Osteoporosis and its associated fractures is a major public health problem worldwide. Hong Kong is no exception to this problem. The age-specific hip fracture rates among Hong Kong Chinese population have increased 2-fold in the past few decades(Reference Lau and Cooper1). The cost for the acute care of hip fracture totalled HK$150 million in 1995, and the cost is projected to increase to HK$960 million in 2010(Reference Lau and Woo2). It is therefore important to identify dietary and lifestyle factors that could increase or optimize bone stability and probably reduce the risk of developing osteoporosis later in life.
During adolescence, tremendous skeletal growth occurs, and approximately 90 % of adult bone mineral density (BMD) is acquired by the end of adolescence(Reference Bailey, Faulkner and McKay3). Genetic factors account for an estimated 60–80 % of the variability in adult BMD with diet, physical activity and hormonal status being important modifiable factors of bone accrual(Reference Bachrach4, Reference Abrams5). The role of Ca has long been recognized to be important for bone health(Reference Greer and Krebs6, Reference Prentice7). However, there is increasing evidence to highlight the role of overall diet instead of individual nutrient on bone metabolism and Ca balance(Reference Palacios8–Reference Massey10). The interplay among diet composition and its effect on net endogenous acid production (NEAP) and bone health has been reviewed(Reference Oh11–Reference Lemann, Bushinsky and Hamm13). It has been hypothesized that diets high in acid-forming components, including several amino acids in protein foods, P and Cl, and low in base-forming components, such as fruit and vegetables, K and Mg, could increase net acid and urinary Ca excretion, which may lead to lower BMD and higher fracture risk.
The diet of Hong Kong Chinese has undergone rapid changes in the past decades. Consumption of meat by Hong Kong population increases markedly whereas daily intake of fruit and vegetables is inadequate(14). Previous studies also revealed that dietary practices and lifestyle of the Hong Kong youth population are worse than are those of their adult counterparts(Reference Lee and Tsang15, Reference Adab and Macfarlane16). Since no studies have been done to explore the relationship between diet composition, NEAP and other determinants of bone health in adolescents of Chinese origin, the aim of this study was to investigate the association of dietary estimates of NEAP with bone mineral status in a group of Hong Kong Chinese adolescents.
Materials and methods
Subjects
The subjects were the 171 boys and 180 girls who participated in a longitudinal Hong Kong Adolescent Bone Health Cohort Study. The study was conducted by the Jockey Club Centre for Osteoporosis Care and Control, the Chinese University of Hong Kong. Boys aged 11–12 years and girls aged 10–11 years were recruited from nine primary schools in Hong Kong on a voluntary basis. Subjects suffering from any conditions known to influence bone or receiving prior therapy with a known effect on bone metabolism were excluded from the study. All subjects were measured at baseline and followed up annually. Results of this study were based on baseline data on anthropometry, dietary intake and bone mineral status collected between November 2003 and October 2004. Informed consent was obtained from both the subjects and their parents or guardians. The study was approved by the Ethics Committee of the Chinese University of Hong Kong.
Anthropometric measurements
Body weight was measured to the nearest 0·1 kg by the Physician Balance Bean Scale (Healthometer, IL, USA) with subjects wearing a light gown. Body height was measured to the nearest 0·1 cm by Holtain Harpenden Stadiometer (Holtain Ltd, Crosswell, UK) with subjects barefoot. Subject's sexual development was assessed by the researcher using the Tanner grading system. This included assessments of the pattern of development of pubic hair in all adolescents and of breast development in girls and penile and testicular size in boys(Reference Tanner, Forfar and Arnell17). If discrepancies existed among criteria, greater emphasis was placed on the degree of breast development in girls and on testicular and penile size in boys, for determination of Tanner stage.
Dual-energy X-ray absorptiometry of total hip, lumbar (L1–L4) spine and whole body
Dual-energy X-ray absorptiometry (DXA) was performed by using a Hologic QDR-4500 W densitometer (Hologic, Waltham, Mass., USA) to measure bone area (BA), bone mineral content (BMC), and BMD of total hip, lumbar (L1–L4) spine and whole body. The machine was calibrated daily in accordance with the manufacturer's instructions. Repositioning the subjects, reproducibility of the DXA scan results was within 1·5 % for all measured sites. In the present study, bone mineral apparent density (BMAD) rather than BMD was presented to reflect more accurate changes in volumetric bone density during growth. Total hip BMAD was calculated as BMC/A 2, spine BMAD was calculated as BMC/A 3/2 and whole body BMAD was estimated as BMC/(A 2/h), where A is the projected bone area and h is height(Reference Katzman, Bachrach, Carter and Marcus18, Reference Carter, Bouxsein and Marcus19).
Nutrient analysis
Dietary intake was assessed by a modified version of the FFQ based on data obtained in the Hong Kong Adult Dietary Survey in 1995(Reference Leung, Ho, Woo, Lam and Janus20). The FFQ had been validated with the basal metabolic rate calculation and the 24 h Na/creatinine and K/creatinine analysis(Reference Woo, Leung, Ho, Lam and Janus21). Subjects were asked about their usual frequency and consumption in the past 12 months from the food list. Standard portion size was listed and a food photo album was provided to assist assessment. Daily nutrient intake was calculated by the Food Processor Nutrition analysis and Fitness software version 7.9 (Esna Research, Salem, USA), with the addition of composition of some local foods based on a food composition table from China(22). Intakes of Na were calculated from food, drinks and the amount of salt used in cooking or at table. To assess the validity of the reported energy intakes, the intake divided by basal metabolic rate was calculated(Reference Goldberg, Black, Jebb, Cole, Murgatroyd, Coward and Prentice23). A ratio of less than 1·35 was used to indicate potential under-reporters of energy intake.
Dietary estimates of net endogenous acid production
Several algorithms have been developed to estimate NEAP from diet(Reference Frassetto, Lanham-New, Macdonald, Remer, Sebastian, Tucker and Tylavsky24). Frassetto et al. calculated the estimated NEAP from the diet's protein:K ratio(Reference Frassetto, Todd, Morris and Sebastian25) whereas Remer et al. estimated NEAP from average intestinal absorption rates of ingested protein and other minerals as well as an anthropometry-based estimate for organic acid excretion(Reference Remer, Dimitriou and Manz26). Each has its rationale and limitations(Reference Frassetto, Morris and Sebastian27).
In this study, Frassetto's method was adopted to allow for the particular dietary focus of the present study. The method calculates the diet's protein:K ratio expressed as g/mEq and exclusively estimates the diet-dependent net acid load to the metabolic system. The rationale and algorithm of this method have been described previously and the estimated NEAP could account for about 70 % of the variation in renal net acid excretion(Reference Frassetto, Todd, Morris and Sebastian25).
Statistical analysis
Data analysis was performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). Data are presented as means and standard deviations, unless indicated otherwise. Normality of the data was checked by histograms. Log transformation was applied for intakes of vitamins C, D and K for analysis. The independent t test and non-parametric Mann–Whitney U test were used to compare characteristics between boys and girls. Analysis of covariance (ANCOVA) was used to compare the nutrient intakes between boys and girls after adjustment for daily energy intake.
Multiple regression analysis was conducted to examine the contribution of estimated NEAP in explaining the variance of BMC and BMAD in each measured site. Either BMC or BMAD was the dependent variable. For the model with BMC, the following predictor variables were entered into a multiple linear regression analysis: BA, weight, height, pubertal stage (stage I as reference category), dietary protein, Ca and K intakes and estimated NEAP. The same set of predictor variables was entered for the regression model of BMAD, except for BA. Protein, Ca and K intakes and estimated NEAP were adjusted for dietary energy intake by the residual method(Reference Willett, Howe and Kushi28). Statistical tests were considered significant if P < 0·05 (two-sided).
Results
Subject characteristics
The anthropometric and bone characteristics of the study population are presented in Table 1. These characteristics differed significantly between boys and girls, with boys being older and having higher weight, height and BMI than girls. Boys had significantly higher hip and whole body BMC but lower spine and whole body BMAD than girls.
Table 1 Anthropometric and bone characteristics of the study population
(Mean values and standard deviations for 171 boys and 180 girls)
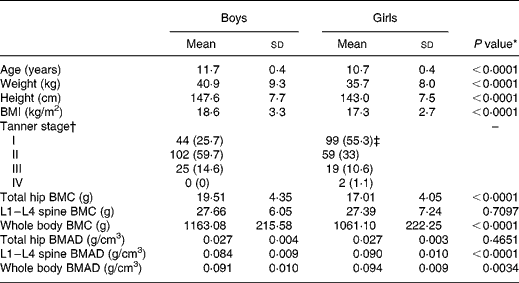
BMC, bone mineral content; BMAD, bone mineral apparent density; L1–L4, lumbar regions.
* Gender difference by independent t test.
† n (%).
‡ One girl refused assessment of pubertal development.
Daily nutrient intakes and estimated net endogenous acid production
Table 2 shows the daily nutrient intakes and the estimated NEAP of the study population. There was no significant difference in the energy-adjusted intakes of most nutrients and estimated NEAP between boys and girls, except for intakes of vitamins C and D. In all, 13·5 % of the boys (n 23) and 8·3 % of girls (n 15) were found to be potential under-reporters, but no significant sex difference was observed. Subjects with higher BMI were more likely to be under-reporters (details not shown).
Table 2 Estimated net endogenous acid production (NEAP) and daily nutrient intakes for 171 boys and 180 girls*
(Mean values and standard deviations; medians and interquartile ranges (IQR))

* For details of procedures see Materials and methods.
† Two samples t test.
‡ Analysis of covariance (ANCOVA) adjusted for energy intake.
§ The intake of these nutrients was not normally distributed. Vitamins C, D, K are log transformed for comparison in ANCOVA.
∥ Mann–Whitney U test.
Factors predicting bone mineral content or bone mineral apparent density at different sites
No significant association was observed between BMC and energy-adjusted NEAP or other nutrients (Table 3). BA was significantly and positively associated with BMC at all sites in both boys and girls. A significant positive association was found between weight and BMC in hip and spine in both sexes. Height showed negative correlation with BMC at all sites, but significant difference was only observed in hip for boys and in whole body for girls. Pubertal stage was significantly and positively associated with BMC in different sites in both sexes. The model accounted for 80–90 % of the variability in BMC of different measured sites in boys and 85–94 % in girls. No significant association was observed between BMAD and energy-adjusted NEAP or other nutrients (Table 4). Weight and height contributed most of the variability in BMAD at different sites. The models explained about 17–54 % of the variability in BMD at different sites in boys, and 23–62 % in girls.
Table 3 Regression models of energy adjusted estimated net endogenous acid production (NEAP) and nutrients in predicting bone mineral content (BMC) at hip, lumbar region L1–L4 spine and whole body for boys (n 171) and girls (n 179)*

β, Parameter estimate; r 2, partial R 2.
* For details of procedures see Materials and methods.
Table 4 Regression models of energy adjusted estimated net endogenous acid production (NEAP) and nutrients in predicting bone mineral apparent density (BMAD) at hip, lumbar region L1–L4 spine and whole body for boys (n 171) and girls (n 179)*

β, Parameter estimate; r 2, partial R 2.
* For details of procedures see Materials and methods.
Discussion
The results of this study suggested that anthropometric characteristics and pubertal stage were the more influential factors than dietary NEAP and dietary intake of protein, Ca and K in determining bone mineral status of Hong Kong Chinese adolescents.
Anthropometric measurement and pubertal stage were the main predictors for BMC and BMAD in the study population. The results were supported by previous studies(Reference Cheng, Maffulli, Leung, Lee, Lau and Chan29, Reference Carter, Whiting, Drinkwater, Zello, Faulkner and Bailey30). Cheng et al.(Reference Cheng, Maffulli, Leung, Lee, Lau and Chan29) examined the effects of puberty, physical activity, physical fitness and Ca intake on bone mineral acquisition in 179 healthy Hong Kong Chinese adolescents aged 12–16 years. Pubertal stage overrode all external factors in determining bone mass accretion in their studied subjects. Carter and colleagues(Reference Carter, Whiting, Drinkwater, Zello, Faulkner and Bailey30) investigated the effect of Ca intake on BMC in 227 children aged 8–17 years in Saskatoon, and found that over 90 % of the variability in total body and spine BMC were accounted for by BA, weight and height. The results illustrated the importance of adjusting for size-related variables in multivariate analyses of BMC before examining the effect of dietary intake as proposed by Prentice et al. (Reference Prentice, Parsons and Cole31). Prentice and colleagues suggested that the relationship between BMC and BA at various sites may change when adjustments are made to account for differences in body size between individuals over and above differences in bone size.Where weight and height were not allowed for, BA considered as part of bone stability provided information about the overall size of the individual and the size of the bone being scanned.
The present findings failed to show an association between bone variables and dietary Ca intake. The results were in line with those reported by Cheng et al. (Reference Cheng, Maffulli, Leung, Lee, Lau and Chan29) and Cvijetic et al. (Reference Cvijetić, Colić Barić, Bolanča, Jureša and Dekanić Ožegović32), in which no significant effect of Ca intake on bone mass accretion or bone stiffness was found. The effect of increasing Ca and dairy product intake for promoting child and adolescent bone mineralization, especially for non-white populations however remains controversial(Reference Lanou, Berkow and Barnard33). Instead, there is increasing evidence to show that bone development is influenced by the overall diet and not just by a single nutrient. Nutrients may interact with each other and with other genetic and environmental factors, and these complex interactions are likely the main reason for inconsistent results in studies of the role of single nutrients in bone health. Therefore, quantifying the acid–base contents of diets generally consumed by populations is useful for identifying the diets' effects on bone health(Reference Remer34). As NEAP is difficult to measure directly, we applied the simple algorithm by Frassetto et al. (Reference Frassetto, Todd, Morris and Sebastian25) to estimate the NEAP from the diet's protein:K ratio.
Our data do not support the growing interest in the importance of acid–base homeostasis to skeletal integrity. No significant association was observed between estimated NEAP and BMC or BMAD in our subjects. The result was in line with that of the study by Ginty et al. in which no evidence for a negative association between bone mineral status and indirect estimates of renal net acid excretion was found in 212 adolescent boys and girls(Reference Ginty, Prynne, Muniz-Terrera, Mishra, Prentice and O'Connell35). However, previous studies examining dietary acidity and bone variables in children(Reference Alexy, Remer, Manz, Neu and Schoenau9) and women(Reference New, MacDonald, Campbell, Martin, Garton, Robins and Reid36, Reference McDonald, New, Fraser, Campbell and Reid37)showed different results. Alexy et al. (Reference Alexy, Remer, Manz, Neu and Schoenau9) showed that children with higher dietary acid load had significantly less cortical area and BMC. However, the influence of dietary acid load on the growing bone was not very strong and could only be unmasked after adjustment for the stronger protein-anabolic impact on bone(Reference Alexy, Remer, Manz, Neu and Schoenau9). In women, it was shown that diets with a lower protein content but higher K content (i.e. lower acidity or higher alkalinity) were associated with greater bone mass and a tendency to less bone resportion(Reference New, MacDonald, Campbell, Martin, Garton, Robins and Reid36, Reference McDonald, New, Fraser, Campbell and Reid37). Higher K content could simply be a marker of higher fruit and vegetable intake. The beneficial role of fruit and vegetable intake on bone might be related to the alkalinizing effect(Reference MacGartland, Robson, Murray, Cran, Savage, Watkins, Rooney and Boreham38), or factors secondary to the fruit and vegetable intake, such as a healthier lifestyle(Reference New39), the presence of vitamins C and K(Reference Palacios8, Reference New, Robins, Campbell, Martin, Garton, Bolton-Smith, Grubb, Lee and Reid40) and other plant-based compounds(Reference Muhlbauer, Lozano and Reinli41, Reference Arjmandi and Smith42). Therefore, it was proposed that the balance between the amount of protein in the diet and the dietary acid load in part determines whether the diet as a whole has a net anabolic or catabolic effect on bone(Reference Sebastian43). High protein intake by Hong Kong children and low intake of fruits and vegetables have long been documented(14, Reference Leung, Chan, Lui, Lee and Davies44). The mean protein intake of the sample studied was also higher than the Chinese dietary reference intake(Reference Chan, Woo, Chan, Cheung and Lo45). Considering the tracking effect of diet from childhood to adulthood, the effect of high protein intake and low fruit and vegetable intake on the long-term bone health of Hong Kong Chinese adolescents warrants attention.
We failed to show any association between bone variables and estimated NEAP or nutrient intakes in our adolescent subjects. Issues on the methodological weaknesses should be considered in interpreting the results. The DXA method used for the measurements may not have been accurate enough to specifically identify the association as it provides only two-dimensional projection that is affected by the subject's size(Reference Fewtrell46, Reference Wren, Liu, Pitukcheewanont and Gilsanz47). Its analyses in the growing years require adjustment for bone size, which itself is also a parameter of bone stability. Thus, architectural bone structures responsible for bone strength cannot be determined specifically with this kind of analysis. We tried to minimize the problem of size effects by using BMAD(Reference Wren, Liu, Pitukcheewanont and Gilsanz47); the results however remained the same. Second, the use of FFQ in children requires attention. Correlation between FFQ and other diet-assessment methods (e.g. food weighed dietary record) is small-to-modest(Reference Rockett and Colditz48). Poor agreement has been observed in estimating protein intake in children and adolescents by FFQ as compared with food weighed dietary record, and FFQ is primarily used for ranking individual diet, not for quantifying individual intake(Reference Lietz, Barton, Longbottom and Anderson49, Reference Bertoli, Petroni, Pagliato, Mora, Weber, Chiumello and Testolin50). We tried to minimize the reporting errors by using the set of photographs and by adding enough items for better discrimination of individual consumption. However, careful interpretation of results is needed. Although the subjects were not recruited on a random basis, they were recruited in nine primary schools of various school types and different geographic locations in Hong Kong. Their anthropometric and dietary data were also comparable to those reported previously for Hong Kong adolescents(51, Reference Sung, Yu, Choi, McManus, Li, Xu, Chan, Lo, Chan and Fok52). Therefore, we speculated that the present findings could reflect the current dietary practice of adolescents of similar age in Hong Kong.
In conclusion, the findings of this study suggest that anthropometric characteristics and pubertal stage are the more influential factors than dietary NEAP in determining bone mineral status of Hong Kong Chinese adolescents. However, issues on the methodological weaknesses regarding the use of DXA and FFQ in the present study require attention.
Acknowledgements
This study was supported by the Jockey Club Centre for Osteoporosis Care and Control and the Centre for Nutritional Studies, the Chinese University of Hong Kong. The authors thanked Dr Edith Lau for setting up the Hong Kong Adolescent Bone Health Cohort Study, and Ms Winny Lau and Ms Liz Li for supervision and assistance in the administration of the FFQ and setting up the nutrient analysis database.
There is no conflict of interest.






