Ageing of the population is a global phenomenon. By the year 2050, it is predicted that the proportion of the population over the age of 60 years will double to 22 %(1). Health care expenditure for this subgroup of the population is disproportionately high, increasing exponentially as end of life approaches(Reference Van Weel and Michels2). The cost of accidental falls and fall-related orthopaedic injuries contribute largely to the overall cost, with increasing frequency and severity with increasing age(3) and the association of these events with poorer outcomes overall(Reference Marottoli, Berkman and Cooney4, Reference Magaziner, Simonsick and Kashner5).
There is evidence that achieving and maintaining a desirable nutritional status can assist in the prevention of falls and fractures and increase life expectancy(Reference Huang, Himes and McGovern6). While there is no ‘gold standard’ measure to define desirable nutritional status, there have been numerous screening and assessment instruments developed in an effort to capture established risk factors and clinical signs and symptoms of malnutrition(Reference Green and Watson7). The complexity of these instruments vary but most require significant resources in terms of training, time to administer and interpret, and few have demonstrated an ability to predict outcomes among the oldest old. At a time when resources are scarce and expected to become even more so, questions should be asked about the need to use these relatively intensive methods of identifying malnutrition.
The BMI (kg/m2) is commonly used across a variety of settings and age groups to describe the extremes of adiposity and the level of morbidity risk(8). An index of weight for height, the BMI is included across a range of nutrition screening and assessment instruments for use among older adults despite concerns about its feasibility and predictive ability.
Concerns have been raised about the physiological changes and episodes associated with ageing that can lead to inaccuracy in the measurement of both weight and height, in addition to the variability in equipment and possible observer error associated with both measurement and interpretation(Reference Cook, Kirk and Lawrenson9). While most of these concerns can be overcome with the use of surrogate measures of height (e.g. knee height), regular training and equipment calibration along with the use of nomograms designed to remove the need to calculate BMI, there is still uncertainty about the ability of BMI to predict meaningful outcomes among the oldest old(Reference Cook, Kirk and Lawrenson9).
The aim of the present study was to investigate the relationship between BMI and risk of falls, fractures and all-cause mortality among older Australians in residential aged care facilities.
Materials and methods
Study participants
The study sample was taken from the Fracture Risk Epidemiology in the Elderly (FREE) study, which aimed to evaluate falls and fracture risk in very frail older people in residential care facilities. The FREE study comprised 2005 participants (473 males and 1532 females), aged 65–104 years (85·7 (sd 7·1)) from thirty hostels (intermediate care facilities) and fifty-two nursing homes in northern Sydney, Australia. Full details of the study protocol have been published elsewhere(Reference Lord, March and Cameron10, Reference Sambrook, Cameron and Chen11). In brief, all nursing care facilities in the Northern Sydney Area Health Service region were randomly assigned to blocks of ten nursing homes and five hostels. During recruitment, the facilities were approached one block at a time to maintain randomisation. Facilities were invited to participate by the researchers, and out of the ninety-five facilities invited, eighty-two (88 %) agreed to participate. Reasons for non-participation included: two rebuilding at the time; seven closed down after the randomisation; four refused without reason. The facilities not participating were similar to participating facilities. Informed consent was obtained from participants, or the person legally entitled to make decisions on behalf of the participant, with an overall consent rate of 55 %. Consent rate was higher for residents able to provide consent independently (78 %) compared with residents requiring proxy consent (33 %). Non-participants were similar in age and sex to participants but had higher care needs. Ethical approval was obtained from the local human research ethics committee for each participating facility and informed consent obtained from the participants or next of kin.
Anthropometry
A single measurement of body weight was performed using calibrated scales to the nearest 1 kg in light clothing and no footwear. Participant's height was estimated using equipment with a fixed footplate and an adjustable, sliding end plate. The distance from the base of the heel to the anterior surface of the thigh above the condyles of the femur and slightly proximal to the patella was measured to the nearest 0·5 cm with knee flexed at 90°. Age- and sex-specific equations were used to estimate height from knee height(Reference Chumlea and Guo12). BMI was calculated as weight in kilograms divided by height squared in metres and the participants classified as BMI < 22 kg/m2 (underweight) and BMI ≥ 22 kg/m2 (desirable weight for height)(Reference Lipski13). Data are also presented to describe the number of participants with a BMI>27 kg/m2 for comparison with other datasets; however, it must be acknowledged that there is very little evidence for an upper limit on BMI for the oldest old, so these data should be used with caution.
Falls, fractures and mortality
Falls were ascertained from incident reports and nursing records at 6-week intervals, and were defined as events resulting in a person coming to rest unintentionally on the ground or other lower level, not as the result of a major intrinsic event or an overwhelming hazard(14). Fracture data were collected via regular liaison with staff at the residential care facilities and confirmed by radiology reports. Deaths were noted according to documentation in the residents' nursing records.
Potential confounding variables
The following baseline variables were considered potential confounders in the first round of statistical analyses: sex (male, female); age (continuous); level of residential care (hostel or nursing home). All models where the relationship between BMI and outcome remained significant were adjusted further to include cognition (continuous, as measured by mini-mental state examination(Reference Folstein, Folstein and McHugh15)), number of medications (continuous, as measured by review of resident files) and falls in the 2-year follow-up period (yes, no) for fracture and cognition, number of medications and fracture in the 2-year follow-up period (yes, no) for mortality.
Statistical methods
Data are expressed as mean or median and 95 % CI according to data distribution. Difference in age and sex across level of care were evaluated using t tests and χ2 analysis, respectively. Separate logistic regression analyses were performed, taking baseline BMI as the dependent variable and baseline sex (male, female), age (continuous) or level of care (hostel or nursing home) as potential predictors. Cox proportional hazards regression models using time to fall, fracture and death from the baseline interview as the endpoint was used to analyse the relationship between BMI and falls, fracture and mortality. We used 2 years after the baseline interview as the censoring date. We also calculated incidence rate ratios using negative binomial regression models to assess the association between BMI and number of falls. Time at risk was entered into the model as an offset variable. All analyses were conducted using the SPSS statistical package version 14.0 for Windows, except for the negative binomial regressions which were performed in SPSS version 15.0.1.1 (2007).
Results
Of the 2005 participants enrolled in the FREE study, estimated height and therefore estimated BMI was available for 1846 participants. There were no differences in age, sex or falls for those 159 FREE participants screened out of subsequent analyses compared with the study sample for this report. There were, however, a greater proportion of FREE participants screened out of subsequent analyses that experienced a fracture (P = 0·035) or died (P = 0·046) in the subsequent 2 years. Table 1 shows characteristics of the study participants. Regardless of setting, males were heavier than females (P < 0·001) and for those in hostel accommodation they also had a higher BMI than females (P = 0·023). Females were older (P < 0·001) and had a greater proportion that suffered a fracture in the subsequent 2 years (P = 0·017 for hostel comparison, P = 0·009 for nursing home comparison) compared with males across both settings. There was no sex difference for the proportion that suffered a fall in the subsequent 2 years (P = 0·257 for hostel comparison, P = 0·780 for nursing home comparison). The most frequently documented cause of death were: cardiac (33 %); infection (29 %); cerebrovascular (16 %).
Table 1 Participant characteristics according to level of residential care and stratified by sex
(Mean values and 95% confidence intervals)

* Comparison across setting (hostel v. nursing home).
Older participants (OR = 1·03, 95 % CI 1·02, 1·05, P < 0·001) and those from nursing home (OR = 1·93, 95 % CI 1·60, 2·34) were more likely to have a low BMI according to the logistic regression analyses. Sex was not found to independently predict BMI (OR = 1·25, 95 % CI 0·99, 1·58).
In the 2-year follow-up, 1246 participants experienced a fall, 321 suffered a fracture and there were a total of 616 deaths. The incidence rate of falls was 2·1 and 1·8 falls per person-year for years 1 and 2, respectively. The incidence rate of fracture was 0·12 and 0·14 fractures per person-year for years 1 and 2, respectively. A low baseline BMI ( < 22 kg/m2) was found to predict time to first fall (hazard ratio (HR) = 1·12, 95 % CI 1·02, 1·28) and time to first fracture (HR = 1·38, 95 % CI 1·10, 1·72) during the 2-year follow-up. After adjusting for age, sex and level of care, the association only remained for time to first fracture (HR = 1·38, 95 % CI 1·11, 1·73). Further inclusion of the number of medications, cognition and fall in subsequent 2 years into the model only slightly reduced the increased risk of fracture at 2 years for those participants with a low BMI (HR = 1·37, 95 % CI 1·09, 1·71). Figure 1 shows the cumulative survival curve over the 2 years according to BMI < 22 kg/m2 and ≥ 22 kg/m2 after adjusting for age, sex and level of care. A low BMI showed an increased risk of death at 2 years, independent of age, sex and level of care (HR = 1·52, 95 % CI 1·30, 1·79). Further inclusion of number of medications, cognition and fracture in subsequent 2 years into the model only slightly reduced the increased risk of death at 2 years for those participants with a low BMI (HR = 1·50, 95 % CI 1·28, 1·77). The negative binomial regression demonstrated an 11 % increased risk of experiencing a greater number of falls in those with a BMI < 22 kg/m2 (incidence rate ratio = 1·114, P = 0·048); however, when age, sex and level of care were entered into the model, this relationship did not persist (incidence rate ratio = 1·087, P = 0·136).
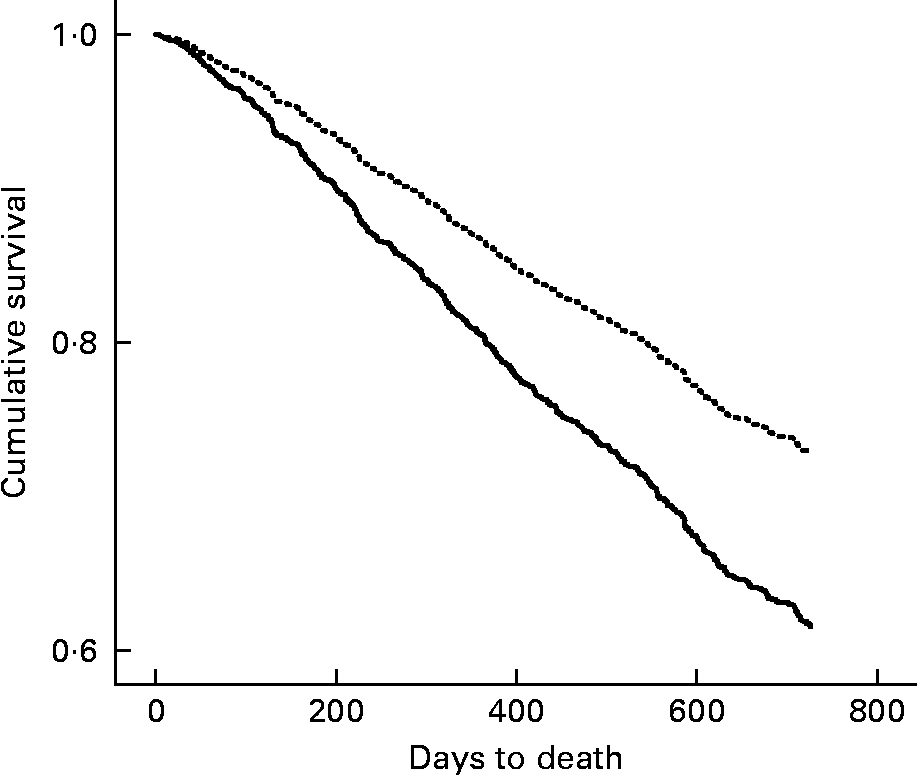
Fig. 1 BMI (—, < 22 kg/m2; …, ≥ 22 kg/m2) and cumulative survival over 2 years (adjusted for age, sex and level of residential care) for the 1846 participants of the Fracture Risk Epidemiology in the Elderly study.
Discussion
The findings of the present study provide clear evidence that a relationship exists between poor nutritional status, as measured by BMI, and subsequent fracture and mortality among the oldest old. At 2-year follow-up, residents of aged care facilities with a low BMI were 38 % more likely to sustain a fracture and 52 % more likely to die, even after adjusting for potential confounders, than residents with a BMI ≥ 22 kg/m2.
The BMI is commonly used across a variety of settings as a measure of nutritional status and is commonly incorporated into nutrition screening protocols; however, BMI categories used to distinguish an acceptable BMI in older adults are inconsistent. Flacker & Kiely(Reference Flacker and Kiely16) identified factors associated with 1-year mortality in nursing home residents and found that a BMI of < 23 kg/m2 was associated with increased 1-year mortality (HR = 1·29, 95 % CI 1·25, 1·34). Volpato et al. (Reference Volpato, Romagnoni and Soattin17) also investigated the relationship between BMI and mortality risk in nursing home residents, realising a significantly increased risk of mortality within 4 years, in residents with a low BMI (P < 0·001). BMI cut-offs of 22 kg/m2 and 21·6 kg/m2 were used to classify a low BMI in females and males, respectively. When BMI was classified into tertiles (cut-offs of 21·6 and 25·6 kg/m2 for men and 22 and 25·4 kg/m2 for women), a reduced risk of mortality was observed in participants in the higher BMI tertiles (relative risk 0·61 (95 % CI 0·43, 0·88) for the highest v. the lowest BMI tertile). While the present study does not investigate the impact of high BMI on the outcomes of interest, it is consistent with Volpato et al. (Reference Volpato, Romagnoni and Soattin17) highlighting that the effect of a low BMI on risk of mortality may persist beyond 1 year.
In addition to the studies described earlier, the WHO conducted a review of a number of European studies that investigated mortality and morbidity risk according to BMI(8). A Norwegian study found that a BMI between 21 and 27 kg/m2 and 23 and 27 kg/m2 for men and women, respectively, resulted in the lowest mortality and morbidity risk, and that the relationship between BMI and mortality is U shaped(Reference Waller18, Reference Waller19). A Finnish study(Reference Rissanen, Knekt and Heliovaara20) found the most favourable BMI to be 27 and 31 kg/m2, and that being overweight did not reduce life expectancy in women aged 65–79 years, a conclusion that supported the results of another study in the same country(Reference Matilla, Haavisto and Rajala21). In Australia, the nutrition screening initiative(Reference Lipski13) suggested that a BMI range of 22–27 kg/m2 be recommended for the management of chronic disease in older adults.
One of the more common chronic conditions in later life is osteoporosis, with fall-related fractures being the most devastating consequence both in terms of loss of independence and quality of life. In the present study, we found that adjusted BMI predicted the time taken to first fracture within a sample of older adults in residential care. These findings are consistent with the previous work that has evaluated the relationship between nutritional status and risk of fracture among older adults in residential care and independent-living older adults. Sambrook et al. (Reference Sambrook, Cameron and Chen11) investigated the influence of body weight on fracture risk in residential aged care residents and found that those who experienced a fracture were significantly lower in weight than those who did not experience a fracture over a median follow-up period of 705 d (P < 0·001). Lower body weight (lowest tertile: 27–52 kg) was an independent risk factor for fracture with an incidence rate ratio of 1·99 (95 % CI 1·49, 2·66) compared with the highest tertile ( ≥ 65 kg). While body weight is a commonly used measure of nutritional status, used in isolation, it can be problematic. Adjustment for height allowing for a standardised index of adiposity or monitoring of weight change are far more valuable alternatives.
Two large prospective studies in Norway also investigated the relationship between BMI and fracture. Meyer et al. (Reference Meyer, Tverdal and Falch22) studied 674 000 Norwegian men and women aged 50–89 years for 16 years with the incidence of hip fracture as an outcome. An inverse relationship between BMI and the incidence of hip fracture was observed. A Cox proportional regression analysis showed a reduced risk of fracture in both men and women in the three highest quartiles of BMI compared with the lowest quartile, with a higher reduction of risk observed in the older age groups. In the 70–79 years age group, the relative risk was 0·57 (95 % CI 0·5, 0·65) in women and 0·48 (95 % CI 0·39, 0·59) in men for the highest quartile of BMI (>29·9 kg/m2 for women and >27·5 kg/m2 for men) compared with the lowest quartile ( < 24·1 kg/m2 in women and < 22·9 kg/m2 in men). The inverse relationship was still present two decades after screening. In the present study, a cut-off of ≤ 22 kg/m2 was used, lower than that used in the study by Meyer et al. (Reference Meyer, Tverdal and Falch22), and also that used by Flacker & Keely(Reference Flacker and Kiely16), which may indicate that a lower cut-off should be used in our population.
The present study did not provide evidence for a relationship between BMI and falls after adjustments were made for potential confounders. This finding remained robust with negative binomial regression indicating that there was also no difference in the frequency of falls between the two BMI categories. While falls does not appear to be associated with BMI, fracture does, indicating that the increased risk may be due to a reduction in ability to absorb the impact of a fall and increased bone fragility as a consequence of starvation(Reference Coin, Perissinotto and Enzi23, Reference Coin, Sergi and Beninca24). The inability of the present study to demonstrate a relationship between nutritional status and falls could be related to the measure used to define nutritional status or the robust analytical approach used to test the relationship.
While the present study has demonstrated good evidence for the clinical utility of BMI, the feasibility of BMI has been a topic for debate over many years. Much of this debate is centred around pragmatic issues including inability or unwillingness of staff to perform the anthropometric measures necessary and/or to calculate BMI. There is also discussion around the reliability of measures used to calculate BMI, particularly height. Of much concern is the issue of inability to weigh older adults with mobility limitations. Despite all of these factors being legitimate concerns, there has been much time spent on developing alternative strategies to increase feasibility including the development of knee height equations for estimating stature, easy-to-use nomograms for calculating BMI and advances in technology such as weigh chairs and beds. Increasing recognition of the clinical utility of BMI as a measure of nutritional status in addition to provision of regular training and resources will assist in improving the perception that BMI is not a feasible measure. Agreement on appropriate cut-offs for BMI should also be a priority – the present study would support a BMI ≥ 22 kg/m2 being desirable.
While the evidence presented in the present study is convincing, the findings must be interpreted with caution as the study was a secondary analysis of a large epidemiological study with different aims to the present study. The primary concern is that due to the difference in aims, not all potential confounders required for the present study were necessarily measured and hence were unable to be adjusted for in the statistical analyses. Further adjustments are likely to reduce the magnitude of effect found in the present study; however, even after adjusting for some risk factors with large independent effects, the magnitude of effect for BMI and fracture and BMI and mortality was negligible. The present study did, however, include measurement of clearly defined and monitored outcome variables and had a large sample size, therefore increasing the power to detect the differences of interest and allowing for appropriate regression models to be calculated with adjustment for some established confounders.
In the context of an ageing population and the increasing incidence of falls and fractures, it is vital that screening methods are utilised to identify individuals who are at increased risk of fractures and mortality so that strategies can be implemented to reduce these risks. It is well documented that nutritional status has a role in the risk of falls and subsequent fractures, and also that poor nutritional status is a predictor of early mortality in older adults. After adjustment for a small selection of important confounders, the present study found that a relatively simple index of nutritional status, the BMI, can predict early mortality and fractures within older adults in residential care. Despite not all possible confounders being adjusted for, it is recommended that residential care staff be aware of how to measure BMI and that BMI be included as a component of admission procedures for residential care facilities as it is a rapid, inexpensive and non-invasive screen with the potential to identify high-risk individuals who may benefit from strategies to improve their nutritional status to subsequently reduce their fracture and mortality risk.
Acknowledgements
We thank Jennifer Schwarz, Jill Makaroff and the aged care staff at the participating residential aged care facilities for their assistance with the present study. We also thank the Flinders University Statistician, Kylie Lange, for her direction with analyses. The present study was partly funded by the Australian National Health and Medical Research Council. No conflict of interest exists for any of the authors. There are no conflicts of interest. M. D. M. and J. M. T. were involved in the concept and design of the secondary analysis, analysis and interpretation of the data and preparation of the paper. I. D. C., P. N. S., L. M. M., R. G. C. and S. R. L. conceived the idea for the FREE study, developed the FREE study protocol, secured funding and supervised data collection. All contributed to the design and interpretation of the secondary analyses presented in this paper in addition to the preparation of this paper. J. S. C. assisted with the data collection and coordinated the data analysis for the FREE study. He also contributed to the design and interpretation of the secondary analyses presented in this paper in addition to the preparation of this paper.




