Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterised by severe impairment in social interaction, deficits in verbal and non-verbal communication and repetitive and restricted patterns of behaviour and interests.
The disorder’s genesis, which is likely heterogeneous(Reference Taylor, Rosenqvist and Larsson1–Reference Wozniak, Leezenbaum and Northrup5), is the result of a confluence of genetic and environmental factors that results in a certain phenotype(Reference Kinney, Barch and Chayka6,Reference Srancikova, Bacova and Bakos7) . In any case, it seems almost certain that changes in the neurotransmitters’ levels play an important role in the pathophysiology of ASD. Thus, alterations in the GABAergic, glutamatergic, serotonergic and dopaminergic systems have been related to ASD(Reference Fung and Hardan8–Reference Bengtsson, Nobin and Falck13).
There are still accounts of how dietary changes can affect autism behaviour or how it can get worse after ingesting various substances like sugar(Reference Kral, O’Malley and Johnson14). The best-known therapeutic intervention is the gluten/casein-free diet(Reference Akhter, Khan and Firdous15,Reference Quan, Xu and Cui16) . Moreover, treatments for autism include melatonin, carnitine, tetrahydrobiopterin, vitamin C, oxytocin, carnosine, multivitamin/mineral complex, PUFA, vitamin B6/Mg, elimination diets and chelation(Reference Rossignol17). The heterogeneity that all of the aforementioned treatments exhibit is a drawback. Several studies do not have a large number of individuals and, in others, their results are conflicting and lack statistical significance. Further studies exploring these treatments are needed. There are reports of successful outcomes for many of the dietary interventions that have been performed on children with autism, but none mentions a report of a complete cure(Reference Esposito, Mirizzi and Fadda18,Reference Evangeliou, Vlachonikolis and Mihailidou19) . Our intervention with branched-chain amino acid (BCAA) adds another weapon to the quiver of weapons that we have against autism.
The BCAA is a group of essential amino acids that take part both in the energy balance of a human being and in the homoeostasis of all the neurotransmitters mentioned above(Reference Harris, Joshi and Jeoung20,Reference Sperringer, Addington and Hutson21) .
At the same time, there have been scientific trials that refer to very low levels of BCAA in people with ASD, which triggered us to supply BCAA to ASD patients(Reference Novarino, El-Fishawy and Kayserili22,Reference Zheng, Wang and Li23) . The fact that BCAA have already been utilised in psychiatric models with a pathophysiology comparable to ASD, like mania(Reference Scarna, Gijsman and McTavish24), supported the initial hypothesis. On the other hand, sodium valproate, a drug that is used in experimental models to cause autistic behaviour, disturbs the balance between BCAA and aromatic amino acids (AAA) in animal models by reducing the BCAA levels and increasing AAA levels, while implicitly increasing the levels of serotonin, dopamine and epinephrine(Reference Bianchi, Marzocchi and Agostini25,Reference Maciejak, Szyndler and Kołosowska26) . Based on these observations, but also from our own experience in the use of BCAA that we gave as an adjunctive treatment to the ketogenic diet and where, in addition to their antiepileptic effect, we also observed an improvement in the children’s behavioural problem without seeing any side effects, we decided to distribute BCAA to children with autism(Reference Evangeliou, Spilioti and Doulioglou27). In cases where the classic ketogenic diet does not produce the desired effects, our clinic uses a combination of the ketogenic diet and BCAA. The improvement in children’s behaviour and academic achievement as observed by instructors caught our attention concerning this combo. The fact that these kids’ behaviour improved following BCAA administration should be stressed at this point. This observation was the main reason for administering BCAA to children with autism.
The purpose of our study was to add one more effective non-pharmacological alternative treatment to the already existing ones in the treatment of autism. In carrying out this study, we were encouraged by the fact that except for some inborn errors of metabolism, no important side effects were recorded while using this group of amino acids(Reference Schopler, Reichler and DeVellis28,Reference Russell, Daniel and Russell29) .
Experimental methods
Study design and participants
A total of fifty-five children with autistic behaviour (49 boys and 6 girls), between 6 and 18 years of age (median age 10·03 years), participated in a prospective study of the role of BCAA in autistic behaviour. The pool of our patients came from our hospital and from the child psychiatric clinics of two other hospitals during the period from May 2015 to May 2018.The study was conducted according to 1975 declaration of Helsinki and received prior approval from the ethics committee of our university (No. 4/4112010). Our initial goal was to do a double-blind study, but the majority of the children’s parents were opposed to it, and this is a limitation of the study.
All subjects were referred to our hospital’s psychologist prior to admission, for a general psychiatric evaluation according to the Childhood Autism Rating Scale (CARS) as adapted by Schopler et al. in 1980(Reference Evangeliou, Spilioti and Doulioglou27). According to this scale, scores between 30 and 36 indicate mild to moderate cases, whereas scores of 37 and over indicate severe cases (Table 1). The main reason we used the CARS scale is that it can easily be used and although many new methods of assessing autism have been proposed, CARS remains reliable. We started using the CARS scale several years before the start of this project and found it to be quite reliable in all respects. This is also in line with international literature, which has found that the CARS scale has excellent psychometric qualities. High levels of internal consistency, good inter-rater agreement and excellent test–retest reliability are all indicated by reliability statistics(Reference Russell, Daniel and Russell29,Reference Chu, Bian and Yan30) . In an attempt to strengthen the reliability of the study which has the disadvantage of not being double blind, the psychologist participating in this study evaluated and followed up on all of the enrolled autistic children, who were among others with autistic behaviour, without knowing which of the children were included in the study.
Table 1 Childhood Autism Rating Scale (CARS) (Schopler et al. 1980)

Legend to table: ranking of symptoms: 1, normal for age; 2, mild disorder; 3, moderate disorder; 4, serious disorder. For each of these fifteen items, a cumulative score is derived by summing 1–4 points for each item.
At the time of admission, other than the psychiatric evaluation, all children underwent a thorough clinical paediatric examination, somatometric data collection, hearing examination with audiogram and auditory evoked potentials, ophthalmologic examination with fundoscopy and optical acuity testing. A detailed laboratory investigation was then carried out, including the following: complete blood count, biochemical tests (electrolytes, blood glucose, transaminases, cholesterol, TAG and thyroid hormones), electrocardiogram and alert-phase electroencephalogram. Finally, more in-depth tests were conducted, including those to measure the amino acid carnitine, serum purine and pyrimidine, urine amino acids and urine organic acids, as well as brain MRI + MRS in some selected cases.
Inclusion–exclusion criteria
The children of this study had no change in their treatment (any kind of treatment pharmaceutical or non-pharmaceutical) at least 6 months prior to the initiation of the BCAA supplement and none had any changes in the CARS scale either. Furthermore, no other form of treatment was provided during the BCAA supplementation, as well as for the 3 months prior to and the 3 months following it.
Patients with chromosomal abnormalities and any other genetic disorders, patients with perinatal damage, patients with epileptic seizures and patients with maple syrup urine illness were all excluded from the study.
Branched-chain amino acid administration
Before the study started, three meetings were held among the participants to better coordinate and discuss the way of administration and dosage of BCAA. We used a carbohydrate-free BCAA-powdered mixture containing 45·5 g of leucine, 30 g of isoleucine and 24·5 g of valine. The daily dose was 0·4 g/kg of body weight given every morning mixed into the patient’s food or beverage to be consumed at each meal. This dosage was chosen based on an earlier study of our clinic which showed that this specific amount of BCAA had no side effects(Reference Maciejak, Szyndler and Kołosowska26). One drawback of the approach was that the amino acids were administered via the parents. However, as already mentioned above, prior to the study’s commencement, three meetings were arranged among the participants before to better coordinate and go over the administration and dosage of BCAA. In addition, in order to feel secure, the morning dose was given in the presence of the health care workers. Following the initiation of BCAA administration, children were submitted to a monthly psychological examination.
Assessment criterion: statistics
As an assessment criterion, we set an optimistic target of maintaining the administration of amino acids beyond 4 weeks and for a maximum supplementation time of 6 months. Statistical analysis was done using the Statistical Analysis Systems statistical software package, version 20 (SAS Institute). Results were regarded as significant when P < 0·05. The difference between the baseline and follow-up CARS was assessed by the t test for paired comparisons. In our study, we have to compare two variables for the same subject in terms of time and in this case the paired t test was used. The difference between the baseline and follow-up CARS was assessed for paired comparisons. In our study, we have to compare two variables for the same subject in terms of time and in this case the paired t test is useful.
Results
At the beginning of the study, the paediatric psychiatric evaluation according to the CARS scale yielded twenty-six patients (47·27 %) with scores of 30 and 36, which corresponds to mild to moderate autistic condition, whereas twenty-nine patients (52·73 %) had scores between 37 and 54, which corresponds to more severe cases. Thirty-two patients (58·18 %) received BCAA beyond 4 weeks, whereas the remaining twenty-three patients (41·82 %) did not. The justification for discontinuing the BCAA, for ten patients, was in disagreement over the testing, while for thirteen patients was the high BCAA cost.
The study’s ten participants stopped because they objected to the entire process, which they found painful (frequent psychological assessments and administration of the morning dose in the presence of the health therapist) while the study’s thirteen participants simply considered the treatment expensive in relation to what it offers.
For the six of thirty-two patients who discontinued BCAA due to lack of improvement, non-improvement was assessed not only by the psychologists on the CARS scales which did not change during the course, but also by the observations of the parents and health professionals who did not see any improvement.
Thus, patients receiving the BCAA were 58·18 %, significantly higher than the set target of 50 % (Z = 1·391; P < 0·001) (Fig. 1). Of these thirty-two patients, six (10·9 %) discontinued the application after 4–10 weeks, owing to a lack of improvement. The remaining twenty-six patients (47·27 %), who concluded the BCAA ingestion for more than 10 week, presented improvements in their social behaviour and interactions, speech, cooperation, stereotypy and, principally, in their hyperactivity, which contributed significantly to their improvement in learning. Specifically, of the twenty-six patients (47·27 %) who received the BCAA for the entire recommended duration, one patient (1 boy) (1·81 %) experienced the most significant improvement, with a reduction of more than twelve units on the CARS. This improvement was so significant that the child could attend a school for non-mentally handicapped children. Seven patients (12·72 %) (6 boys and 1 girl) experienced an average improvement (8–12 units on the CARS), and eighteen (32·72 %) (15 boys and 3 girls) displayed minor improvement (2–8 units on the CARS) (Table 2, Fig. 2).

Figure 1. Fifty-five children participated in the study. From the thirty-two children received BCAA beyond 4 weeks, twenty-six (47·27%) improved according to the Childhood Autism Rating Scale (CARS). BCAA, branched-chain amino acid.
Table 2 Patients with improvement after BCAA supplementation. Overall average improvement in terms of CARS based on 32 patients was 5·39 (standard error 0·6155)(t = 0·0003; df = 31)


Figure 2. Combined boxplot representing the scores (Y axis) before and after BCAA administration for those twenty-six children who had improvement. BCAA, branched-chain amino acid.
In the children who improved, the improvement became visible in a period of 5–15 d and reached the maximum in the period of 20–30 d. After this time, we saw no further improvement and this improvement remained to the end of treatment as well as 3 months after its discontinuation. Moreover, these children did not have any special characteristics compared with those who did not improve, and also during the study we did not see any remarkable metabolic change related to the clinical picture of the patients.
Discussion
Numerous times it has been noted that nutritional supplements and dietetic protocols may have an effect on the behaviour in some patients with autism(Reference Coppola, Wenner and Ilkayeva31–Reference Fujiwara, Morisaki and Honda34). BCAA supplementation in children with autistic behaviour constitutes an attempt to find an additional or alternative treatment for children who exhibit minimal to no improvement with conventional methods of treatment. The original thought was to conduct a double-blind trial; however, as children with autistic behaviour are part of a vulnerable social group, it was extremely challenging to conduct that kind of study since their parents were negative to that perspective. Our prior study, which showed improvement in children’s behaviour and academic performance as reported by teachers, provided us the rationale for administering BCAA to autistic children, and thus served as the motivation for us to carry out this study(Reference Evangeliou, Spilioti and Doulioglou27). In carrying out this study, we were encouraged by the fact that except for some inborn errors of metabolism, no important side effects were recorded with the use of this group of amino acids(Reference Gee and Deniel35,Reference Riazi, Rafii and Clarke36) . Eight patients in this study had great success with BCAA supplementation; one had a considerable improvement, seven had a moderate improvement and eighteen patients had a little improvement. This is significant because the patients in question had not improved for a while. Additionally, neither major nor minor negative effects were observed in our patients when taking BCAA. There is only one main contradiction for BCAA administration, specifically in people suffering from maple syrup urine disease and none of our patients was suffering from this disease.
The first question that arises is how the BCAA were able to benefit some of the patients and why not all of them. It was not the same for everyone, even for those who saw some improvement. The different degrees of improvement might be the result of changes at the level of neurotransmitters, considering that BCAA affect the neurotransmitters in a complex way(Reference Varesio, Grumi and Zanaboni37–Reference García-Espinosa, Wallin and Hutson39).
As already mentioned earlier, BCAA could affect serotonin homoeostasis. BCAA compete with AAA to transport across the blood–brain barrier(Reference Hawkins, O’Kane and Simpson40,Reference Kandasamy, Gyimesi and Kanai41) . The result of an increased level of BCAA in the blood is the decreased entrance of tryptophan, tyrosine and phenylalanine in the brain which leads to decreased levels of certain neurotransmitters produced by these AAA, serotonin, dopamine and epinephrine(Reference Dalangin, Kim and Campbell42,Reference Fernstrom43) . High levels of the above-mentioned neurotransmitters, especially serotonin, may play an important role in autism and other psychiatric conditions(Reference Bijl, Thys and Wittevrongel44–Reference Muller, Anacker and Veenstra-VanderWeele48).
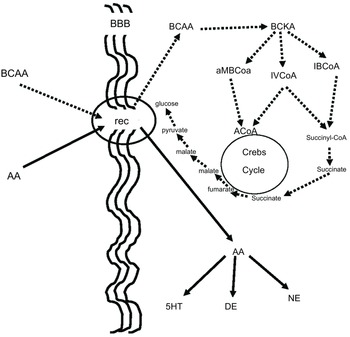
BCAA, in addition to their aforementioned effect on neurotransmitters, play an important role in many aspects of the body’s energy balance. They can be both ketotic and glucogenic. Thus, through valine and partially through isoleucine, BCAA produce succinate, which after several reactions ends up in glucose. This is believed to be a very important pathway because glucose is produced in the cell, bypassing the insulin system, which is energy-consuming, while it is already known that autism is connected to dysfunction in glucose energy production. On the other side, the BCAA, through leucine and partially through isoleucine, can be ketotic and the increase of ketone bodies may contribute to the beneficial effect of BCAA in children with autistic behaviour(Reference Evangeliou, Vlachonikolis and Mihailidou19,Reference Holeček49,Reference Fernstrom50) . Figure 3 illustrates our concept of how BCAA influence the metabolism of neurotransmitters, glucose and ketone bodies.
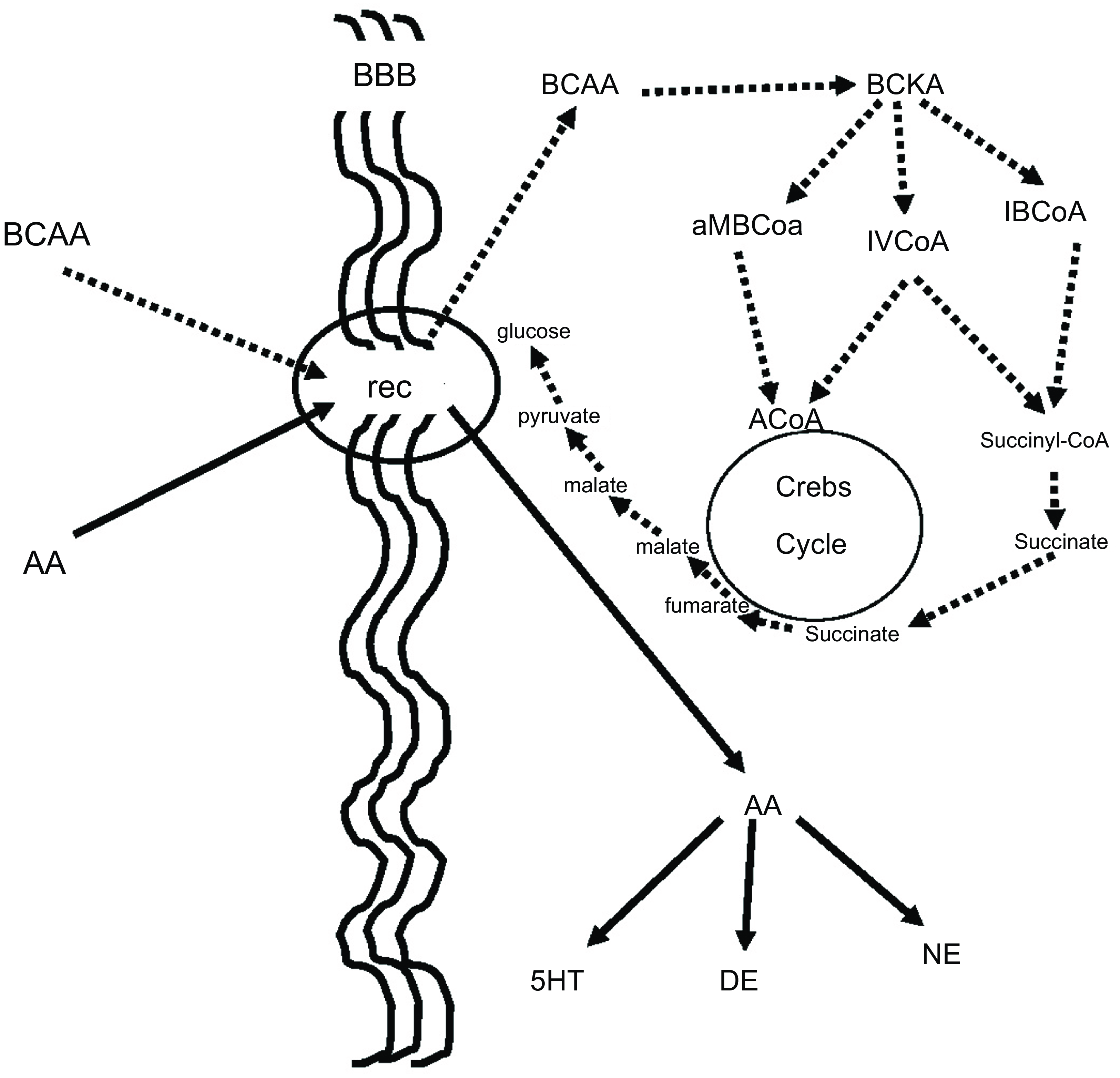
Figure 3. BCAA effect on serotonin and glucose metabolism. BCAA and AAA have common receptors in the BBB. It is to be expected that the increased flow of BCAA (dashed lines) will decrease the flow of AAA (solid lines), resulting in a decrease in serotonin, which is known to increase in some autistic patients. Furthermore, the increase in intracellular flow of BCAA results in increased succinate production, resulting in increased intracellular glucose production bypassing the energy-borne insulin system, which is also implicated in the pathogenesis of autism. BCAA, branched-chain amino acid; AAA, aromatic amino acid; BBB, blood brain barrier; Rec, common receptor for branched-chain and aromatic amino acids; BCKA, branched-chain ketoacids; aMBCoA, a-methylbutyryl-CoA; IVCoA, isovaleryl-CoA; IBCoA, isobutyryl-CoA; 5HT, serotonin; DE, dopamine; NE, norepinephrine.
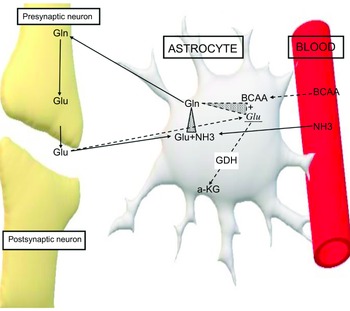
In addition, BCAA play an important role in the homoeostasis of glutamate. Glutamate is one of the most important stimulating neurotransmitters and under normal conditions it is metabolised in the glutamate-glutamine cycle. Increased levels of this neurotransmitter have been measured in patients with both epilepsy and autism. It is well known that high levels of glutamate play an important role in the pathogenesis of autism and epilepsy(Reference Cai, Ding and Zhang51–Reference Zheng, Zhu and Qu58). BCAA counteract the overflow of glutamate by either transmuting it into glutamine in astrocytes, playing the role of ammonia donors or by activating GDH, which transmutes glutamate into α KG(Reference Yudkoff59,Reference Zhou and Thompson60) (Fig. 4).

Figure 4. Effect of the BCAA on glutamate. Solid arrows. Glutamate metabolism under normal conditions in glutamine-glutamate cycle. Dashed arrows. Metabolism of excessive amount of glutamate in interfering with BCAA. Glu, glutamate; Glu1, excessive amount of glutamate; Gln, glutamine; NH3, ammonia; BCAA, branched-chain amino acids; GDH, glutamate dehydrogenase; a-KG, alpha-ketoglutarate.
Moreover, BCAA could facilitate GABAergic neurotransmission by decreasing the effects of glutamatergic neurotransmission(Reference Dufour, Nalecz and Nalecz61,Reference Dufour, Nalecz and Nalecz62) . GABAergic neurotransmission is a fundamental regulator of neuronal networks and dysfunction at this level may contribute to autism pathophysiology(Reference Cellot and Cherubini63–Reference Uzunova, Pallanti and Hollander65). It has been mentioned that BCAA play a complex role in several neurotransmitters that are involved in autism. The ideal scientific way to prove the action of BCAA in our group of patients would be to attempt lumbar puncture and measure the levels of neurotransmitters in CSF before and after the supply of BCAA, but this would neither be ethical nor easy. There was also a thought to measure neurotransmitters in the blood, but glutamate levels differ between blood and CSF(Reference Shulman, Grant and Seres66). Nevertheless, there is a correlation between serotonin levels in blood and CSF. This has been proved in experimental models by measuring lower serotonin levels after the supply of BCAA(Reference Berry, Novak and Withrow32,Reference Smriga, Kameishi and Torii67) . Technically speaking, this operation is challenging, and it is also too expensive for our budget(Reference Mumtaz, Narasimhachari and Friedel68). The optimal duration and dosage for BCAA supplementation are still questionable. The dose of 0·4 g was arbitrary, and the reason we chose this dose was from our experience of using BCAA as adjunctive treatment to the ketogenic diet in children with refractory epilepsy. Under this dose, we did not notice any side effects and at the same time we saw an improvement in the autistic symptoms that existed in some of these children(Reference Evangeliou, Spilioti and Doulioglou27).
Also, another point in our research that is worth commenting on is the fact that the majority of patients are male. We looked for a logical explanation for this. It is well known that autism is significantly more common in boys than in girls. This skewed sex ratio has been acknowledged since the first cases of autism were described in 1943(Reference Kanner69). The exact reasons for the ratio remain unclear. It might stem from biological differences between the sexes. A recent study done in our country regarding the prevalence of autism showed that for 2021 8588 males and 2195 females were diagnosed, so the proportion of diagnoses of ASD in relation to sex for the entire age group of 2–17 years was 78·82 % for boys and 21·18 % for girls(Reference Kouznetsov, Angelopoulos and Moulinos70). In our case, this relationship was a little higher and it is a matter that needs further investigation. It is possible that this is due in part to the fact that many of our patients came from isolated rural areas where certain socio-economic conditions still remain prevalent.
Also, in our study there is a significant difference between a 6-year-old and an 18-year-old. It is known that the younger the age, the greater the plasticity of the brain. From other studies of alternative therapies, we see that the earlier a treatment starts, the better the results, but this does not mean that older brains are not capable of adaptation. Based on these data, we would like to see which ages are the most favourable for starting the BCAA administration so that a more systematic targeted study can be done along the way. Looking at our data, we saw that the children who had the greatest improvement belonged to the youngest ages (Fig. 1, Table 2).
Remarkable is the fact that improvement after the BCAA administration was seen very quickly at most up to 3 weeks after the initiation of their administration. Positive effects persisted long after the BCAA treatment was over. This makes us ponder the ideal duration of a BCAA regimen. Perhaps a brief period of 1–2 months would be sufficient for them to contribute what they can without requiring further administration. This would significantly reduce the cost of treatment. This is a reasonable consideration that remains to be proven in a future study.
The limitations of our study are similar to those of the vast majority of studies on people with autism using alternative therapy. The majority of these studies lack a sizeable patient population or are open labelled, and their findings are controversial. What needs to be done is to take advantage of our promising results with a more systematic double blind study.
The high cost of BCAA, which was not covered by insurance, presented a challenge in the interpretation of our data. Because only high-income families were eligible to participate, this could have an adverse effect on the validity of our findings. However, this is a universal problem with all alternative therapies, affecting both wealthy and poor nations. All the participants in our study had received other alternative treatments significantly more expensive than the administration of BCAA, and before the treatment began, they had been informed about the cost of the treatment and the impossibility of administering it through the insurances both state-covered and private. The thirteen patients who left because of the cost did not belong to low-income families; they simply considered the treatment expensive in relation to what it offers.
Despite all these concerns arising from our study, we believe that it is necessary to broaden our treatment options for autistic behaviours and that BCAA supplementation may be promising. One factor supporting this opinion is the fact that although there are no studies which have applied the use of BCAA in autism, there has however been an increase in studies showing a disturbance in the BCAA metabolism in children with autism. A recent study demonstrated that the median serum levels of BCAA were lower in autistic children(Reference Meguid, Hashem and Ghanem71).This opens the door for discussion about the role of the BCAA in autism pathophysiology. Furthermore, there are no studies on the use of BCAA in children with autism, and these early encouraging results motivate us to further explore the role of BCAA in the treatment of these children.
Finally, the different degrees of improvement in the patients can be explained by the different metabolic biochemical profiles of each patient. A study of our clinic among 187 children supported this fact(Reference Spilioti, Evangeliou and Tramma72).We strongly believe that metabolic biochemical profiles should be examined in every child with autism. This will enable us to enhance already used treatments and create individualised approaches for every patient.
Acknowledgements
None.
The author(s) reported there is no funding associated with the work featured in article.
The authors declare that there are no conflicts of interest.