 20–25 ml/kg/d.
In all newborns with less than 32 PMA, the recommended nutrition was mother’s own milk (MOM). Some preterm infants of mothers with little or no milk secretion were able to receive donor human milk (DHM) up to week 32 PMA, before continuing with premature formula milk. In these cases, when the mother did not authorise the use of DHM, nutrition with premature formula milk was indicated.
At our hospital, mother’s milk composition is determined according to the Standardised Reporting of Neonatal Nutrition and Growth checklist, and formula composition is assessed according to commercial notifications(Reference Cormack, Embleton and van Goudoever29). In all cases, the aim is that during the first week of life the minimum nutritional requirements to ensure growth should be met, according to standard recommendations(Reference Thureen30,Reference Thureen31) . When necessary, enteral nutrition is administered via a nasogastric or orogastric tube, but under the standard protocol, preference is given to feeding with fresh breastmilk. When this is not possible, frozen breastmilk (MOM) or (if authorised) DHM is supplied. Frozen MOM is warmed in a bain-marie and stirred before each feeding. DHM is pasteurised by the Holder method (62°C for 30 min and cooling); a microbiological control is then performed, after which the milk is labelled and stored for up to 3 months.
The fortification procedure is started at 1 week of chronological age and when enteral nutritional intake exceeds 80 ml/kg. Breastmilk is fortified using 4 g of PreNan Human Milk Fortifier (Nestlé, Spain) per 100 ml of breastmilk. This product contains 3·6 g/100 kcal of partially hydrolysed cow’s milk whey proteins(Reference Narbona, Uberos and Armada32).
Morbidity
In accordance with the thresholds proposed by NIHCD(Reference Jensen, Dysart and Gantz7), BPD is defined as a need for supplemental oxygen > 21 % or for positive airway pressure at week 36 PMA.
A diagnosis of clinical sepsis is made when an NOSEP-1 score > 8 is recorded. On this scale, the presence of CRP > 0·014 g/l is assigned five points; that of neutrophils > 50 %, three points; thrombocytopaenia < 150 × 109/l, five points and fever > 38·2°C, five points(Reference Mahieu, De Muynck and De Dooy33). The clinical risk index for babies (CRIB II score) for each newborn is obtained using the following variables: sex, gestational age (in weeks), birth weight (in grams) and excess base, producing a total CRIB II score ranging from 0 to 27(Reference Brito, Matsuo and Gonzalez34). Persistent ductus arteriosus is diagnosed by Doppler ultrasound and treated when clinical repercussions are observed or when the diameter is > 2 mm. For the diagnosis of NEC, patients are classified according to Bell’s criteria(Reference Caplan and Jilling35). Cases classified as spontaneous intestinal perforations are excluded from this diagnosis.
Statistical analysis
The descriptive data are summarised using medians (p50) and the interquartile range (p25–p75) for the continuous values and frequency distribution for the categorical ones. In addition, the quartiles for Δwt up to week 36 PMA were determined. Univariate comparisons of the continuous variables were performed using the Mann–Whitney test and by the chi-square test for the categorical ones. The risk assessment of BPD, retinopathy of prematurity, NEC, IVH and late sepsis was obtained first by means of univariate regression analysis and later by multivariate logistic regression analysis. Collinearity was assessed by the variance inflation factor > 5. An adjustment was made to account for the variables that did not present multicollinearity with the other predictor variables when variance inflation factor < 5 was obtained(Reference Backhaus, Erichson and Plinke36). All statistical analyses were performed using IBM SPSS 28.0 for Windows (IBM).
Results
The results align with the main objective of this study, which was to explore the relationship between breast milk feeding in the first 2 weeks of life and the appearance of BPD in VLBW newborns.
Fig. 1 shows the flow chart of the study population. During the study period (January 2008 to December 2021), 566 newborns weighing < 1500 g were attended in the neonatal intensive care unit at our hospital. Of these, seventy four died during the neonatal period and three were excluded from the analysis due to the non-availability of the nutritional data required.
The rate of BPD in the entire population was 39·8 %. A significant downward trend was observed during the study period ranging from 52·9 % in 2008 to 35·1 % in 2021 (Fig. 2). The rate of days of invasive mechanical ventilation per patient per year has decreased in our cohort from 11·1 d in 2008 to 1·3 d in 2021.
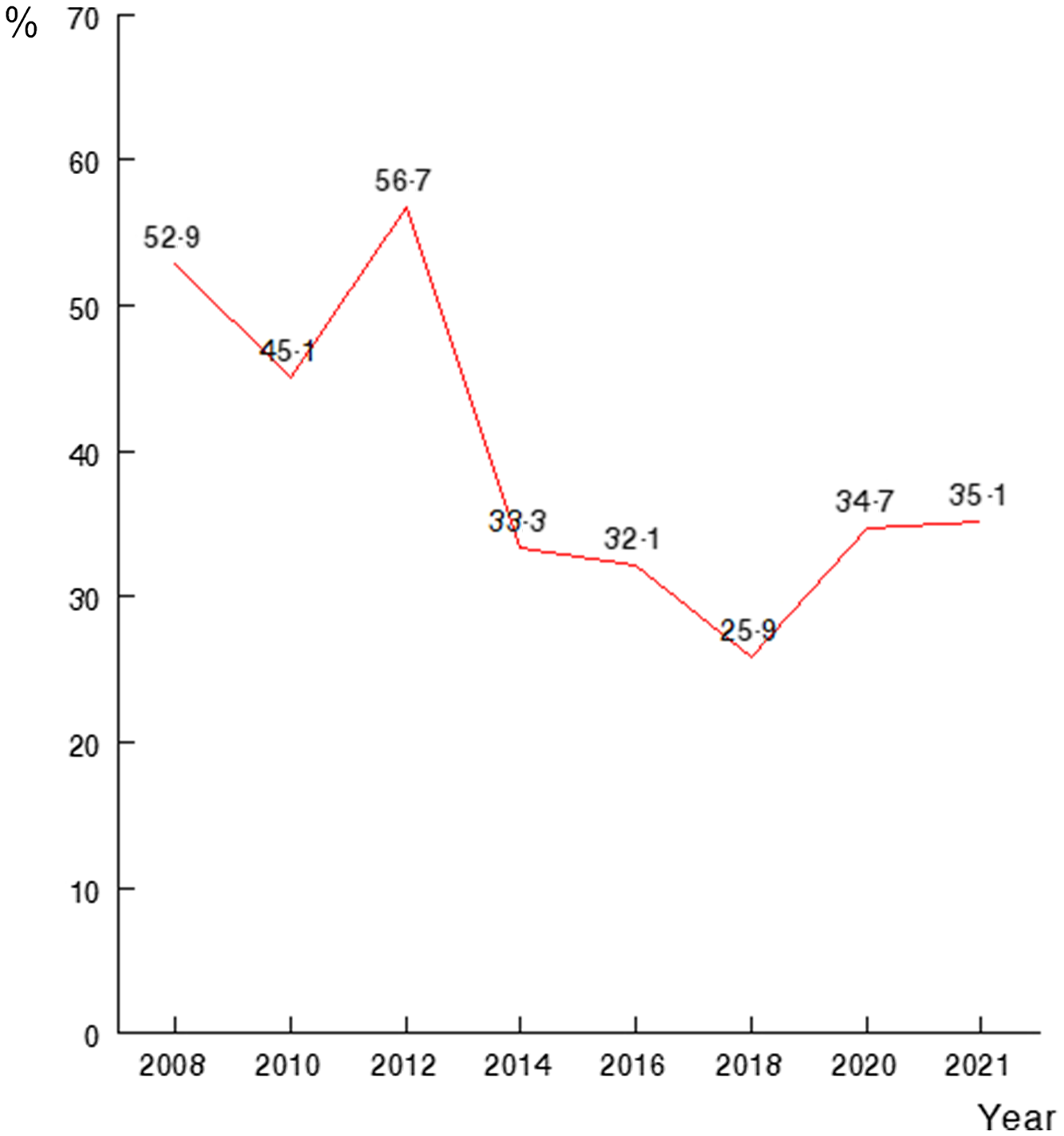
Fig. 2. Bronchopulmonary dysplasia rates (%) over the study period, P < 0·05. Historical analysis of our population. Occurrence from 2008 to 2021 of any degree bronchopulmonary dysplasia among VLBW infants. VLBW, very low birth weight.
Infants’ characteristics
Table 1 shows the characteristics of the total population and the differences between those with BPD (n: 195, 39·8 %) and those with no BPD (n: 294, 60·2 %). On average, the gestational age of neonates in our sample was 29·6 (± 2·3) weeks of gestation, and their birthweight was 1241 g (±317). In total, 48·3 % of them were girls. The rate of multiple births was 40·0 %.
The results of univariate analysis showed an inverse association of birth weight, gestational age and Apgar score with BPD. On the other hand, the bivariate analysis shows that the factors associated with BPD were history of chorioamnionitis, male gender, history of NEC ≥ stage 2, history of late sepsis, Persistent ductus arteriosus, oxygenotherapy and mechanical ventilation.
Enteral fluid intake in the first week of life was significantly lower in neonates with BPD, who received significantly greater volumes of parenteral nutrition (Table 1). 48·6 % of the newborns received MOM in the first week of life; in 21·4 % of the cases this nutrition was supplemented with. During the second week of life, 64·2 % of the newborns received MOM and 16·7 % of the cases this nutrition was supplemented with DHM. The remaining newborns received premature formula, 51·5 % during the first week of life and 35·8 % during the second week. Figure 3 shows the percentage of feeding with MOM in the 1st and 2nd week, which goes from 18 % and 35 % in 2008 to 73 % and 79 %, respectively, in 2021.
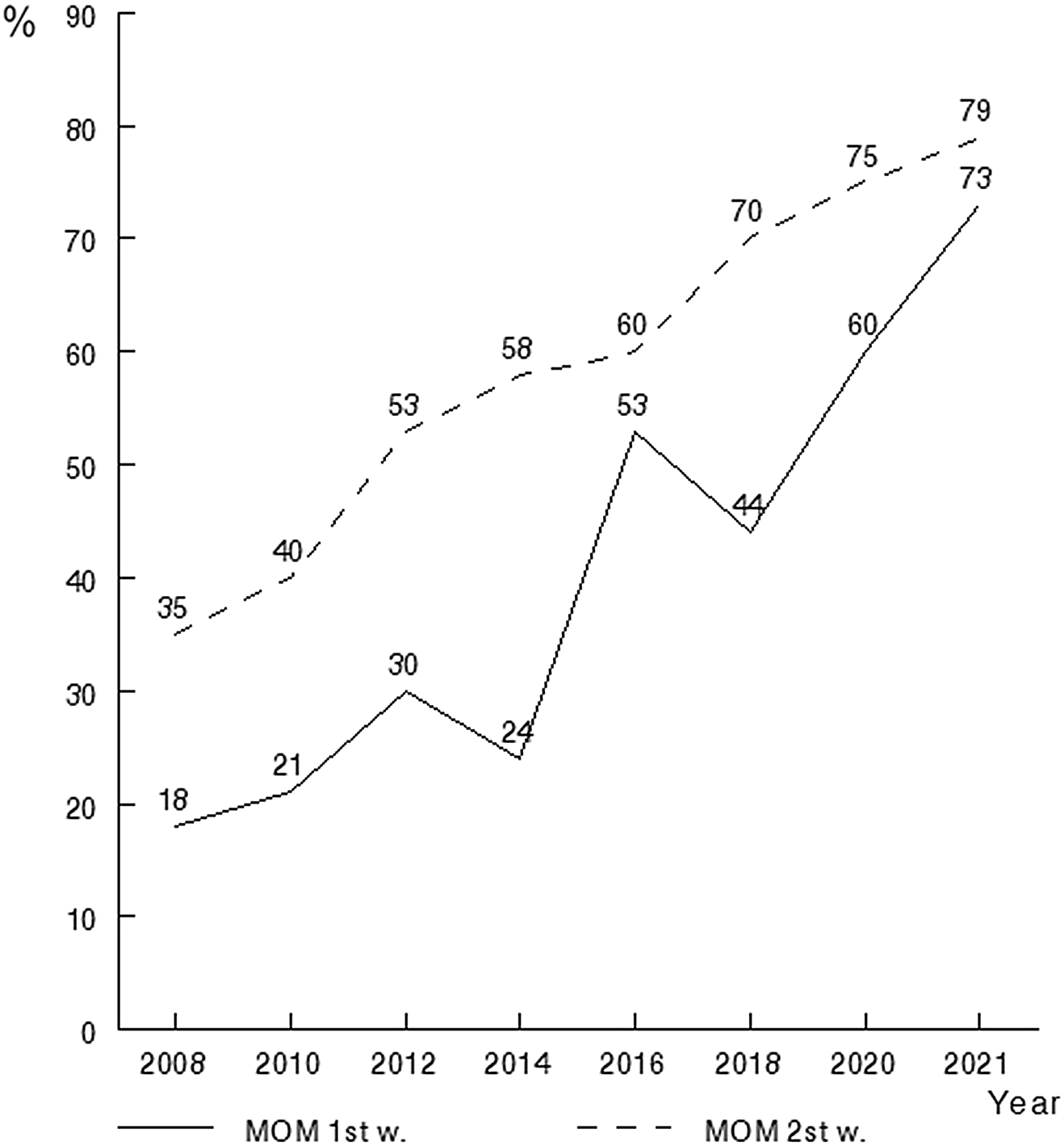
Fig. 3. Historical analysis of the prevalence of mother’s own milk feeding in the first and second week of life.
A statistically significant proportion of newborns who developed BPD had not received MOM nutrition in the first weeks of life (61·0 % v. 66·6 %) (Table 1). However, 49·6 % of all newborns received milk fortification (whether MOM or DHM), and among these there were no significant differences between those with or without BPD. The percentage of newborns who received DHM during the second week of life was also lower among those who developed BPD (9·3 % v. 21·8 %). At 36 weeks PMA, 46·5 % of infants without BPD were breastfed, compared to 52·3 % of infants with BPD, with no significant differences between groups (P = 0·21).
Table 2 shows the results of the multinomial logistic regression analysis for the different degrees of BPD and for the administration of MOM during the first and second postnatal weeks. The regression models were adjusted for chorioamnionitis and gestational age. The analysis shows that the administration of MOM in the first and second weeks of life is associated with lower odds of BPD and significantly so both for mild BPD (OR 0·16; 95 % CI 0·03, 0·71) and for severe BPD (OR 0·08; 95 % CI 0·009, 0·91).
Table 2. Relationship between feeding with mother’s own milk in the first two weeks of life and the risk of bronchopulmonary dysplasia
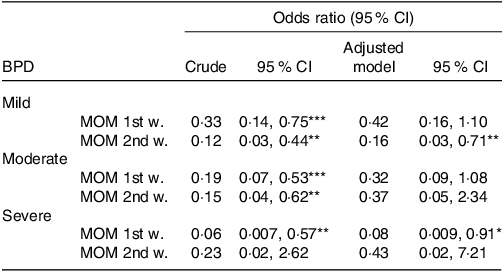
BPD, bronchopulmonary dysplasia; MOM, mother’s own milk; 1st w.: first week of life, 2nd w.: second week of life. Odds ratio (95 % CI) obtained by logistic regression analysis (crude and adjusted for gestational age and chorioamnionitis).
* P < 0·05.
** P < 0·01.
*** P < 0·001.
Table 3 shows the results of the multinomial logistic regression analysis for BPD grades, together with the Δwt z-score quartiles to week 36 PMA. Our results show less weight gain in VLBW and severe BPD infants, which appears to be a consequence of greater respiratory effort and greater energy expenditure. We observed a significant association between quartile 1 of the Δwt z score from birth to week 36 PMA and severe BPD (OR 17·6; 95 % CI 2·92, 105·5).
Table 3. Relationship between weight gain from birth to week 36 PMA and the risk of bronchopulmonary dysplasia
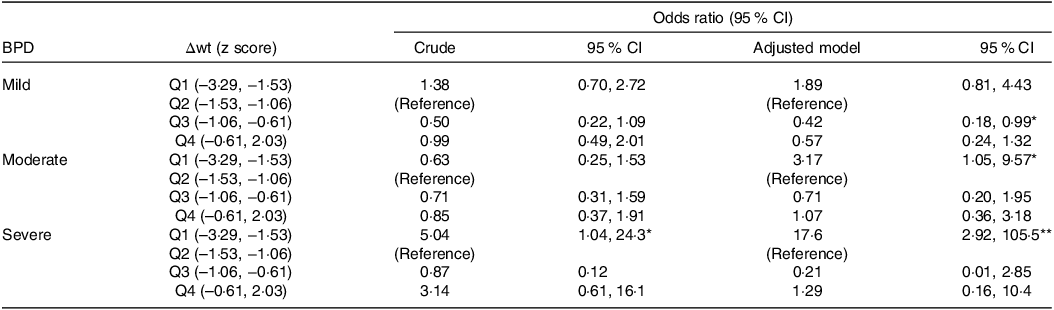
PMA, postmenstrual age; BPD, bronchopulmonary dysplasia; Q 1 to 4, quartiles 1 to 4 of weight gain (Δwt); odds ratio (95 % CI) obtained by logistic regression analysis (crude and adjusted for gestational age and chorioamnionitis).
* P < 0·05.
** P < 0·01.
Discussion
In this cohort study, we observed that preferential nutrition with MOM in the first and second week of life is associated with a lower probability of developing BPD, at the same time, we confirmed the observation reported by various authors(Reference Al-Jebawi, Agarwal and Groh Wargo37,Reference Malikiwi, Lee and Davies-Tuck38) that BPD is associated with greater energy expenditure and less weight gain.
Changing epidemiology of bronchopulmonary dysplasia over the study period
In the period studied, we have witnessed a decrease in the prevalence of BPD in our study cohort. Most studies of temporal trends in respiratory outcomes in VLBW preterm infants have reported similar results(Reference Avila-Alvarez, Zozaya and Pertega-Diaz39,Reference Smith, Zupancic and McCormick40) . Several reasons could explain this finding in our results and in the series consulted, first, the use of ventilatory modes with guaranteed volume, a greater use of non-invasive ventilation, the incorporation of probiotics as NEC prophylaxis and a higher percentage of feeding with MOM in our newborns.
Ongoing research into the pathogenesis of BPD examines the potential value of human milk in preventing and controlling this neonatal complication(Reference Villamor-Martinez, Pierro and Cavallaro41). Antioxidant activity may be the main mechanism by which breast milk decreases the risk of BPD(Reference Yang, Jiang and Deng42). Although BPD is multifactorial in origin, it has been suggested that the association between breast-feeding and BPD is related to the presence of antioxidant components such as the exosomes in breast milk, which exert a protective role on the type II alveolar epithelium(Reference Zhou, Liu and Xu43). From an epidemiological standpoint, the prevalence of late sepsis and NEC is reported to be lower in VLBW infants fed with breast milk(Reference Uberos, Campos-Martinez and Fernandez-Marin44,Reference Altobelli, Angeletti and Verrotti45) , due to the shorter period spent in the neonatal intensive care unit, with fewer days of mechanical ventilation and oxygen therapy(Reference Uberos, Aguilera-Rodriguez and Jerez-Calero46), and hence a lower risk of BPD, observations in line with our results. Moreover, the anti-inflammatory factors present in breast milk may not only reduce inflammation but also strengthen the immune response and thus decrease the prevalence of late-onset sepsis(Reference Cortez, Makker and Kraemer47).
Studies have shown that the early provision of energy and protein to VLBW infants is associated with a lower risk of BPD(Reference Uberos, Jimenez-Montilla and Molina-Oya10,Reference Lin, Xiong and Lu16) . In our cohort, the Δwt to week 36 PMA was significantly lower in the neonates with BPD, probably because this condition increases energy expenditure in breathing(Reference Biniwale and Ehrenkranz48). In this respect, Gianni et al.(Reference Gianni, Roggero and Colnaghi4) observed that enteral energy intake, the severity of BPD and the presence or otherwise of NEC are the main determinants of the speed of weight gain in the first weeks of life. In VLBW infants, breast-feeding and the use of probiotics are associated with a lower prevalence of NEC(Reference Uberos, Campos-Martinez and Fernandez-Marin44,Reference Lucas and Cole49,Reference Boundy, Anstey and Nelson50) . Achieving adequate weight gain to week 36 PMA is associated with favourable neurodevelopment(Reference Uberos, Jimenez-Montilla and Machado-Casas12), while BPD is known to have an unfavourable impact on cognitive outcomes in very preterm infants(Reference Schmidt, Roberts and Millar51).
Other authors(Reference Peng, Han and Li52) have associated breast-feeding in VLBW infants with a reduced prevalence of NEC, BPD and retinopathy of prematurity. Moreover, the randomised double-blind study conducted by Sun et al.(Reference Sun, Cao and Han53) concluded that VLBW infants fed with fresh breast milk present better outcomes in terms of reduced mortality, NEC ≥ stage 2, late-onset sepsis, retinopathy of prematurity and BPD, together with increased weight and height gain and head circumference, reduced time to full enteral feeds and fewer and less severe critical incident reports, including feeding errors.
Lower prevalence of bronchopulmonary dysplasia among premature newborns fed human milk
Our study shows that feeding with MOM in the first 2 weeks of life significantly reduces the risk of BPD, which is in line with the findings of Huang et al.(Reference Huang, Zheng and Zhao54) and Patel et al.(Reference Patel, Johnson and Robin55), who reported a 9·5 % reduction in the probability of BPD for each 10 % increase in the volume of breast milk consumed. Another meta-analysis and retrospective study also found that breast-feeding was associated with a lower risk of BPD(Reference Huang, Zhang and Tang56). A retrospective study of 1363 preterm infants found that those who received 50 ml/kg/d of breast milk during the first 4 weeks of life had lower odds of BPD. In contrast, the authors found no effect with volumes less than 50 ml/kg/d of breast milk on BPD(Reference Xu, Yu and Li57). In our study, enteral intake in the first week of life is significantly lower in newborns with BPD, where MOM intake was also significantly lower.
Currently, the standard handling procedure for fresh milk expressed by mothers of preterm infants is to freeze it, and sometimes to pasteurise it, depending on where it is expressed. However, many components of human milk change with freezing, pasteurisation and subsequent reheating. While carbohydrates remain relatively intact, bioactive proteins, lipids and energy content are all reduced by these milk processing actions(Reference Bertino, Coppa and Giuliani58). Furthermore, there is no immune or stem cell activity after pasteurisation, freezing or heating(Reference Ewaschuk, Unger and Harvey59).
Villamor-Martinez et al.(Reference Villamor-Martinez, Pierro and Cavallaro60) conducted a systematic review and meta-analysis of randomised clinical trials and observational studies, observing some disparity in the results presented. For example, observational studies show that supplementation of breast milk with donated milk decreases the risk of BPD, compared with supplementation with preterm formula; however, meta-analyses of randomised clinical trials do not reveal differences in this respect. Our results show a lower prevalence of newborns fed with MOM in the first and second week of life among the group of infants with BPD, who more frequently received preterm formula feeding.
Among other limitations affecting our study, the analysis of VLBW infants considered only one Level 3 neonatal intensive care unit, a narrowness of focus that may reduce the external validity of the findings obtained. In addition, the retrospective nature of this study, conducted over the course of 15 years, may have introduced temporal and data recording biases. As a second limitation, during the period evaluated in our study, changes have been introduced in medical practices related to BPD, mainly related to the use of non-invasive nasal positive pressure and high-frequency ventilation, which were rarely used before 2010.
As a final conclusion, the study results obtained support the hypothesis that breast-feeding during the first weeks of postnatal life is associated with a reduced prevalence of BPD, which is frequently associated with less weight gain as a result of greater respiratory effort with greater energy expenditure.
Acknowledgements
The authors thank the neonatologists and nursing staff involved for their invaluable collaboration.
No external funding was received for this study. The authors state that they have no financial relationships relevant to this article to disclose.
J. U. F. designed the analysis and interpretation of data and wrote the article and critically reviewed it for important intellectual content. They approve the version to be published and agree to be responsible for all aspects of the paper to ensure that any questions related to the accuracy or completeness of any part of the work are properly investigated and resolved. A. C-M., A. R-L., I. C-M. and E. F-M. made substantial contributions to the conception, design and writing of the article and critically reviewed it for important intellectual content. They approve the version to be published and agree to be responsible for all aspects of the work to ensure that any questions related to the accuracy or completeness of any part of the work are properly investigated and resolved.
The authors have no relevant conflicts of interest to declare.
The authors affirm that the work is original and is not being evaluated in any other journal.









