Human milk is a complex species-specific biological fluid adapted to perfectly satisfy the nutritional and immunological needs of the neonate. It has been demonstrated that breast milk confers protection against different infectious diseases since the incidence of these disorders is lower in breast-fed than in formula-fed infantsReference Lopez-Alarcon, Villalpando and Fajardo1, Reference Wright, Bauer, Naylor, Sutcliffe and Clark2. It has been suggested that this anti-infective effect is due to several bioactive compounds present in colostrum and/or in mature milk. These include immunoglobulins, immune cells, antimicrobial acids, polyamines, oligosaccharides, lysosyme, glycoproteins such as lactoferrin and bioactive peptides, which, acting individually or synergistically, could inhibit pathogenic microorganismsReference Saavedra, Räihä and Rubaltelli3, Reference Isaacs4.
Recent studies have demonstrated that human milk, far from being a sterile fluid, constitutes an excellent and continuous source of commensal bacteria for the infant gutReference Hekkilä and Saris5, Reference Martin, Langa, Reviriego, Jimenez, Marin, Xaus, Fernandez and Rodriguez6. These bacteria could also play an important role in the reduction of incidence and severity of infectious diseases in breastfed children. This hypothesis is supported by relatively old studies reporting the loss of antimicrobial activity in pasteurised human milkReference Ford, Law, Marshall and Reiter7.
Among the bacteria found in human milk, those belonging to the species Staphilococcus, Lactococcus, Enterococcus and Lactobacillus are the most frequentReference Hekkilä and Saris5, Reference Martin, Langa, Reviriego, Jimenez, Marin, Xaus, Fernandez and Rodriguez6 (Table 1). There is increasing interest in some of these breast milk lactobacilli, such as L. gasseri, L. salivarius, L. rhamnosus, L. plantarum and L. fermentum, because they are considered as potentially probiotic species (Table 1).
Table 1 Bacterial species generally isolated from the breast milk of healthy women
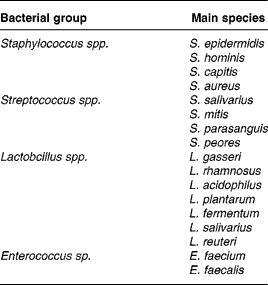
Modified from Martin et al. Reference Björksten, Naaber, Sepp and Mikelsaar32.
Breastfeeding and protection against diseases
In developing countries, one of the main causes of death in the paediatric age group is the infectious disease, specially gastroenterocolitis and respiratory infections. Newborns who have not been breastfed show a 17-fold higher risk of being hospitalised due to pneumonia than those who exclusively received human milkReference Cesar, Victoria and Barros8. Similarly, the risk of death due to diarrhoea increases 14.2-fold in weaned infantsReference Victoria, Smiyh and Vaughan9. Breastfeeding has also been related to a lower incidence of acute otitis mediaReference Duncan, Ey, Holberg, Wright, Martinez and Taussing10, urinary tract infectionReference Pisacane, Graziano, Mazzarella, Scarpellino and Zona11 and meningitis caused by Haemophilus influenzae Reference Silfverdal and Olcen12.
Besides its anti-infective properties, it has been demonstrated that human milk modulates the immune system of the newbornReference Grazioso, Werner and Alling13. Although an anti-inflammatory activity has not yet been demonstrated in vivo, several epidemiologic studies suggest that breast-fed children are protected against infections without the observation of evident lesion of the intestinal or respiratory mucosa due to an inflammatory responseReference Garofalo and Goldman14. This is probably the result of an anti-inflammatory system better regulated by bioactive components of human milk.
The immunomodulatory action of breast milk could also explain the better antibody production response in breast-fed compared to formula-fed infants after vaccination against poliomyelitis, tetanus and diphtheriaReference Hahn-Zoric, Fulconis and Minoli15.
Neonates who received breast milk also have a more favourable intestinal microbiota than those fed infant formulaReference Harmsen, Wildeboer-Veloo and Raangs16, which is probably due to presence of lactic acid bacteria in human milk, besides other bifidogenic compounds such as oligosaccharidesReference Kunz and Rudloff17. It has been suggested that these differences in intestinal microbiota could be responsible for some of the beneficial effects seen in breast-fed infants. It has been known for several decades that lactobacilli and bifidobacteria inhibit the growth of pathogen microorganisms such as Staphylococcus aureus, Salmonella typhimurium, Yersinia enterocolitica and Clostridium perfringens Reference Gilliland and Speck18. These bacteria competitively colonise the intestine of the child, thus preventing the adhesion of pathogens. Moreover, a competition for nutrients is established and this is another mechanism that inhibits the growth of pathogenic microorganismsReference Conway19.
Intestinal colonisation by commensal bacteria also plays a key role in the maintenance of immune system homeostasis. These bacteria stimulate TH1 responses and compensate the trend towards TH2 responses characteristic of the neonatal immune system. It has been reported that the administration of specific probiotics to newborns reduces the incidence of atopic manifestationsReference Kalliomaki, Salminen, Arvilommi, Kero, Koskinen and Isolauri20 and also of inflammatory processes where a TH2 response is involved, such as necrotizing enterocolitisReference Dani, Biandaioli and Firmito21.
Probiotics isolated from breast milk
The description of the presence of bacteria in human milk dates back 30 years, but then it was assumed to be a contamination occurring during sample extractionReference Gavin and Ostovr22. At the beginning of the 21st century, two European groups independently demonstrated the presence of lactic acid bacteria in human milk and their probiotic potentialReference Hekkilä and Saris5, Reference Martin, Langa, Reviriego, Jimenez, Marin, Xaus, Fernandez and Rodriguez6. Thus Heikkilä et al. reported that these human milk bacteria protect both mother and newborn from infections caused by Staphilococcus aureus Reference Hekkilä and Saris5. In a similar way and in a series of reports, Martín et al.Reference Martin, Langa, Reviriego, Jimenez, Marin, Xaus, Fernandez and Rodriguez6 described the isolation of lactic acid bacteria from human milk, namely, L. gasseri CECT5714Reference Martin, Olivares, Marin, Fernandez, Xaus and Rodriguez23, L. salivarius CECT5713Reference Martin, Jimenez, Olivares, Marin, Fernandez, Xaus and Rodriguez24 and L. fermentum CECT5716Reference Martin, Olivares, Marin, Fernandez, Xaus and Rodriguez23. Besides these strains, there are other commercial strains related to human milk. One of them is L. reuteri ATCC55730, which is claimed to be derived from human milk but its origin has not been published yet. L. rhamnosus LGG, although originally isolated from intestinal sourcesReference Conway, Gorbach and Goldin25, has also been found in human milk by the Finnish groupReference Hekkilä and Saris5. Nevertheless, this work focuses on the beneficial effects of L. gasseri CECT5714, L. salivarius CECT5713 and L. fermentum CECT5714, which have been summarised in Table 2.
Table 2 Beneficial effects of some breast milk-isolated probiotic strains

Anti-microbial effects
Protection against viral or bacterial infections is one of the most frequent claims made for probiotic consumption. Different mechanisms have been suggested to explain this anti-microbial activity (Fig. 1). In vitro studies demonstrate that certain probiotic strains produce anti-microbial compounds, such as organic acids, H2O2 and/or bacteriocinsReference Fons, Gomez and Karjalainen26, that have been reported to inhibit the growth of E. coli, Salmonella spp. and Listeria monocytogenes Reference Olivares, Diaz-Ropero, Martin, Rodriguez and Xaus27. No bacteriocin-producing lactobacillus has been found in human milk, although a high production of H2O2 has been reportedReference Martin, Olivares, Marin, Fernandez, Xaus and Rodriguez23. In addition, those strains belonging to the species L. reuteri produce reuterin, another antimicrobial compoundReference Talarico and Dobrogosz28. It has also been shown that some bacteria present in human milk improve the intestinal barrier function by increasing mucine production and reducing intestinal permeabilityReference Olivares, Diaz-Ropero, Martin, Rodriguez and Xaus27. However, competition with entero-toxigenic bacteria for nutrients and for epithelial intestinal cell receptor binding sites is probably the main anti-infective mechanism of probiotic bacteriaReference Fons, Gomez and Karjalainen26, Reference Olivares, Diaz-Ropero, Martin, Rodriguez and Xaus27(Fig. 1).

Fig. 1 Intestinal anti-infective mechanisms of probiotic bacteria.
The human milk-isolated probiotics L. gasseri CECT5714, L. salivarius CECT5713 and L. fermentum CECT5714 have been reported to inhibit the adhesion of Salmonella cholerasuis to mucins and to increase the survival of mice infected with this pathogenReference Olivares, Diaz-Ropero, Martin, Rodriguez and Xaus27. It was demonstrated that the protective effect of L. salivarius CECT5713 is significantly higher than the effect of a reuterin producing strainReference Martin, Olivares, Marin, Xaus, Fernandez and Rodriguez29. This is probably due to the combination of the immunomodulatory role and the competitive activity reported for L. salivarius CECT5713Reference Diaz-Ropero, Martin, Sierra, Lara-Villoslada, Rodriguez, Xaus and Olivares30.
Different clinical trials have demonstrated that, when breastfeeding is not possible, infant formula supplemented with probiotics protect children from infectious diseases. To our knowledge, most of the studies have involved supplementation with L. rhamnosus LGG, which have demonstrated a reduction in the incidence of rotavirus infection and in the duration of diarrhoeaReference Senok, Ismaeel and Botta31. Currently, clinical studies are in progress to evaluate the tolerance and effectiveness of other breast milk strains, such as L. reuteri ATCC55730 and L. salivarius CECT5713.
Immunomodulatory properties
Intestinal colonisation is often the result of the first contact of the newborn with microorganisms, which is crucial for the development of the immune system of the neonate. It has been reported that differences in the composition of intestinal microbiota influence the incidence of certain pathologies with an important immunological component, such as allergic or inflammatory processesReference Björksten, Naaber, Sepp and Mikelsaar32. The anti-allergic effect of probiotics could be explained on the basis of the Hygiene Hypothesis and the TH1/TH2 balance. Probiotics induce a TH1 response, and thus down-regulate the production of TH2 cytokines, responsible for the allergic response.
In contrast, the anti-inflammatory effect of probiotics is more difficult to explain. In vitro studies have demonstrated that the immunomodulatory effects of probiotics depend on the cell environment. Thus, in the absence of additional stimulus, the breast milk probiotics L. salivarius CECT5713 and L. fermentum CECT5716 enhance the production of TH1 cytokines such as IL-2 and IL-12 and the inflammatory mediator TNF-αReference Diaz-Ropero, Martin, Sierra, Lara-Villoslada, Rodriguez, Xaus and Olivares30. However, when cells are incubated in the presence of lipopolysaccharide, together with the probiotics, a reduction of TH1 cytokines is observedReference Diaz-Ropero, Martin, Sierra, Lara-Villoslada, Rodriguez, Xaus and Olivares30. This regulatory mechanism is probably based on the production of IL-10, an immunosuppressive cytokine, which has been reported to be increased by these probiotic strainsReference Diaz-Ropero, Martin, Sierra, Lara-Villoslada, Rodriguez, Xaus and Olivares30.
The immunomodulatory effects of probiotics have also been reported in animal models of pathologies where the immune system is involved. Different probiotic strains isolated from human milk have been reported to enhance the immune defence of mice, increasing both natural and acquired immune responsesReference Diaz-Ropero, Martin, Sierra, Lara-Villoslada, Rodriguez, Xaus and Olivares30. This immune-stimulating activity could be also involved in the anti-infective role previously mentioned for these bacteria in an animal model of Salmonella infectionReference Olivares, Diaz-Ropero, Martin, Rodriguez and Xaus27. In addition, the breast milk probiotic L. gasseri CECT5714 in combination with L. coryniformis CECT5711 reduces the incidence and severity of the allergic response in an animal model of cow's milk protein allergyReference Olivares, Díaz-Ropero, Lara-Villoslada, Rodriguez and Xaus33. In a recent report, L. fermentum CECT5716 showed a beneficial effect in an animal model of intestinal inflammation, reducing the inflammatory response and the intestinal damageReference Peran, Sierra and Comalada34.
Probiotics have also been reported to modulate the immune response of healthy humans, as shown by a recent study which reports an increase in phagocytic activity, in the number of natural killer cells and in the plasma concentration of IgA in healthy humans consuming human milk-isolated probiotics daily for 3 monthsReference Olivares, Diaz-Ropero, Gomez, Lara-Villoslada, Sierra, Maldonado, Martin, Rodriguez and Xaus35. A more recent report demonstrates that the consumption of L. fermentum CECT5716 enhances the response to influenza vaccination in healthy volunteers aged 26-40 and reduces the incidence of influenza-like illnessReference Olivares, Díaz-Ropero, Sierra, Lara-Villoslada, Fonolla, Navas, Rodriguez and Xaus36.
In addition, the beneficial effect of probiotics in allergic processes has been widely reported. In this sense, the consumption of probiotics present in human milk, especially L. rhamnosus LGG, has been shown to reduce the incidence and severity of atopic dermatitis in childrenReference Kalliomaki, Salminen, Arvilommi, Kero, Koskinen and Isolauri20. Although less is known about other allergic disorders, there is data to support a positive effect of L. gasseri CECT5714 in adults with respiratory allergyReference Olivares, Díaz-Ropero, Lara-Villoslada, Rodriguez and Xaus33.
Gastrointestinal benefits
There is increasing interest in the manipulation of intestinal microbiota with the aim of improving gastrointestinal function and nutrient absorption. Different reports demonstrate that human milk probiotics colonise the intestine and increase faecal lactobacilli counts thus modifying intestinal microbiota both in rodentsReference Peran, Camuesco, Comalada, Nieto, Concha, Diaz-Ropero, Olivares, Xaus, Zarzuelo and Galvez37 and humansReference Olivares, Díaz-Ropero, Gomez, Lara-Villoslada, Sierra, Maldonado, Martin, Lopez-Huertas, Rodriguez and Xaus38. In addition, molecular analysis show that these bacteria are metabolically active in the human gut, increasing the production of functional metabolites such as butyrateReference Olivares, Díaz-Ropero, Gomez, Lara-Villoslada, Sierra, Maldonado, Martin, Lopez-Huertas, Rodriguez and Xaus38, which is the main energy source for colonocytes and plays a key role in the modulation of intestinal function. In the previously mentioned clinical trialReference Olivares, Díaz-Ropero, Gomez, Lara-Villoslada, Sierra, Maldonado, Martin, Lopez-Huertas, Rodriguez and Xaus38, an increase in faecal moisture, and in stool frequency and volume was observed which could be related to the increase in the faecal concentration of butyric acid.
Similarly, the administration of L. gasseri CECT5714 also caused an increase in faecal lactobacilli counts in a clinical trial in children aged 3-12Reference Lara-Villoslada, Sierra, Boza, Xaus and Olivares39. In the same study the cytotoxicity of the faecal water of children who received the probiotic has been shown to be lower than that of the control childrenReference Lara-Villoslada, Sierra, Boza, Xaus and Olivares39. Finally, in another clinical trial the supplementation of infant formulas with L. rhamnosus LGG has been demonstrated to improve neonate growth pattern, which could suggest an increased bioavailability of nutrients in these infantsReference Vendt, Grünberg, Tuure, Malminiemi, Wuolijoki, Tillmann, Sepp and Korpela40.
Conclusions
Breastfeeding is the main determinant of the intestinal colonisation of the neonate, which, apart from other components, is due to the recently discovered presence of probiotic bacteria in human milk. In addition to gastrointestinal benefits, modulation of microbiota by probiotic bacteria has been shown to regulate the immune function and to enhance defence against intestinal pathogens. Thus, the addition of breast milk probiotics to infant formulas could be a new alternative to mimic some of the functional effects of human milk in children who are not breastfed.
Conflict of interest statement
All the authors except JMR are employees at Puleva Biotech SA. All the studies presented have been funded by Puleva Biotech's own founds. This review has mainly been written by JX with collaboration of all the other authors.





