Plasma leptin, mainly produced by adipose tissue, is involved in the regulation of body energy homeostasis by its effects on appetite, body composition, energy expenditure and nutrient partitioning (Kershaw & Flier, Reference Kershaw and Flier2004). Circulating leptin concentration is strongly related to body lipid content in man and rodents (Barsh & Schwartz, Reference Barsh and Schwartz2002), as in ruminants (Chilliard et al. Reference Chilliard, Delavaud and Bonnet2005). This relationship exists over a long-term regulation process, and seems to be also modulated in the short term by feeding level, whatever the body fat content. In the ewe, leptinaemia was indeed decreased after medium-term underfeeding in parallel with changes in body fatness, and was increased at shorter term (several days) by re-feeding (Blache et al. Reference Blache, Tellam, Chagas, Blackberry, Vercoe and Martin2000; Delavaud et al. Reference Delavaud, Bocquier, Chilliard, Keisler, Gertler and Kann2000). At the same level of adiposity, leptinaemia was increased in adult sheep fed ad libitum when compared with animals fed to maintain a constant body composition (Archer et al. Reference Archer, Rhind, Findlay, Kyle, Thomas, Marie and Adam2002) and a 48 h fasting period was shown to abruptly decrease leptinaemia in initially fat or lean adult sheep (Daniel et al. Reference Daniel, Whitlock, Baker, Steele, Morrison, Keisler and Sartin2002). Therefore, the feeding level can be considered to be positively related to leptinaemia at either short (daily timescale) or medium term (weekly timescale).
Elsewhere, it was shown that in lean hypoleptinaemic ewes a 32 h fasting period failed to decrease plasma leptin (Henry et al. Reference Henry, Goding, Tilbrook, Dunshea, Blache and Clarke2004), and that 7 d re-feeding on a diet supplying about double the maintenance requirement failed to increase it (Zhang et al. Reference Zhang, Blache, Blackberry and Martin2005). So it seems that the plasma leptin response to rapid changes in feeding level depended on body condition and/or other metabolic factor(s). In the ewe, since contemporary circulating leptin constitutes a feedback signal able to modulate hypothalamic gene expression involved in appetite regulation (Archer et al. Reference Archer, Rhind, Findlay, Kyle, Thomas, Marie and Adam2002), it is of great interest to understand its regulation, particularly during animal adaptation to periods of fluctuation in food availability. Therefore, we hypothesised that the plasma leptin response to short-term changes in energy intake level could be dependent on regulations involving medium-term (feeding level) and/or long-term (body fatness) nutritional history. In order to test this, we studied the short-term impacts of meal distribution and intake, of feeding level, and of re-feeding a high-energy diet in fat and lean adult sheep that had been previously well fed or underfed for several weeks and for which the body lipid content was measured. In addition, and because of the existence of contradictory results in the literature (Blache et al. Reference Blache, Tellam, Chagas, Blackberry, Vercoe and Martin2000; Marie et al. Reference Marie, Findlay, Thomas and Adam2001; Daniel et al. Reference Daniel, Whitlock, Baker, Steele, Morrison, Keisler and Sartin2002), plasma leptin individual episodic profiles were examined according to initial body fatness and feeding level.
Methods
Animals and diets
Experiment 1
Twenty ovariectomised dry adult INRA 401 ewes (5·1 (sd 0·9) years, 57·2 (sd 1·5) kg, body condition score (BCS) 2·8 (sd 0·4)) were randomly allocated to four equivalent groups matched for body weight (BW) and adiposity, and placed in individual pens under natural photoperiod conditions (from November to May). During the pre-experimental period (28 d), all ewes were fed first growth dactyl cocksfoot (Dactylis glomerata) hay (16·5 (sd 1·1) g DM/kg BW per d) providing 100 % estimated maintenance energy requirements (MER; INRA, Reference Jarrige1989). Then, during the first part of the experimental period (166 d), half of the ewes were maintained on the pre-experimental diet (well-fed group, W; n 10), and the others were fed the same hay, but with quantities restricted to provide 40 % estimated MER (underfed group, U; n 10). All diets were supplemented with a mineral and vitamin premix (20 g/d). At the end of this period, half of the well-fed and underfed ewes were killed by decapitation for brain tissue preparation and subsequent neuropeptide Y-containing neuron analysis by immunohistochemistry (for results, see Chaillou et al. Reference Chaillou, Baumont, Chilliard and Tillet2002a), and those remaining were re-fed (W/R (n 5) and U/R (n 5), for previously well-fed and underfed ewes, respectively) ad libitum on a diet composed of second growth dactyl cocksfoot (D. glomerata) hay, wheat and soyabean meal (76:22:2, by weight) containing 928, 138, 249, 548 and 301 g/kg DM as organic matter, crude protein, crude fibre, neutral-detergent fibre and acid-detergent fibre, respectively. This diet was formulated to provide at least 150 % estimated MER for 3 d (days 167 to 169). During the whole experimental period, ewes had free access to fresh water and diets were given once per d in the morning. BW and BCS of the ewes were registered every week during the first 166 d, and then on days 168 and 170. Body lipid content was estimated by the 2H-labelled water-dilution technique (Bocquier et al. Reference Bocquier, Guillouet, Barillet and Chilliard1999) at day 162. Briefly, 2H-labelled water (Euriso-top; CEA, St Aubin, France) was flushed at 9 h through a catheter into the jugular vein at 0·5 g/kg BW, ewes were weighed and four blood samples were obtained by venepuncture at 5, 7, 29 and 31 h after infusion, i.e. before recovery of basal conditions within 2 d. Ewes were fed normally during all that period. Blood water was then extracted by freeze-drying, and 2H-labelled water concentration was determined by IR spectrophotometry at 4 μm (MIRAN-1 FF; Foxboro Co., East Bridgewater, MA, USA). The 2H-labelled water space (kg), corresponding to the amount of 2H-labelled water injected divided by the concentration of 2H-labelled water at zero infusion time (obtained by extrapolation of the regression between log 2H-labelled water concentration and sampling time), was combined with the BW at infusion time (BWi) in order to determine body lipid content (body lipids (kg) = 0·863 BWi − 0·865 2H-labelled water space − 9·1; R 2 0·92, ± 1·40 kg). BCS was assessed by palpation of the lumbar region by trained scorers, and subjective estimates were reported on a 0 to 5 scale (Bocquier et al. Reference Bocquier, Guillouet, Barillet and Chilliard1999).
Experiment 2
Twenty-four dry adult Lacaune ewes were selected 4 months before the start of the experiment according to BW and BCS. Four groups of six ewes having the same initial BW (63·4 (sd 3·6) kg) and BCS (3·04 (sd 0·34)) were conducted as follows to obtain two levels of body condition (fat or lean): twelve ewes grazed natural pasture given in large amounts (fat objective), and the twelve remaining ewes were kept in a barn and received low-quality hay and straw (lean objective). According to INRA (Reference Jarrige1989) in relation to observed BW and BCS changes, the % MER covered by these diets can be estimated to be 151 % for fattened ewes and 57 % for restricted ewes. At the start of the experiment, fat v. lean groups of ewes differed both for BW (70·5 (sd 3·91) v. 57·6 (sd 3·59) kg, respectively) and for BCS (3·38 (sd 0·20) v. 2·23 (sd 0·31, respectively). Thereafter, ewes were placed in individual pens under natural photoperiod conditions (from September to December). In order to avoid any putative remnant effect of previous feeding management on subsequent nutritional experimentation, all ewes were well fed during 15 d with the same diet, i.e. 18·7 (sd 1·1) g DM/kg BW per d of a diet comprising 50 % hay (first cycle summer natural meadow grass hay) and 50 % straw, and providing 115 % of the estimated MER (INRA, Reference Jarrige1989). During the following 94 d, six lean (WL; BCS 2·1 (sd 0·3)) and six fat (WF; BCS 3·3 (sd 0·2)) ewes were maintained on that pre-experimental diet, whereas the other six lean (UL; BCS 2·3 (sd 0·3)) and six fat (UF; BCS 3·5 (sd 0·2)) ewes were underfed on the same diet given in lower amounts (9·2 (sd 0·4) g DM/kg BW per d comprising 50 % hay and 50 % straw), providing 51 % estimated MER. All diets were supplemented with a mineral and vitamin premix (30 g/d). At the end of this period, all ewes were re-fed a diet composed of hay, barley grain, rapeseed meal, fish meal and palm oil Ca soaps (52·5:20·0:16·3:3·6:7·6, by weight, respectively) containing 913, 167, 198, 446 and 245 g/kg DM in organic matter, crude protein, crude fibre, neutral-detergent fibre and acid-detergent fibre, respectively. These re-feeding diets, that were totally consumed, were distributed in order to provide 80 % estimated MER on the first day (day 95), 157 % estimated MER on the second day (day 96), and then 235 % estimated MER during 9 d (days 97 to 105). The groups re-fed (/R) ewes were then coded as follows: WL/R, WF/R, UL/R and UF/R. During all the experimental periods, ewes had free access to fresh water and diets were given daily at 10.00 hours. BW and BCS were checked weekly. Body lipid content was also estimated by the 2H-labelled water dilution technique (Bocquier et al. Reference Bocquier, Guillouet, Barillet and Chilliard1999) at day 91.
The overall design of both experiments is given in Fig. 1, where levels of feeding, initial body fatness and experimental period durations are summarised.

Fig. 1 Summary of the experimental designs of experiments 1 and 2. MER, estimated maintenance energy requirements, on the basis of 0·193 MJ net energy/kg body weight0·75 (INRA, Reference Jarrige1989) for dry adult ewes; W, well-fed ewes; U, underfed ewes; W/R, previously well-fed ewes that are re-fed; U/R, previously underfed ewes that are re-fed; F, fat; L, lean; WF, well-fed fat ewes; WL, well-fed lean ewes; UF, underfed fat ewes; UL, underfed lean ewes; WF/R, previously well-fed fat ewes that are re-fed; WL/R, previously well-fed lean ewes that are re-fed; UF/R, previously underfed fat ewes that are re-fed; UL/R, previously underfed lean ewes that are re-fed.
Adjustments of feed intake
The feed intake of ewes was individually adjusted to their BW, in both experiments. Offered feeds and refusals were registered daily and individually, thus allowing an individual calculation of energy intake. Energy requirements and balances were calculated on the basis of 0·193 MJ net energy/kg BW0·75 (INRA, Reference Jarrige1989). During the underfeeding period for experiments 1 and 2, the feeding level of underfed ewes was re-adjusted weekly to measured BW, if BW varied by over 5 %. All experimental procedures were conducted according to the French recommendations for the use of experimental animals including animal welfare and appropriate conditions (Guidelines 18 April 1988), under the guidance of the Animal Care and Use Committee of INRA.
Blood sample collection
In order to study the effects of underfeeding and re-feeding on leptin variation, jugular blood samples were collected before the morning feed into 10 ml tubes containing EDTA (K3) (21 mg/tube, Terumo®; Terumo Europe, Haasrode, Belgium). After centrifugation (3000 g; 20 min; +4°C), plasma was collected and stored at − 20°C before leptin and metabolite assays. Insulin was only determined in plasma samples of experiment 1. For experiment 1, the samples were collected on days − 3, 14, 98, 162, 167 and 170 for all ewes, and also on days 168 and 169 for W/R ewes. On day 162, blood was collected just before 2H-labelled water flushing. For experiment 2, the samples were collected on days 94 (end of underfeeding period, 3 d after 2H-labelled water flushing), 99 and 105 (during the re-feeding period).
In order to study leptin pulse parameters and the 24 h leptin profile, a unilateral catheter was implanted in a jugular vein of the twenty-four ewes in experiment 2 the evening before serial sampling. Blood samples were collected into 10 ml tubes containing heparin (100 IU/tube; heparin Choay®; Sanofi-Synthelabo, Paris, France), every 15 min from 1 h before and for 5 h after food was given on day 88 (towards the end of the underfeeding period) and, in the six WF ewes only, every 2 h from day 87 at 07.30 hours to day 88 at 07.30 hours. As heparin could artificially increase absolute plasma leptin concentrations (Ma et al. Reference Ma, Gingerich, Santiago, Klein, Smith and Landt1996), the plasma leptin concentrations measured in blood samples collected on EDTA were compared with concentrations obtained from heparin samples from the same ewes. This showed that these two determinations were linearly related: heparin leptin = 1·80 × EDTA leptin − 0·68 (R 2 0·79; n 24). Because heparin systematically increased leptin values, the results of this sub-part of experiment 2 cannot be directly compared with the other results for experiment 2 and experiment 1 (obtained on EDTA and presented in Tables 1 and 2 and Figs. 2 and 3). Therefore the use of heparin as anticoagulant has been specified in the corresponding Results sections. For all blood samples, and as previously described, plasma was collected and stored at − 20°C pending plasma assays.
Table 1 Plasma leptin, body weight (BW), body condition score, body lipid content and plasma non-esterified fatty acid concentration in ewes of experiments 1 and 2 at the end of underfeeding period (Mean values with their standard errors)

W, well-fed ewes; U, underfed ewes; WF, well-fed fat ewes; UF, underfed fat ewes; WL, well-fed lean ewes; UL, underfed lean ewes.
a,b Mean values within a row with unlike superscript letters (experiment 1) are significantly different (P < 0·05).
c,d,e,f Mean values within a row with unlike superscript letters (experiment 2) are significantly different (P < 0·05).
Table 2 Net energy intake (EI), hormones and metabolites measured during re-feeding in previously well-fed (W/R) or underfed (U/R) ewes (experiment 1)† (Mean values with their pooled standard errors)

PFL, previous feeding level; ND, not determined.
a,b,c,d Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Within-day mean values are significantly different (P < 0·05).
† W/R ewes re-fed at 210 (sd 38) % MER; U/R ewes re-fed at 207 (sd 53) % MER.
‡ Day 0 corresponds to the end of the underfeeding part of the experiment (day 166).
§ The Tukey–Kramer test (α = 0·05) was used.

Fig. 2 Experiment 1. Plasma leptin in ewes either well fed (W, ▲, n 10) at 100·1 (sd 1·1) % theoretical maintenance energy requirements (MER) or underfed (U, Δ, n 10) at 41·0 (sd 0·7) % MER after 14, 98 and 162 d of the feeding treatment. Values are means with their standard errors represented by vertical bars. The effect of time was significant (P < 0·001). The effect of feeding level × time was significant (P < 0·005). a,b Mean values of W ewes with unlike letters were significantly different (P < 0·05). c,d,eMean values of U ewes with unlike letters were significantly different (P < 0·05). *Mean value was significantly different from that of the U ewes at the same time point (P = 0·052).

Fig. 3 Experiment 2. (A) Plasma leptin variation after 5 (day 99) and 11 (day 105) days of re-feeding of previously either well-fed lean (WL/R, ■, n 6) and fat (WF/R, ●, n 6) ewes, or underfed lean (UL/R, □, n 6) and fat (UF/R, ○, n 6) ewes at 217 (sd 52) % maintenance energy requirement (MER) (WL/R), 219 (sd 50) % MER (UL/R), 216 (sd 53) % MER (WF/R) and 204 (sd 48) % MER (UF/R) on average for the 11 d. The effect of time was significant (P < 0·001). The effect of initial body fatness was significant (P < 0·001). The effect of previous feeding level was significant (P < 0·01). The effect of time was significant (P < 0·001). The effect of initial body fatness × time was significant (P < 0·02). (B) For the same groups of ewes, relationship between body lipids (percentage body weight (%BW)) and plasma leptin concentration at day 11 of re-feeding (y = − 1·68+1·004x − 0·0710x 2+0·00 182x 3, R 2 0·59, n 24, P < 0·01).
Plasma assays
Plasma leptin concentration was determined in duplicate on 100 μl samples according to a previously described disequilibrium double-antibody ovine-specific RIA (Delavaud et al. Reference Delavaud, Bocquier, Chilliard, Keisler, Gertler and Kann2000). Briefly, this assay is based on a rabbit anti-ovine leptin antibody at a final dilution of 1:30 000. Recombinant ovine leptin (Gertler et al. Reference Gertler, Simmons and Keisler1998) was either used as standard or was iodinated (chloramine T method modified previously; Kann, Reference Kann1971) and used as tracer. After 44 h of incubation, bound and free ligands were separated by the addition of specific ram anti-rabbit plasma, unbound [125I]leptin was removed by aspiration after centrifugation (3000 g; 25 min; +4°C), and the bound radioactivity was counted with a Cobra II γ counter (Packard, Downers Grove, IL, USA). The intra- and inter-assay CV were 6 and 9 %, respectively. The limit of detection, determined as the lowest leptin quantity able to generate a 5 % reduction in the B:Bo ratio was 0·83 ng/ml for a 100 μl sample size. Plasma insulin was determined using the commercial INSI-PR RIA kit (ORIS Group, Gif-sur-Yvette, France), which had been validated for sheep plasma by using a test for parallelism to the standard curve of the kit. Plasma samples were analysed in duplicate according to the manufacturer's instructions and intra- and inter-assay CV were 8·4 and 5·7 %, respectively. The limit of detection was 13·6 pmol/l. Plasma glucose and NEFA concentrations were determined enzymically by the glucose dehydrogenase method (E. Merck, Darmstadt, Germany) and the acyl-CoA synthetase method (Oxoid SAS, Dardilly, France), respectively, using an ELAN multi-analyser (Merck-Clevenot SA, Nogent-sur-Marne, France). The intra- and inter-assay CV were 0·5 and 2 % for glucose, and 0·5 and 7 % for NEFA, respectively (Ferlay & Chilliard, Reference Ferlay and Chilliard1999). The linearity was between 0 and 16·7 mmol/l for glucose and between 0 and 2 mmol/l for NEFA. Plasma 3-hydroxybutyrate (β-OH-butyrate) levels were determined according to Barnouin et al. (Reference Barnouin, el Idilbi, Chilliard, Chacornac and Lefaivre1986), using an ELAN multi-analyser (Merck-Clevenot SA). Intra- and inter-assay CV were 4·1 and 4 %, respectively (Ferlay & Chilliard, Reference Ferlay and Chilliard1999), and the linearity was between 0·08 and 8 mmol/l.
Statistical analysis
Results are presented in the text as means and standard deviations. Plasma leptin, insulin and metabolite variations over several days or hours (Fig. 2, Table 2, Fig. 3 and Fig. 4) were analysed by the PROC MIXED procedure of SAS (version 8.1; SAS Institute Inc., Cary, NC, USA; 2000) for unequally spaced time data (covariance structure: sp(pow)). Fixed effects were ‘feeding level’ or ‘body fatness’, and the random effect was animals within groups. Non-significant factors and/or interactions (P>0·05) were removed from the model and least square means for significant factors or interactions were compared by t test (α = 0·05). For unbalanced data, the Tukey–Kramer test was used. Plasma leptin and NEFA measured at the end of the underfeeding period (Table 1) were analysed by the GLM procedure of SAS (SAS Institute, 2000) separately for each experiment, and pair-wise means comparisons were assessed by the least square difference test. The PROC CORR and GLM procedures of SAS (SAS Institute, 2000) were used for correlation and regression analysis, respectively.

Fig. 4 Experiment 2. Plasma leptin variation during 24 h in six well-fed fat ewes under natural photoperiod conditions between days 87 and 88 of the experimental period. ↑ , Time at which the meal was given. Blood was collected on heparin.
The individual pulsatile profiles of leptin (Table 3) were analysed using the Munro software package (Zaristow Software; Haddington, East Lothian, UK). This software package uses the Munro algorithm (Taylor, Reference Taylor1987), the principle of which is to calculate the baseline and pulse amplitudes and their duration according to an iterative process that separates highest values from a secretory pattern. Number of pulses, pulse amplitude, between-pulse intervals and nadir were determined for each individual leptin profile. These results were then analysed using the GLM procedure of SAS (SAS Institute, 2000). The statistical model included the effects of ‘initial body fatness’, ‘feeding level’ and their interaction. The means obtained for each group (WL, WF, UL, UF) were compared by t test (α = 0·05).
Table 3 Leptin pulse parameters analysed on day 88 (experiment 2) from plasma leptin concentrations measured every 15 min, from 1 h before to 5 h after meal distribution
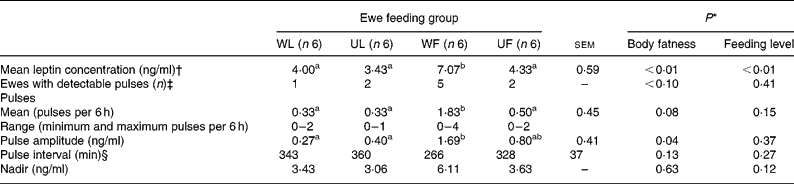
WL, well-fed lean ewes; UL, underfed lean ewes; WF, well-fed fat ewes; UF, underfed fat ewes.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05, t test, α = 0·05).
* Statistical significance of effects determined by the GLM procedure of SAS (SAS Institute, 2000).
† Heparin was used as anticoagulant.
‡ χ2 Test was used to analyse initial body fatness and feeding level effects on frequency in observed pulsatility.
§ If number of peaks is ≤ 1, then interval value is 360 min.
Results
Plasma leptin responses to medium-term underfeeding, and to subsequent short-term re-feeding
Experiment 1
Plasma leptin concentration was decreased by 30 % (P < 0·05; Fig. 2, experiment 1) after 14 d of severe underfeeding (i.e. 41 (sd 0·7) % MER) and remained depressed when underfeeding was maintained for 98 or 162 d (Table 1 and Fig. 2). Rapid re-feeding to 265 (sd 0·4), 167 (sd 0·3) and 189 (sd 0·3) % MER on days 167, 168 and 169, respectively, did not increase leptinaemia (Table 2). By contrast, in previously well-fed ewes, re-feeding to 254 (sd 0·2), 180 (sd 0·1) and 196 (sd 0·3) % MER increased plasma leptin concentration significantly (P < 0·001) and continuously by 42, 109 and 153 % (v. leptinaemia before re-feeding) after 1, 2 and 3 d of re-feeding, respectively (Table 2).
Experiment 2
The plasma leptin response to underfeeding was studied in both fat and lean ewes. Plasma leptin was significantly (P < 0·05) decreased in underfed fat ewes (UF) after they were fed for 94 d on a diet providing only 50·6 (sd 1·1) % MER, in comparison with well-fed fat ewes (WF) that were at 112·3 (sd 3·1) % of their MER (Table 1). By contrast, leptinaemia remained at the same level in lean ewes that were underfed (UL) at 53·2 (sd 1·8) % MER, and in lean ewes that were well fed (WL) at 114·8 (sd 2·3) % MER. Thus, at the end of the underfeeding period, no differences were observed in plasma leptin levels between UF, WL and UL groups (Table 1). During the following period, the total amounts of distributed feed were gradually increased during the first 2 d, and was then kept constant to provide on average 235 (sd 11) % MER for the last 9 d (days 96 to 105) of re-feeding. Plasma leptin measured on days 99 and 105 (Fig. 3 (A)) was significantly affected by time from the start of feeding treatment (P < 0·001): it was significantly increased after 5 d of re-feeding in WF/R (+144 %; P < 0·001), UF/R (+121 %; P < 0·02) and WL/R (+123 %; P < 0·05) ewes, remained high after 11 d of re-feeding in WF/R (+129 %; P < 0·001) and UF/R (+162 %; P < 0·002), but was slightly decreased in WL/R ewes and did not differ from their initial level (Fig. 3 (A)). No significant variation in leptinaemia was observed in UL/R ewes after either 5 or 11 d of re-feeding (Fig. 3 (A)). Plasma leptin response to re-feeding was significantly modulated by initial body fatness (P < 0·001), being significantly higher in WF/R than in WL/R ewes on days 99 (P < 0·001) and 105 (P < 0·001), and significantly higher in UF/R than in UL/R ewes, but only on day 105 (P < 0·01). Furthermore, the plasma leptin response to re-feeding was modulated by the previous feeding level (P = 0·009) but only among fat ewes, so that leptinaemia was significantly higher in WF/R than in UF/R ewes on days 99 (P < 0·002) and 105 (P < 0·05).
Plasma leptin in relation to body composition and blood parameters during feeding experiments
The severity of underfeeding assigned in both experiments caused important changes in animal body condition (Table 1). At the end of the underfeeding period, leptin concentrations measured in experiments 1 and 2 (n 44) were significantly and positively correlated with all body composition parameters: BW (r 0·43; P < 0·01), BCS (r 0·47; P < 0·01) and body lipid content expressed either in kg (r 0·52; P < 0·001) or in percentage BW (r 0·47; P < 0·01). All these correlations were also significant within each experiment, 1 (n 20) and 2 (n 24).
Experiment 1
Underfeeding for 162 d significantly (P < 0·001) decreased BW ( − 36 %), BCS ( − 50 %), body lipid content expressed either in mass (kg) ( − 66 %,) or in proportion (%) of BW ( − 47 %), and plasma NEFA were dramatically increased (+755 %) (Table 2).
As previously observed for leptinaemia, re-feeding increased plasma insulin significantly (+328 % v. insulinaemia before re-feeding; P < 0·001) after 2 d in previously well-fed but not significantly in previously underfed ewes (Table 2). Plasma NEFA were significantly decreased ( − 81 % v. NEFA before re-feeding; P < 0·001) as early as the first day of re-feeding in previously underfed ewes, and after 2 d of re-feeding in previously well-fed ewes ( − 90 %; P < 0·001) (Table 2). Plasma glucose and βOH-butyrate were significantly (P < 0·001) increased compared with their pre-refeeding values as early as the first day of re-feeding and were maintained at high values for 3 d whatever the previous feeding level. Throughout the re-feeding experiment, glycaemia remained higher (P < 0·05) in previously well-fed than previously underfed ewes (Table 2). Leptin concentrations were positively correlated to plasma insulin at the end of the underfeeding period (r 0·66; n 20; P < 0·002), and after 2 d of re-feeding (r 0·93; n 10; P < 0·001). No correlation was found between plasma leptin and glucose, NEFA or β-OH-butyrate.
Experiment 2
Underfeeding for 94 d significantly (P < 0·001) decreased BW ( − 26 %), as well as body lipid mass ( − 31 %), without any significant change in BCS ( − 13 %) or body lipid content expressed as percentage of BW ( − 8·7 %), and plasma NEFA were significantly increased (+645 %; P < 0·001) (Table 1). At the end of re-feeding (day 105), plasma leptin was curvilinearly related to body lipid content (R 2 0·59; P < 0·01; Fig. 3 (B)). Fig. 3 (B) shows that in fat ewes, leptinaemia of previously underfed animals was much lower than in previously well-fed ones, whereas no such difference due to previous feeding level was observed among lean ewes having an adiposity lower than 20 % body lipids, in which leptinaemia remained at its basal level.
Within-day variation of plasma leptin (Experiment 2)
On day 88 of the underfeeding period, plasma leptin was measured every 15 min in fat (Fig. 5 (A)) and lean (Fig. 5 (B)) ewes that were either well fed (WF and WL) or underfed (UF and UL) from 1 h before to 5 h after the meal was given. The use of heparin as anti-coagulant for this sub-part of experiment 2 (see Methods) led to higher absolute values of plasma leptin concentrations, as shown by the means of WF, WL, UF and UL groups of ewes reported in Fig. 5 when compared with those in Table 1. Plasma leptin variation was not altered by food distribution whatever the feeding level and initial body fatness (Fig. 5). When individual 6 h leptin profiles were analysed, pulses were detected in only ten animals out of twenty-four, with the highest proportion of animal having at least one pulse being found in WF ewes (five out of six). Despite these observations, the frequency of pulse detection and pulse intervals were modulated by neither initial body fatness nor feeding level (Table 3; Fig. 5), but fat ewes had a higher pulse amplitude (P < 0·05). Consequently, no clear pulsatility of plasma leptin was shown by these results. The 24 h leptin profile measured in fat and well-fed ewes (n 6; Fig. 4) showed a large inter-individual variability and an absence of significant plasma leptin modulation by the hour of day.

Fig. 5 Experiment 2. Plasma leptin variation from 1 h before to 5 h after the meal was given, measured at day 88 of the underfeeding period. Plasma leptin was determined from blood collected on heparin every 15 min (A) in fat ewes fed at 110 (sd 2·9) % (well fed, WF, ●, n 6) and 50 (sd 1·1) % (underfed, UF, ○, n 6) estimated maintenance energy requirement (MER) and (B) in lean ewes fed at 113 (sd 1·3) % (WL, ■, n 6) and 52 (sd 1·3) % (UL, □, n 6) estimated MER. Values are means with their standard errors represented by vertical bars. Plasma leptin determined from blood of ewes no. 5 (C), 14 (D), 11 (E) and 24 (F) from groups WF, UF, WL and UL, respectively. *Pulses.
Discussion
Plasma leptin pattern during 24 h period
In the present study, meal distribution and intake did not influence plasma leptin concentrations within the following 5 h at least, whatever the initial body fatness or the feeding level of the dry ewe, which is in accordance with previous studies conducted in crossbred wethers (Tokuda et al. Reference Tokuda, Delavaud and Chilliard2002) or adult rams (Blache et al. Reference Blache, Tellam, Chagas, Blackberry, Vercoe and Martin2000). Furthermore, the observation of plasma leptin over a 24 h period did not allow showing of significant diurnal variation in plasma leptin. However, in adult Soay rams, diurnal variations of plasma leptin were shown to be dependent on timing of meals (Marie et al. Reference Marie, Findlay, Thomas and Adam2001) and an increase of leptinaemia was observed 6–7 h after feeding time in male Merino sheep (Kadokawa et al. Reference Kadokawa, Briegel, Blackberry, Blache, Martin and Adams2003). Altogether, these results suggest that ovine plasma leptin should be slightly modulated at the day scale by meal intake but this modulation does not seem to be related to any endogenous circadian rhythm, contrary to what was suggested in man (Sinha et al. Reference Sinha, Ohannesian, Heiman, Kriauciunas, Stephens, Magosin, Marco and Caro1996; Saad et al. Reference Saad, Riad-Gabriel, Khan, Sharma, Michael, Jinagouda, Boyadjian and Steil1998; Shea et al. Reference Shea, Hilton, Orlova, Ayers and Mantzoros2005).
Episodicity was observed in the present study for well-fed and fat ewes but not in the other groups (underfed and/or lean), and, when they did occur, the number and amplitude of peaks remained low. In a previous study (Daniel et al. Reference Daniel, Whitlock, Baker, Steele, Morrison, Keisler and Sartin2002), plasma leptin episodicity was clearly found in both fat and thin Blackface ewes either fed or fasted for 48 h, and all pulsatile parameters were shown to be positively regulated by both nutritional status and level of adiposity. Differences with the present study could be partly due to the fact that the Blackface ewes were fatter than our Lacaune ewes, thus leading to higher leptin levels. Moreover, it could be also explained by the difference in ovarian activity, with plasma leptin and number of pulses lower in our ovariectomised ewes, compared with the intact ewes studied by Daniel et al. (Reference Daniel, Whitlock, Baker, Steele, Morrison, Keisler and Sartin2002). In human subjects, leptin episodicity differed with sex (Saad et al. Reference Saad, Riad-Gabriel, Khan, Sharma, Michael, Jinagouda, Boyadjian and Steil1998) and was decreased by gonadectomy in the rat (Bagnasco et al. Reference Bagnasco, Kalra and Kalra2002). To date, the mechanisms and biological significance of leptin episodicity are not fully understood and need further investigation in single-stomached and also in ruminant species.
Plasma leptin regulations during underfeeding
The decrease in plasma leptin during underfeeding periods is in accordance with previous studies conducted in ruminant species, as reviewed by Chilliard et al. (Reference Chilliard, Delavaud and Bonnet2005). The present study showed in addition that the down regulation was rather rapid, within 14 d, followed by stabilisation with time. Moreover, this down regulation was not related to BW change which was linearly decreased from the beginning to the end of the underfeeding period (Chaillou et al. Reference Chaillou, Baumont, Tramu and Tillet2002b). The present results confirm and complete previous studies showing that plasma leptin down regulation by energy intake level could occur in the absence of any loss in body condition, as observed after 4 weeks of maintenance of BW and BCS when compared with ad libitum feeding in adult sheep (Archer et al. Reference Archer, Rhind, Findlay, Kyle, Thomas, Marie and Adam2002) or after 3 weeks of mild underfeeding in adult cows (Delavaud et al. Reference Delavaud, Ferlay, Faulconnier, Bocquier, Kann and Chilliard2002). The present work used diets of the same composition and energy density, but distributed in different quantities to achieve either normal or underfeeding. Underfeeding due to distributing a very-low-energy-density diet ad libitum could putatively act differently on the satiety mechanisms, and thus result in a different leptin response. However, Chelikani et al. (Reference Chelikani, Ambrose, Keisler and Kennelly2004) showed that partial evacuation of ruminal contents did not alter plasma leptin (and other plasma variables) before and during response to feed deprivation. Further studies in fat and lean ewes are needed to unravel this issue.
During experiment 2, plasma leptin was significantly depressed by underfeeding in fat ewes, whereas no change was observed in lean ewes. This may be due to fatness per se since underfed fat and lean ewes were subjected to a similar theoretical energy deficit, confirmed by plasma NEFA concentrations (Table 1). We previously observed a similar lack of response of plasma leptin to underfeeding in lean Holstein cows (Delavaud et al. Reference Delavaud, Ferlay, Faulconnier, Bocquier, Kann and Chilliard2002). This was also observed on a shorter time scale, when an absence of plasma leptin modulation was observed in lean ewes (lipids 15 % of BW), in contrast to a significant decrease in fat ewes (lipids 35 % of BW) that were fasted for 32 h (Henry et al. Reference Henry, Goding, Tilbrook, Dunshea, Blache and Clarke2004). When studied on a medium-term time scale (weeks), no leptin down regulation was observed during underfeeding of lean animals, even though BW and body lipid mass (present study) or backfat thickness (Blache et al. Reference Blache, Tellam, Chagas, Blackberry, Vercoe and Martin2000) were greatly diminished. From the results of the present experiments, initial body fatness seems to play a major role in plasma leptin response to medium-term underfeeding due to the fact that when leptin secretion is already low due to low adiposity, it does not decline further due to underfeeding. Moreover, body composition measurements allowed us to estimate that a threshold between 16 and 22 % body lipids (Table 1) should be critical for plasma leptin regulation in the adult ewe, fitting well with equations calculated in man (Morio et al. Reference Morio, Gachon, Boirie, Rousset, Gachon, Beaufrere and Ritz1999; Gavrila et al. Reference Gavrila, Chan, Miller, Heist, Yiannakouris and Mantzoros2005).
Time course of plasma leptin regulation during re-feeding
In experiment 1, the ewes were re-fed ad libitum, whereas in experiment 2, feeding level was progressively increased and then only slightly limited in re-fed ewes. Despite this difference in the pattern of re-feeding between the two experiments, the results show a stepwise and strong increase in circulating levels of leptin during the first 4 d of re-feeding, which stabilised after 10 d. This positive effect of re-feeding is in accordance with the increase in leptinaemia observed after 14 d of re-feeding at 190 % estimated MER in adult ewes (Bocquier et al. Reference Bocquier, Bonnet, Faulconnier, Guerre-Millo, Martin and Chilliard1998), after 10 d of re-feeding at 180 % estimated MER in wethers (Tokuda et al. Reference Tokuda, Delavaud and Chilliard2002), and after 2·5 or 5 d on a high-energy diet in adult rams (Blache et al. Reference Blache, Tellam, Chagas, Blackberry, Vercoe and Martin2000; Zhang et al. Reference Zhang, Blache, Blackberry and Martin2004). However, the rapid evolution we observed in leptin response was dependent on the ewes' previous energy status. Indeed, no response in plasma leptin to re-feeding was observed in ewes who had been previously underfed at 40 % MER for 166 d, and this was despite an important increase in energy intake level, and clear responses of other blood parameters such as a decrease in NEFA, and increases in glucose and β-OH-butyrate as early as after 1 d of re-feeding. A similar absence of leptin response to 7 d of re-feeding at a high energy level was recently observed in underfed rams with a low BCS (Zhang et al. Reference Zhang, Blache, Blackberry and Martin2005). When considering experiment 2, it appears that the leptin response to re-feeding is in fact modulated by both current body lipid content and previous energy intake level, so that at the same adiposity the leptin response was amplified in previously well-fed as compared with previously underfed fat ewes (Figs. 3 (A) and (B)). This response was also linked to the current body lipid content within the same previous feeding group, so that the plasma leptin response to re-feeding mirrors the above observations concerning leptin down regulation by underfeeding. Furthermore, it appears that the fatness threshold for response to re-feeding (Fig. 3 (B)) is close to 20 %, in agreement with the range (16–22 %) observed in underfed ewes (Table 1).
Energy intake level calculations made in the present study were based on BW0·75 of ewes without taking differences in body fatness into account. As MER/kg BW0·75 were shown to be slightly higher in fat than in lean ewes (Chilliard et al. Reference Chilliard, Ferlay, Faulconnier, Bonnet, Rouel and Bocquier2000), it is likely that the actual feeding level of fat ewes during re-feeding was slightly lower than that calculated. Hence, this reinforces the above suggestion for a role of body fatness per se in the higher response of fat ewes to a given feeding level. The high plasma leptin levels maintained for 11 d in fat ewes due to the high energy allowance were probably not sufficient to induce a negative feedback able to limit food intake level below 235 % MER (experiment 2).
Putative biological significance of medium- or short-term leptin nutritional regulation
The results obtained in experiments 1 and 2, respectively, lead to the same overall conclusions, despite differences between the protocols for the two experiments (season, level and duration of restriction and/or re-feeding, diet composition, etc.). In fat animals, plasma insulin could partly mediate plasma leptin response to re-feeding, although the correlation observed in experiment 1 between these two hormones does not prove any cause-and-effect relationship. However, in vitro studies showed that leptin production by adult ovine adipose tissue explants (Faulconnier et al. Reference Faulconnier, Delavaud and Chilliard2003) or bovine adipose tissue leptin gene expression (Houseknecht et al. Reference Houseknecht, Portocarrero, Ji, Lemenager and Spurlock2000) is stimulated by insulin. The opposition between the rapid response of circulating leptin to medium-term underfeeding or re-feeding in fat ewes, and the absence of response in lean ewes, is likely to be due to the fact that leptin synthesis is related to adipocyte volume which is lower in lean animals (Delavaud et al. Reference Delavaud, Ferlay, Faulconnier, Bocquier, Kann and Chilliard2002). In this respect, it could be hypothesised that the maintenance of plasma leptin at low levels in lean animals would allow a stimulation of appetite and energy intake for reconstitution of body fat reserves. Furthermore, a role for hypoleptinaemia in improved energy utilisation efficiency was previously suggested (Chilliard et al. Reference Chilliard, Delavaud and Bonnet2005), due to the persistent hypoleptinaemia observed in late lactation goats, despite the fact that these animals were in positive energy balance (Bonnet et al. Reference Bonnet, Delavaud, Rouel and Chilliard2005), and due to the more efficient use of metabolisable energy in dry, non-pregnant lean ewes compared with fat ewes (Chilliard et al. Reference Chilliard, Ferlay, Faulconnier, Bonnet, Rouel and Bocquier2000). Altogether, current knowledge concerning ruminants is consistent with the notion that nutritional regulation of leptin secretion is more effective in protecting against underfeeding than against increasing energy balance.
Conclusion
These experiments clearly show that plasma leptin in the ewe is under the control of a long-term static effect of adiposity and a short-term dynamic effect of instant energy balance. Long-term underfeeding stimulates body lipid mobilisation to sustain energy requirements and, as a consequence, leptinaemia is decreased by additive effects of negative energy balance and decreased body fatness. A biological threshold (likely to be about 20 % body lipid) under which plasma leptin remains low whatever the energy status should contribute to spare metabolisable energy. In ewes above this fatness threshold, the response of leptinaemia to up or down regulation by nutritional level is rapid with amplitudes that are modulated by both the ewe's adiposity and the medium-term nutritional history. There is very scant evidence for any pulsatile or episodic mode of leptin secretion in sheep; the diurnal variation is weak if any, and postprandial plasma leptin regulation in ruminants seems neither to exist within 5 h after food distribution and intake, nor to be dependent on body fat content or daily energy intake level. The present study confirms the major role of body fat reserves in the regulation of leptinaemia. Furthermore, it shows that body lipids interact with medium-term nutritional history, and contributes to the notion that changes in plasma leptin might play a key role in the efficiency of dietary energy utilisation in situations of large variation of food availability and/or body fatness.
Acknowledgements
We thank A. Gertler for the kind gift of ovine recombinant leptin, G. Kann for kind help in RIA, M. Tourret and D. Bany for sample preparation and plasma assays, as well as J. Rouel, A. Ollier, J. P. Pezant, A. Combeau, D. Roux, D. Thomas and G. Sauvage for animal management and sample collection. The present study was supported by an INRA grant for studies on lipogenesis in farm animals.










