Ginger rhizomes are widely used in foods for their nutritional and medicinal benefits, especially in Asia(Reference Badreldin, Gerald and Musbah1); for example, as a source of Fe and Ca for women during the post-natal period and also for treating morning sickness and other gastrointestinal disorders(Reference Chan, Nelson and Leung2). More recently, ginger juice was shown to have an antidiabetic effect in alloxan-induced diabetic rats(Reference Badreldin, Gerald and Musbah1). In a similar study, ginger juice was reported to cause significant reduction in the fasting glucose levels and an increase in the insulin levels in streptozotocin (STZ)-induced type 1 diabetic rats(Reference Asha, Krishnamurthy and Devaru3). Al-Amin et al. (Reference Al-Amin, Thomson and Al-Qattan4) also found that ginger possesses hypoglycaemic, hypocholesterolaemic and hypolipidaemic potential. They showed, in addition, that raw ginger was effective in reversing proteinuria in diabetic rats(Reference Al-Amin, Thomson and Al-Qattan4).
Though a subsection of non-insulin-dependent diabetes mellitus patients can be managed by diet alone, most patients require an oral hypoglycaemic agent such as insulin. Insulin therapy affords effective glycaemic control, yet its shortcomings such as ineffectiveness on oral administration, short shelf-life, requirement of constant refrigeration and in the event of excess dosage, fatal hypoglycaemia, limit its usage. Treatment with sulphonylurea and biguanides is also associated with side effects(Reference Rang and Dale5).
In addition, studies on the hypoglycaemic properties of ginger in animals have reported variable results(Reference Al-Amin, Thomson and Al-Qattan4, Reference Weidner and Sigwart6, Reference Akhani, Vishwakarma and Goyal7) and there has been no report on the effect of ginger extract on the selected glycolytic enzymes involved in carbohydrate metabolism. Therefore, the present study was undertaken to determine the effectiveness of ginger (Zingiber officinale (ZO) Roscoe) in the treatment of diabetes mellitus using animal models, by investigating its effect on the key enzymes of carbohydrate metabolism, which shows its beneficial effect in correcting the nutritional disturbances in diabetes.
Materials and methods
Animals
Male Sprague–Dawley rats (body weight 250–300 g) were acclimatised inside a room at 22 ± 2°C for a period of 7 d. All animals were fed with standard rat chow in the form of pellets and animals described as fasting were deprived of food for at least 16 h but were allowed to drink filtered tap water. The standard rat chow containing carbohydrate 4·7 %, protein 18·9 % and fat 3·5 % was purchased from Liaz Bhd. The total energy content of the chow was 17·7 kJ/g. All handling and management procedures were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Kulliyyah of Medicine, International Islamic University Malaysia (IIUM).
Preparation of the rhizome extract
Fresh ginger was bought from the wet-market of Chow Kit, Kuala Lumpur; and 2·0 kg of the fresh ginger rhizomes were cut into pieces, air-dried and powdered. Then, 375 g of the powdered material were cold-macerated in 4 litres of distilled water and intermittently stirred thoroughly. The mixture was left at room temperature for 48 h to allow the active ingredients to be completely dissolved. The macerated pulp was first filtered by mesh cloth and then suction-filtered through Whatman no. 1 filter paper and the filtrate was freeze-dried. To increase the shelf life and uniformity, the extract was lyophilised completely by a continuous freeze-drying operation for 54 h and the yield was 25·3 % (w/w), which was stored at − 20°C until use.
Acute toxicity test (LD50 determination)
The acute toxicity test (lethal dose 50 %; LD50) of the aqueous extract of ginger was determined according to the procedure described by Lorke(Reference Lorke8). This method involved an initial dose-finding procedure, in which animals were divided into three groups of three animals per group. Doses of 10, 100 and 1000 mg/kg of ginger extract were administered through oral administration, one dose for each group. The treated animals were monitored for 24 h for mortality. Even the highest dose of 1000 mg/kg was not found to be toxic to the animals. So, the next round of toxicity test was conducted with four different doses of 800, 1600, 3200 and 6400 mg/kg, which were administered orally to four groups of one rat per group. The treated animals were again monitored for 24 h. The LD50 was then calculated as the geometric mean of the lowest dose showing death and the highest dose showing no death (Table 1).
Table 1 Acute toxicity test of the aqueous extract of ginger*
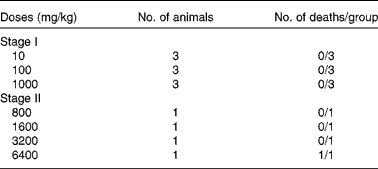
LD50, lethal dose 50 %.
* LD50 = (3200 × 6400)1/2 = 4525·5 mg/kg.
Induction of diabetes
Animals were divided into five different treatment groups (Table 2). Body weights were measured after a 16 h fasting period. The animals in groups 2–5 were lightly anaesthetised with diethyl ether and were injected intraperitonially with 65 mg/kg body weight of STZ which was freshly dissolved in citrate buffer with a pH of 4·5. After injection, they were allowed free access to food and water and were given 5 % glucose solution to drink overnight to counter the hypoglycaemic shock. Then, 3 d after STZ injection, diabetes was confirmed in rats by measuring the normal fasting blood glucose levels with a glucometer (Roche Accu-Check Advantage). All rats showing a fasting blood glucose level ≥ 13 mmol/l were considered diabetic and selected for the experimentation.
Table 2 Grouping of animals into five treatment groups

NCNT, normal control rats; STZ, streptozotocin; DCNT, diabetic control rats; ZO, Zingiber officinale.
Experimental design
Table 2 shows the experimental design. Normal control animals in group 1 received distilled water only. Diabetic animals in groups 3–5 received ginger extract according to the dose stipulated in the table. Diabetic control animals in group 2 received neither distilled water nor the extract. All animals received either water or the extract through oral administration.
Sample collection
Fasting blood glucose level
Fasting blood glucose in blood was measured on days 0, 7, 15, 21 and 30 during the experiment with a glucometer. Intra- and inter-assay percentage CV using ten repeated measures and ten sessions were 1·89 and 4·30 %, respectively. Percentage reduction in blood glucose level was calculated as (BGC at 0 d − BGC at N day/BGC at 0 d) × 100, where BGC = blood glucose concentration; and N = treatment days.
Collection of tissues
Animals were anaesthetised by exposing them to diethyl ether in an air-tight chamber. Thereafter, they were dissected, and the liver, kidney and skeletal muscles were collected in pre-cooled normal saline, and then blotted. Tissues were weighed and finally preserved with liquid N2 and frozen at − 80°C until used for the determination of metabolic changes.
Sample preparation
A small part of the liver tissue was cut and perfused with ice-cold 0·15 m-KCl and 1 mm-EDTA solution and homogenised with twice its weight of ice-cold buffer (0·01 cysteine and 1 mm-EDTA in 0·1 ml Tris–HCl, pH 7·4) and centrifuged (at 20 000 g) for 20 min at 4°C. The supernatant was filtered and frozen at − 80°C.
Biochemical and enzymatic estimations
Assay of glucokinase (EC 2712) activity
The liver glucokinase activity was measured at 340 nm in a reaction mixture containing 75 mm-Tris buffer (pH 9·0), 27 mm-NADP+, 120 mm-ATP, 600 mm-MgCl2, 100 units/ml of glucose-6-phosphate dehydrogenase and 360 mm-glucose at 30°C. Glucose phosphorylation was then assayed by means of the glucose-6-phosphate-dependent spectrophotometric method(Reference Crane, Sols, Colowick and Kaplan9). The %CV for this assay was 2·68 % at 19·40 U/l and 4·10 % at 28·11 U/l for the intra- and inter-assay, respectively.
Assay of fructose-6-phosphate kinase (EC 27111) activity
Assay of fructose-6-phosphate kinase was carried out in a reaction mixture of 100 mm-Tris buffer (pH 8·0), 40 mm and 140 mm of a mixture of MgSO4–KCl, 30 mm-phosphoenol pyruvate solution, 20 mm-fructose-1,6-diphosphate solution, 30 mm-fructose-6-phosphate solution, 20 mm-ATP, 7·0 mm-NADH and appropriately diluted pyruvate kinase (PK)/lactic dehydrogenase enzyme suspension. The fructose-6-phosphate kinase activity was assayed spectrophotometrically by the method of Racker(Reference Racker10); and %CV were 5·07 at 11·69 U/l and 5·39 % at 8·30 U/l for the ten intra- and inter-assays.
Assay of pyruvate kinase (EC 27140) activity
The sample was added to a reaction mixture containing 100 mm-potassium phosphate buffer, pH 7·6 at 37°C, 1·3 mm-NADH, 44 mm-ADP, 100 mm-MgSO4, 5000 units/ml of lactic dehydrogenase and 17 mm-phosphoenol pyruvate freshly prepared. PK assays were then conducted with a thermostated recording spectrophotometer by coupling the enzyme activity to lactate dehydrogenase, using the procedure reported by Bergmeyer et al. (Reference Bergmeyer, Gawehn, Grassl, Bergmeyer and Gawehn11). Intra- and inter-assay %CV using ten measurements were 4·57 % at 9·77 U/l and 8·21 % at 3·81 U/l, respectively.
Estimation of tissue glycogen level
Tissue glycogen level was measured using the glycogen assay kit (Bio Vision) based on hydrolysis of glycogen to glucose by glucoamylases, which is then specifically oxidised to produce a product that reacts with oxy-red probe to generate a colour at λmax = 570 nm(Reference Huijing12). The precision as estimated by %CV for intra- and inter-assay was 1·66 and 2·60 %, respectively, at 82·54 mg/ml.
The protein contents of the tissue homogenates were estimated following the standard method of Lowry et al. (Reference Lowry, Rosebrough and Farr13). Intra- and inter-assay %CV were 7·74 and 11·05 %, respectively, at 6·25 mg/ml.
Data and statistical analysis
The data are presented as mean values with their standard deviations or errors. The statistical analysis was carried out using the software SPSS (version 11; SPSS, Inc.). Groups were compared using one-way ANOVA and Tukey's post hoc test was performed for multiple comparisons. Statistical significance was set at P < 0·05.
Results
The safety limit (LD50) of ginger extract in rats
The percentage yield for the extract was 25·3 % (w/w) as mentioned under ‘Preparation of the rhizome extract’. The oral LD50 was estimated to be 4525·5 mg/kg in rats (Table 1), accordingly:
Ginger reduced blood glucose levels in streptozotocin-induced diabetic rats
The basal levels of blood glucose of the rats in all groups before STZ injection were not significantly different. However, 72 h after STZ injection, blood glucose levels were significantly higher in the STZ-induced rats. In contrast, normal control rats (NCNT) remained persistently euglycaemic throughout the course of the study (Table 3).
Table 3 Changes in the blood glucose levels (mmol/l) in streptozotocin-induced (65 mg/kg) diabetic rats over a period of 30 d administration of ginger extracts Zingiber officinale (ZO)
(Mean values, standard deviations and percentages)

NCNT; normal control rats, DCNT; diabetic control rats.
* Mean values were significantly different compared with normal control rats (P < 0·05).
† Mean values were significantly different compared with the diabetic control rats (P < 0·05).
‡ The percentage reduction in blood glucose from the level at 0 d.
Table 3 also shows that ZO extract has dose-dependent antihyperglycaemic effect on the treated diabetic rats. Injection of STZ (65 mg/kg) led to over 4-fold elevation of blood glucose levels (P < 0·05) which were maintained over a period of 4 weeks in the DCNT. On the 7th day of treatment, DCNT showed a significant increase (P < 0·05) in blood glucose level as compared to NCNT. The ZO-treated (100, 300 and 500 mg/kg) diabetic groups had their blood glucose significantly lowered (P < 0·05) when compared to DCNT, with 10·63, 25·71 and 25·14 % reduction, respectively, on the 7th day. Again, there were significant reductions in blood glucose levels between the DCNT and (100, 300 and 500 mg/kg) ZO-treated diabetic groups, with 10·69, 38·05 and 38·01 % falls at the end of the 15th day, respectively, and 34·47, 52·38 and 53·26 % falls at the end of the 21st day, respectively. After the 4th week of daily treatment with the aqueous extract of ZO, ginger led to a fall in blood glucose levels by 48·30, 62·43 and 67·85 % in the 100, 300 and 500 mg/kg-treated groups, respectively. The maximum antihyperglycaemic effect was observed in the 500 mg/kg-treated group, which was not significantly different when compared with NCNT.
Ginger improved body, liver and kidney weights of streptozotocin-induced diabetic rats
Fig. 1 shows the effect of ZO extract (500 mg/kg) on body weight of STZ-induced diabetic rats. The diabetic control group (DCNT) did not gain any significant weight during the 30 d experimental period while NCNT and ZO (500 mg/kg)-treated rats gained significant weights (P < 0·05). However, the increase in body weight in ZO (500 mg/kg)-treated rats was significantly lower than in NCNT (P < 0·05). When liver and kidney weights were expressed as percentages of body weight, there were observable variations. There was a significant increase in kidney weights of DCNT (P < 0·05) compared to NCNT, whereas kidney weights were found to be reduced significantly (P < 0·05) in ZO (500 mg/kg)-treated rats. Liver weight of DCNT showed a significant increase (P < 0·05) compared to NCNT, but this increase in the liver weight was not significantly altered (P>0·05) by ZO (500 mg/kg) treatment as compared to the DCNT group (Table 4).

Fig. 1 Body weights of streptozotocin (STZ)-induced diabetic rats treated with an aqueous extract of ginger over the experimental period. Weights were measured in normal rats (□), STZ-induced diabetic rats (![]() ) and ginger-treated STZ-induced diabetic rats (500 mg/kg Zingiber officinale; ■). The animals were weighed after STZ injection on 0, 15 and 30 d of experimental period. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly decreased compared with normal control at days 15 and 30 (P < 0·05). † Mean value was significantly different between diabetic control and ginger-treated diabetic rats (P < 0·05).
) and ginger-treated STZ-induced diabetic rats (500 mg/kg Zingiber officinale; ■). The animals were weighed after STZ injection on 0, 15 and 30 d of experimental period. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly decreased compared with normal control at days 15 and 30 (P < 0·05). † Mean value was significantly different between diabetic control and ginger-treated diabetic rats (P < 0·05).
Table 4 Effect of 30 d administration of Zingiber officinale (ZO) extract (500 mg/kg) on liver weight (LW) and kidney weight (KW) in streptozotocin (65 mg/kg) diabetic rats
(Mean values with their standard errors)

BW, body weight; NCNT; normal control rats, DCNT; diabetic control rats.
* Mean values were significantly different compared with NCNT (P < 0·05).
† Mean values were significantly different compared with DCNT (P < 0·05).
Ginger lowered kidneys' glycogen content with no effect on liver and skeletal muscle
Kidney glycogen content increased by over 10-fold while liver and skeletal muscle glycogen content significantly (P < 0·05) decreased by 75 and 53 % in DCNT v. NCNT. There was no significant difference in the glycogen content of liver and muscle in the ginger-treated diabetic group, although kidneys showed a significant decrease in glycogen content due to ginger treatment (Fig. 2).
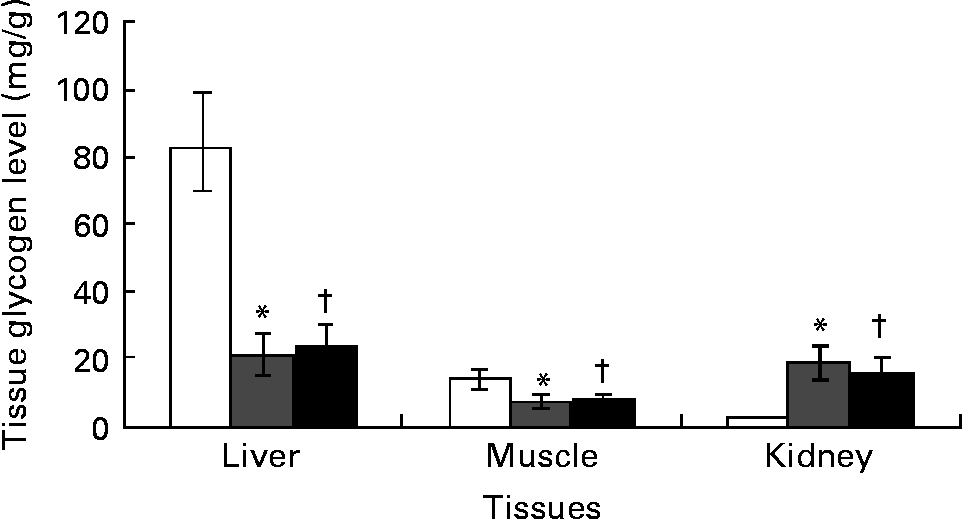
Fig. 2 Tissues’ glycogen was estimated in streptozotocin (STZ)-induced diabetic rats treated with an aqueous extract of ginger over the experimental period. Tissues’ glycogen in normal rats (□), STZ-induced diabetic rats (![]() ) and ginger-treated STZ-induced diabetic rats (500 mg/kg Zingiber officinale; ■). Tissues’ glycogen was estimated at the end of the experimental period. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different compared with normal control (P < 0·05). † Mean value was significantly decreased compared with diabetic control (P < 0·05).
) and ginger-treated STZ-induced diabetic rats (500 mg/kg Zingiber officinale; ■). Tissues’ glycogen was estimated at the end of the experimental period. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different compared with normal control (P < 0·05). † Mean value was significantly decreased compared with diabetic control (P < 0·05).
Ginger increased the activities of hepatic glycolytic enzymes in streptozotocin-induced diabetic rats
The DCNT showed significant decrease by 94, 53 and 61 % in the activities of glucokinase, phosphofructokinase and PK (Fig. 3) compared to NCNT values; however, ZO (500 mg/kg) significantly (P < 0·05) increased these enzymes' activities when compared to DCNT.
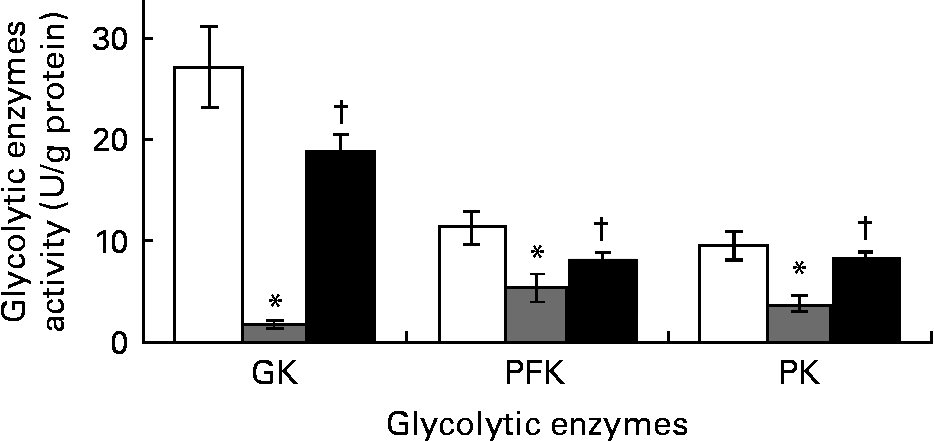
Fig. 3 Hepatic glycolytic enzymes’ activity of streptozotocin (STZ)-induced diabetic rats treated with an aqueous extract of ginger over the experimental period was estimated. Enzymes’ activity was measured in normal rats (□), STZ-induced diabetic rats (![]() ) and ginger-treated STZ-induced diabetic rats (500 mg/kg Zingiber officinale; ■). Enzymes’ activity was analysed at the end of the experimental period. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly decreased compared with normal control (P < 0·05). † Mean value was significantly increased compared with diabetic control (P < 0·05). GK, glucokinase; PFK, phosphofructokinase; PK, pyruvate kinase.
) and ginger-treated STZ-induced diabetic rats (500 mg/kg Zingiber officinale; ■). Enzymes’ activity was analysed at the end of the experimental period. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly decreased compared with normal control (P < 0·05). † Mean value was significantly increased compared with diabetic control (P < 0·05). GK, glucokinase; PFK, phosphofructokinase; PK, pyruvate kinase.
Discussion
Safety limit (LD50) of ginger extract in rats
The high yield of the extract shows that optimal extraction of the constituents requires the use of polar solvents, in consonance with folkloric use of the aqueous infusions. The oral LD50 value of 4525·5 mg/kg in rats, obtained for the aqueous ZO extract falls within the practically non-toxic range(Reference Loomis14). The anti-hyperglycaemic effect observed for all doses (100, 300 and 500 mg/kg) tested was far below the minimum lethal dose (4525·5 mg/kg). The acute toxicity test method by Lorke(Reference Lorke8) utilises a lower number of rats, usually thirteen, and provides a 24 h LD50 value, which is adequate for most practical purposes. With the high margin of safety exhibited by the extract, higher doses can be used to achieve more pronounced anti-hyperglycaemic effect without sacrificing safety. This explains the widespread use of the plant extract in folklore medicine. Based on the results obtained, doses of 100, 300 and 500 mg/kg were selected for the anti-hyperglycaemic screening.
Anti-hyperglycaemic effect of ginger in streptozotocin-induced diabetic rats
Diabetes mellitus is characterised by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both. Strict control of the blood glucose level is considered to be essential in order to delay or prevent the development of diabetic complications(Reference White, Baxter, White and Baxter15). Overt symptoms of diabetes mellitus include hyperglycaemia, increased water intake and polyuria(Reference White, Baxter, White and Baxter15). Chronic complications can involve the kidneys (nephropathy), eyes, nervous system and cardiovascular system(Reference Koda-Kimble, Carlisle, Young, Koda-Kimble, Kradjan and Guglielmo16).
The anti-hyperglycaemic properties of ZO extract were investigated in the present study. STZ was selected as the diabetogenic agent because of its ability to selectively destroy the β-cells of the islet in the pancreas without direct injury to other tissues. A single intraperitoneal injection of 65 mg/kg of STZ in rats leads to hyperglycaemia, 72 h after the injection. The results indicated that the extract possessed significant anti-hyperglycaemic activities. The results also showed a dose-dependent decrease in the plasma glucose concentration from 16·39 (SD 1·97 ) to 5·27 (SD 0·67) mmol/l (67·85 % reduction), which corresponded with the maximum effect of ZO (500 mg/kg) on the 30th day.
Ginger contains potential bioactive compounds; mostly of volatile oils(Reference Evans17, Reference Van Wyk and Wink18). [6]-Gingerol is the most abundant bioactive pungent compound which has been consistently implicated as the antihyperglycaemic agent in ginger(Reference Johji, Michihiko and Rong19–Reference Singh, Akanksha and Singh21). Ginger extract with a higher concentration of [6]-gingerol produces significant reduction in fructose-induced hyperglycaemia and hyperinsulinaemia when compared to another ginger extract with comparably lower concentration of [6]-gingerol(Reference Kadnur and Goyal22, Reference Goyal and Kadnur23).
Earlier studies of the hypoglycaemic properties of ginger in human subjects and animals have produced variable results. The administration of an ethanol extract of ginger (100 or 300 mg/kg) to normal rabbits showed potential hypoglycaemic activity (51 % decrease in serum glucose), 2 h after administration(Reference Mascolo, Jain and Jain24). In contrast, in another study, non-diabetic patients with coronary artery disease showed no decrease in their blood lipid or sugar levels when treated with a daily dose of 4 g powdered ginger for a period of 3 months(Reference Bordia, Verma and Srivastava25). The variability of the results in these studies may be due to the use of different ginger preparations, as it is noted that most of the studies in which anti-hyperglycaemic or hypoglycaemic effects of ginger were reported used ginger juice or water extract of ginger. However, recent studies have consistently reported the hypoglycaemic and antihyperglycaemic effects of ginger. Akhani et al. (Reference Akhani, Vishwakarma and Goyal7) have reported that ginger juice exhibits hypoglycaemic activity in both normal and STZ-induced diabetic rats. Al-Amin et al. (Reference Al-Amin, Thomson and Al-Qattan4) also found that ginger possesses hypoglycaemic, hypocholesterolaemic and hypolipidaemic potential(Reference Al-Amin, Thomson and Al-Qattan4). They also showed that raw ginger is effective in reversing the diabetic proteinuria observed in diabetic rats. More recently, Asha et al. (Reference Asha, Krishnamurthy and Devaru3) and Jafri et al. (Reference Jafri, Abass and Qasim26) reported the antihyperglycaemic and hypoglycaemic activities of ginger in alloxan-induced diabetic rats. Clearly, the results in the present study confirm the observations in the recent studies pertaining to the glucose-lowering effect of ginger in blood. No previous studies have reported changes in tissue glycogen and the selected glycolytic enzymes involved in carbohydrate metabolism, as a result of ginger administration.
Ginger ameliorates body, liver and kidney weights
STZ-induced diabetes is characterised by severe loss in body weight(Reference Chen and Ianuzzo27) and this might be due to loss or degradation of structural proteins, as the structural proteins are known to contribute to body weight. In our study, during the experimental period, NCNT showed an approximately 20 % gain in body weight. On the other hand, DCNT showed no significant gain in body weight over the same time period. ZO-treated rats showed higher and significant gain in body weight in comparison to DCNT. This gain in weight by ZO-treated rats showed in addition that ginger is effective in reversing the body weight loss observed in diabetic rats, which was earlier reported(Reference Asha, Krishnamurthy and Devaru3, Reference Chen and Ianuzzo27, Reference Raju, Gupta and Rao28). STZ-induced diabetic animals tend to show renal hypertrophy. The entry of glucose in the renal tissue is not dependent on the action of insulin; therefore, in the event of hyperglycaemia, there is an increase in the entry of glucose(Reference Belfiore, Rabuazzo and Iannello29). This has been hypothesised to cause increased intra-renal glycogen deposition, which leads to glycosylation of basement membrane collagen in the kidney(Reference Anderson and Stowring30). This is reflected in the present finding as the diabetic rats showed approximately 20 % increase in two-kidney v. body weight ratio in comparison to NCNT as well as more than 10-fold increase in renal glycogen content. Rise in renal weight as well as renal glycogen content has been reported previously(Reference Anderson and Stowring30) though in previous studies(Reference Rasch31, Reference Nielsen, Gronbaek and Osterby32) the degree of renal hypertrophy was much higher in comparison with the present study. The reason for this variation in the present and the past results can be attributed to the difference in the duration of the studies, i.e. 1 month v. 6 and 2 months, respectively. ZO significantly lowered this renal hypertrophy (Table 4). Diabetic rats had significantly higher liver weight/100 g body weight and this alteration in the diabetics was not changed by treatment with ZO. The literature shows conflicting reports on the effect of diabetes on liver weight. While some studies report an increase in liver weight in animals(Reference Chen and Ianuzzo27, Reference Murphy and Anderson33, Reference Sadique, Chandra and Thenmozhi34) as well as in human subjects(Reference Van Lancker and Lancker35), a decrease and no change were also reported in 200 mg/kg alloxan-induced diabetes studied for 21 d(Reference Raju, Gupta and Rao28, Reference Gupta, Kesari and Murthy36). The exact reasons of hepatic hypertrophy are not known. However, increased mobilisation and deposition of fat have been proposed to be the cause(Reference Van Lancker and Lancker35).
Ginger has no significant effect on liver and muscle but kidney glycogen content
The liver plays an important role in buffering postprandial hyperglycaemia and is involved in the synthesis of glycogen. Glycogen is the primary intracellular storable form of glucose and its level in various tissues, especially skeletal muscle, is a direct reflection of insulin activity, as insulin promotes intracellular glycogen deposition by stimulating glycogen synthase and inhibiting glycogen phosphorylase. Since STZ causes selective destruction of β-cells of islets of Langerhans, resulting in marked decrease in insulin levels, it is rational that glycogen levels in insulin-dependent tissues (skeletal muscle and liver) decrease as they depend on insulin for the influx of glucose(Reference Whitton and Hems37–Reference Bishop39). Results showed that liver and skeletal glycogen content decreased drastically in DCNT by approximately three-quarters of their basal levels. This has also been reported earlier(Reference Hikino, Kobayashi and Suzuki40). ZO showed a trend towards an increase in glycogen content but could not significantly increase the glycogen content in muscle and liver.
Ginger increased the activities of hepatic glycolytic enzymes in streptozotocin-induced diabetic rats
In the present study, the activities of glycolytic enzymes in the liver were significantly decreased (P < 0·05) in the DCNT. This confirms the previous findings(Reference Raju, Gupta and Rao28, Reference Grover, Vats and Rathi41, Reference Rathi, Grover and Vats42) that relative deficiency of insulin in the STZ model of type 1 diabetes causes suppression of glucokinase, phosphofructokinase and PK activities. These hepatic glycolytic enzymes are the regulatory factors which drive the metabolic degradation of glucose to form pyruvate. The high-energy compounds ATP and NADH are formed from the free energy released in this process. Maximum suppression was observed in hepatic glucokinase activity, followed by hepatic PK and phosphofructokinase activities. Administration of ZO extract increased significantly the activity of all the three enzymes towards NCNT, suggesting that the anti-hyperglycaemic action observed was as a result of increased glucose utilisation at the liver as well as skeletal muscle. However, it is not possible to deduce from the present findings that the increase in glycolytic enzymatic activity seen in the ZO-treated rats occurred secondary to the ZO-mediated release of insulin or whether a component of ZO had insulinomimetic action. Since STZ diabetes is an insulin-deficient model, the likelihood of insulinomimetic effect seems more possible.
Conclusion
We have demonstrated that the folk medicinal plant ginger is practically non-toxic in rats, with the high margin of safety exhibited by the extract in the present study. The findings also suggest that the administration of ZO rhizome extract to diabetic rats gives good control over tissue glycogen content by enhancing the peripheral utilisation of glucose and correcting the impaired liver and kidney glycolysis and by limiting its gluconeogenic formation similar to insulin. Therefore, raw ginger has significant potential as a phytomedicine in the treatment of diabetes.
Acknowledgements
The present work was made possible through a research grant from the Research Endowment Fund, Research Management Centre (RMC), IIUM. The authors would like to thank Rafidah Hanim Mokhtar for the proof reading. There are no potential conflicts of interest in relation to this paper. N. B. A. carried out the majority of the biochemical analysis, designed the experiment and contributed to the writing. M. M. C. and N. N. W. contributed to the supervision and drafting of the manuscript. R. Z. and M. T. R. contributed with technical support, scientific advice and revised the manuscript.









