Previous studies have demonstrated the critical roles of the high viscosity and molecular weight (MW) of β-glucan in its cholesterol-lowering effect( Reference Wolever, Tosh and Gibbs 1 , Reference Wang, Harding and Eck 2 ). Yet, to date the precise underlying mechanisms responsible for the action of β-glucan reducing blood cholesterol have not been well elucidated.
Cholesterol in humans is mainly determined by cholesterol de novo synthesis, dietary cholesterol intake and conversion to bile acid( Reference van der Velde, Vrins and van den Oever 3 ). It has been proposed that β-glucan may reduce cholesterol via impacting any three of these pathways. β-Glucan is not absorbed by the small intestine, however, the physicochemical properties of β-glucan may alter the luminal environment and lead to increased transit time and thickness of the unstirred water layer (UWL), consequently reducing cholesterol absorption( Reference Eastwood and Morris 4 ). When β-glucan enters the large intestine, gut microbiota present in the colon are responsible for its degradation( Reference Chassard and Lacroix 5 , Reference Cantarel, Lombard and Henrissat 6 ). By-products resulting from β-glucan fermentation, SCFA, might then affect cholesterol synthesis( Reference Illman and Topping 7 – Reference Gunness and Gidley 9 ). In addition, cholesterol is the substrate for bile acid synthesis in the liver, thus β-glucan may affect cholesterol metabolism via interfering bile acid enterohepatic circulation. Approximately 95 % of the bile acids are reabsorbed in the terminal ileum( Reference Martinez-Augustin and Sanchez de Medina 10 , Reference Charlton-Menys and Durrington 11 ) while a small amount of bile acids are excreted with the faeces( Reference Martinez-Augustin and Sanchez de Medina 10 ). The conversion of bile acid from cholesterol occurs at a rate that precisely corresponds to the loss of bile acids within the faeces( Reference Cohen 12 ). β-Glucan has been thought to work as a bile acid sequestrant reducing cholesterol by decreasing bile acid reabsorption and further up-regulating bile acid synthesis and thus consuming more cholesterol( Reference Chen and Huang 13 , Reference Marlett, Hosig and Vollendorf 14 ). The interruption of bile acid enterohepatic circulation resulting from β-glucan consumption has been observed in ileostomy patients( Reference Zhang, Hallmans and Andersson 15 ), yet, evidence from healthy participants in supporting this proposed mechanism is limited.
Cholesterol 7-α hydroxylase (CYP7A1) is the rate-limiting enzyme in the classical bile acid synthetic pathway in humans( Reference Sauter, Berr and Beuers 16 ). Its activity can be indicated by the circulating concentration of 7α-hydroxy-4-cholesten-3-one (7α-HC), a bile acid intermediate of the classical synthesis pathway( Reference Sauter, Berr and Beuers 16 , Reference Axelson, Bjorkhem and Reihner 17 ). Genetic variation of CYP7A1 SNP rs3808607 has been associated with different responses to high-viscosity β-glucan in lowering cholesterol levels in our previous study, where G allele carriers of CYP7A1 SNP rs3808607 showed more pronounced responses in reducing total cholesterol (TC) than T/T allele carriers( Reference Wang, Harding and Eck 2 ). The biological difference between G and T alleles in regulating bile acids and cholesterol metabolism is not entirely clear. Results from De Castro-Oros et al.’s( Reference De Castro-Oros, Pampin and Cofan 18 ) study suggested that the T>G polymorphism might regulate cholesterol catabolism differently due to their varied ability in modulating transcriptional activity of CYP7A gene. G allele was speculated to be more responsive to dietary intervention in elevating the bile acid synthesis( Reference De Castro-Oros, Pampin and Cofan 18 ). However, evidence from different phenotypes, in terms of bile acid synthesis at physiological level, is needed to confirm this speculation.
Collectively, our hypotheses are: (1) β-glucan lowers blood cholesterol levels via suppressing cholesterol absorption, decreasing cholesterol biosynthesis and /or interrupting the enterohepatic circulation of bile acids; and (2) G allele carriers of rs3808607 have a higher level of bile acid synthesis in response to β-glucan intervention. Accordingly, the primary objective of this study was to assess whether lowered serum cholesterol levels resulting from β-glucan consumption are due to inhibition of cholesterol absorption, cholesterol synthesis or increased bile acid synthesis. The additional objective was to assess the phenotype of CYP7A1 SNP rs3808607 in response to β-glucan intervention at a physiological level.
Methods
Diet and clinical trial procedure
A randomised, single-blinded, diet-controlled, cross-over trial was conducted at the Clinical Nutrition Research Unit at the Richardson Centre for Functional Foods and Nutraceuticals (RCFFN), University of Manitoba, Winnipeg. Participants with serum cholesterol between 5·0 and 8·0 mmol/l, LDL-cholesterol between 2·7 and 5·0 mmol/l (n 30) were randomly assigned to receive barley diets containing 3 g high MW (HMW, 13·5×105 g/mol), 5 g low MW (LMW, 2·9×105 g/mol), 3 gLMW (2·9×105 g/mol) β-glucan or a control diet, each for 5 weeks. The washout period between the study phases was ≥4 weeks. Breakfast foods in the format of crepes, tortillas, porridge and chips were formulated from barley to contain β-glucan varying in MW and dose or formulated from wheat and rice to substitute barley ingredients as a control. Lunch and dinner were provided to meet the energy needs of each subject. Overall, participants received study diets containing approximately 30 % of energy as fat, 55 % as carbohydrate and 15 % as protein but with different levels of soluble fibre. Details of nutrition information for the study diets have been reported in a previous paper( Reference Wang, Harding and Eck 2 ) and are shown in Table 1. Participants consumed breakfast at the Clinical Nutrition Research Unit of RCFFN from Monday to Friday. Lunch and dinner for weekdays were picked up by participants and consumed at home. Weekend meals including breakfast were delivered to participant’ home address. This study was conducted according to the principles expressed in the Declaration of Helsinki and all research procedures were approved by the University of Manitoba’s Biomedical Research Ethics Board (Ethic reference no. B2010:057).
Table 1 Nutrient content of the experimental diets
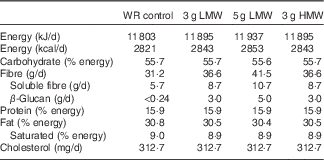
WR, wheat and rice; LMW, low-molecular weight; HMW, high-molecular weight; fibre, total dietary fibre.
Serum lipid and genotype determination
Methods for determining blood lipid concentrations and the genotype of CYP7A1 SNP rs3808607 have been described in the previous study( Reference Wang, Harding and Eck 2 ). In brief, 12 h fasted blood samples were collected on days 1, 2, 34 and 35. Blood samples were centrifuged at 2675 g for 20 min at 4°C, separated into serum, plasma, buffy coat and erythrocytes, and stored at −80°C until analysis. An average of values of days 34 and 35 were used for endpoint analysis for blood lipids. Serum TC, HDL-cholesterol and TAG were measured using the automated enzymatic methods on a Vitros-350 chemistry analyzer (Ortho Clinical Diagnostics). Serum LDL-cholesterol was estimated using the Friedewald equation( Reference Friedewald, Levy and Fredrickson 19 ). DNA used for SNP determination were extracted from the buffy coat of heparinised blood samples following the instructions of the Dneasy® Blood & Tissue kit (QIAGEN). SNPs of rs3808607 for CYP7A1 were genotyped using TaqMan® GTXpress™ Master Mix (Applied Biosystems, Inc.) with allele-specific probes on the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Inc.).
Cholesterol absorption
The cholesterol absorption in response to β-glucan intervention was determined by stable isotope method( Reference Kassis and Jones 20 , Reference Rideout, Chan and Harding 21 ). On day 30 of each study phase, participants ingested approximately 5 g of margarine with 75 mg of [3, 4-13C] cholesterol (99 % atom percentage excess; Cambridge Isotope Laboratories) spread on half of an English muffin. Fasted blood samples were obtained before oral administration of isotope tracers (0 h) and also in the following 96 h on day 31 (24 h), day 32 (48 h), day 33 (72 h), and day 34 (96 h). Free cholesterol was extracted from erythrocytes by modified Folch procedure( Reference Folch, Lees and Sloane Stanley 22 ). The isotopic enrichment of [3,4- 13C] cholesterol was determined using GC isotope ratio MS (GC-IRMS; Thermo Finnigan) where samples were run through a GC unit, a combustion reactor and a mass spectrometer( Reference Kassis and Jones 20 , Reference Rideout, Chan and Harding 21 ). The cholesterol extracts were combusted to13C-enriched CO 2 and the combusted CO2 gas was analysed for 13C enrichment against the international standard Vienna Pee Dee Belemnite limestone. The AUC of 13C enrichment from 0 to 96 h was calculated for determining cholesterol absorption.
Determination of cholesterol biosynthesis
The fractional cholesterol synthesis rate in response to the intervention diets was determined by stable isotope method using deuterated water( Reference Jones, Leitch and Li 23 ). Specifically, cholesterol synthesis was assessed by measuring the rate of 2H derived from deuterium oxide (D2O) within the body water pool into the erythrocyte cholesterol pool( Reference Jones, Leitch and Li 23 ). On day 34 of each study phase, participants ingested 0·7 g of D2O/kg estimated body water (60 % of body weight). Fasting blood samples were collected before D2O oral administration on day 34 (0 h) and day 35 after D2O ingestion (24 h). Blood samples were centrifuged and separated into plasma, buffy coat and erythrocytes, and stored at −80°C until analysis. Total lipids were extracted from erythrocytes by a modified Folch procedure( Reference Folch, Lees and Sloane Stanley 22 ). The lipid extracts were separated using GC and isolated cholesterol was submitted into a pyrolysis reactor to release H 2 gas. Plasma water samples were run through a high temperature conversion elemental analyser. 2H enrichments for both erythrocytes and plasma water were measured by IRMS relative to the reference gas. Normalisation to Vienna standard mean ocean water was performed using a regression equation between the online and offline method with data from the offline method expressed relative to Vienna standard mean ocean water (V-SMOW). The fractional rate of synthesis (FSR) for cholesterol was calculated using the following equation:
where ΔD-Cholesterol is the difference in 2H enrichment between 0 and 24 h for cholesterol; ΔD−PW the difference in 2H enrichment between 0h and 24 h for plasma water; and 0·478 the ratio of labelled H atoms replaced by 2H during in vivo biosynthesis( Reference Kassis and Jones 20 ).
Determination of bile acid biosynthesis
Blood samples for determining bile acid biosynthesis were collected on days 34 and 35. The mean of 7α-HC levels on days 34 and 35 was used for indicating bile acid synthesis level. Bile acid biosynthesis was determined by measuring the serum concentration of 7α-HC using the Ultra-Performance Liquid Chromatography (ACQUITY UPLC System; Waters) coupled with a tandem mass spectrometer (Quattro microTM API; Waters). 7α-HC was extracted by C18-E solid phase extraction (SPE) (Phenomenex®) mounted on a pump (KNF lab pump; KNF Neuberger) connected SPE processor (Agilent Technologies) following the method modified from Burkard et al.( Reference Burkard, von Eckardstein and Rentsch 24 ). 7α-hydroxy-4-cholesten-3-one-d7 (Medical Isotopes. Inc.) was used as an internal standard. In brief, SPE cartridges were pre-conditioned by 2×2 ml methanol, 2×2 ml water and 2×2 ml 100 mm ammonium carbonate buffer pH 9·3. Serum (750 μl) mixed with ammonium carbonate buffer (1:1 v: v) and 150 μl of internal standard (40 ng/ml) were applied to the activated SPE cartridge. Under a vacuum, the speed of the mixture passing the cartridge was controlled within 1 drop/s passing through the cartridge. Subsequently, the cartridges were washed with 2×2 ml of water and dried under N2. Bile acids were desorbed with 3 ml of methanol with the speed of 1 drop/s for passing the cartridge. The eluted substances were dried under N2 and dissolved in 150 μl of methanol before injecting into the UPLC. A reserved phase C18 column (Kinetex™ 1·7 µm XB-C18 100 Å, LC Column 100×2·1 mm; Phenomenex®) with mobile phase water with 0·1 % formic acid (A) and methanol (B) was used for the separation. The ions used for the tracing of unlabelled 7α-HC were m/z 401>177 and the ions for tracing the 7α-hydroxy-4-cholesten-3-one-d7 were 407>177. The mass spectrometer was operated in the positive mode. Corona current was 3 µA, cone voltage 25 V, source temperature 120°C, cone gas flow 62 litres/h, desolvation gas flow 400 litres/h, and collision energy 23 eV. The quantitation was performed with use of a standard curve, plotting the ratio between the response of 7α-HC in the m/z 177 tracing generated from the mother ion m/z 401 (unlabelled) and the response in the m/z 177 tracing generated from the mother ion m/z 407 (labelled).
Statistical analyses
Cholesterol absorption indicated by AUC of 13C enrichment, cholesterol synthesis indicated by FSR and bile acid synthesis indicated by serum concentration of 7α-HC were analysed using linear mixed-models (PROC MIXED, version 9.2; SAS Institute Inc.). Log transformations were used for data that were not normally distributed (7α-HC concentrations). For detecting the diet and genotype effects, diet, genotype and genotype×diet were modelled as fixed factors and participants were modelled as the random factor in the mixed-model. Correlation between repeated measures of these individuals was modelled using the first-order autoregressive (AR (1)) option in the PROC MIXED procedure. Fisher’s least significant difference tests were used for multiple comparisons. In the genotype sub-group analysis, each genotype group was analysed independently with diet as a fixed factor. Statistical analyses for blood lipids were described in the previous study( Reference Wang, Harding and Eck 2 ).
Linear regression of 7α-HC concentration on log(viscosity) was performed using SAS (PROCREG, version 9.2; SAS Institute Inc.) and plotted with GraphPad Prism (GraphPad Prism version 6.00 for Windows; GraphPad Software). The non-parametric correlation was performed by using Spearman’s rank correlation (JMP®, version 10; SAS Institute Inc.). A value of P<0·05 was considered to be significant.
Results
Drop-out rate and genotype distribution
In all, forty-five participants were recruited for the study. Eight participants dropped out from the study with reasons unrelated to the study; seven participants were excluded from the study for reasons including low tolerance to the study diet, missing the study diet, later discovery of ineligibility (e.g. met inclusion criteria at screening but did not meet criteria when day 1 blood samples were analysed). A participant flow chart and baseline characteristics of participants were described in the previous published study( Reference Wang, Harding and Eck 2 ). In the thirty participants who completed four phases of the study, eleven were identified to be TT allele carriers, eleven were GT and eight were GG allele carries of rs3808607( Reference Wang, Harding and Eck 2 ). The distribution of genoptype of CYP7A1 SNP rs3808607 among the participants in this study is similar to the minor genotype frequency reported in the general population( Reference Flicek, Ahmed and Amode 25 ).
Changes in serum cholesterol levels and body weight
Changes in blood lipid levels have been reported in the previous study( Reference Wang, Harding and Eck 2 ). For the convenience of the readers, these data are included in Table 2. Consumption of 3 g HMW/d β-glucan for 5 weeks lowered TC compared with the control (P=0·029) but the LMW β-glucan, at either 3 or 5 g/d, did not change serum cholesterol concentrations (Table 2). In addition, significant genotype-by-diet effect was observed in changing serum TC and LDL-cholesterol levels for 3 gHMW β-glucan (Table 2). Consumption of β-glucan did not change serum HDL-cholesterol or TAG levels (Table 2). Changes in body weight and waist circumference were not statistically different among treatments (data not shown).
Table 2 Changes in serum lipids, 13C-labelled cholesterol enrichment in erythrocytes, fractional synthesis rate (FSR) for cholesterol and serum 7α-hydroxy-4-cholesten-3-one (7α-HC) in response to β-glucan intervention for 5 weeks in mildly hypercholesterolemic adults
(Least squares means (LSM) with their standard errors)
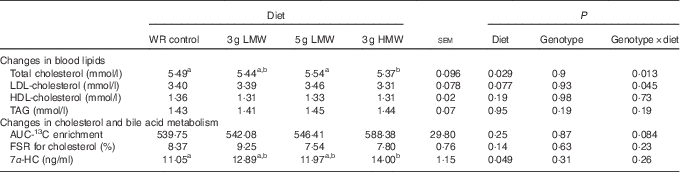
WR, wheat and rice; LMW, low-molecular weight; HMW, high-molecular weight.
a,b LSM in the same row with unlike superscript letters are significantly different among treatments (Fisher’s least significant difference test for multiple comparison, P<0·05).
When the participants were sub-grouped based on their genotypes of CYP7A1 SNP rs3808607, consumption of 3 g/d HMW β-glucan resulted in lower TC levels in G allele carriers (G/T and G/G) and lower LDL-cholesterol in homozygous G allele carriers (G/G) (Table 3). T/T carriers did not respond to β-glucan in reducing serum TC or LDL-cholesterol levels. Serum HDL-cholesterol and TAG levels remained unchanged in all genotype groups.
Table 3 Changes in serum lipids, 13C-labelled cholesterol enrichment in erythrocytes, fractional synthesis rate (FSR) for cholesterol and serum 7α-hydroxy-4-cholesten-3-one (7α-HC) in response to β-glucan intervention for 5 weeks in three genotype groups of cholesterol 7α hydroxylase SNP rs3808607
(Least squares means (LSM) with their standard errors)
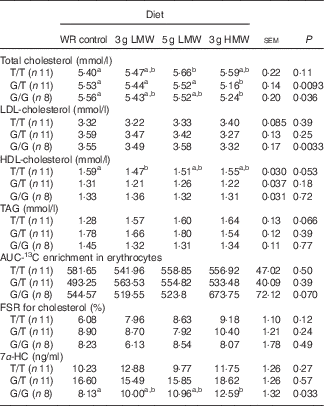
WR, wheat and rice; LMW, low-molecular weight; HMW, high-molecular weight.
a,b LSM in the same row with unlike superscript letters are significantly different among treatments (Fisher’s least significant difference test for multiple comparison, P<0·05).
Changes in cholesterol absorption, cholesterol synthesis and serum 7α-hydroxy-4-cholesten-3-one
Cholesterol absorption was not affected by β-glucan consumption (P=0·25). The AUC of 96 h enrichment of 13C were 539·75, 542·08, 546·41 and 588·38 for the control, 3 g LMW, 5 g LMW and 3 gHMW β-glucan, respectively (Table 2). The FSR for cholesterol was also not affected by β-glucan ingestion (P=0·14). FSR resulting from the experimental diets assessed in a 24-h time window were 8·37, 9·25, 7·54 and 7·80 for control, 3 g LMW, 5 gLMW and 3 g HMW β-glucan, respectively (Table 2). Consumption of 3 g HMW β-glucan resulted in higher 7α-HC levels compared with control (14·00 v. 11·05 ng/ml, P=0·049, Table 2). The 7α-HC levels were not affected by LMW β-glucan, which is consistent with the results of cholesterol changes (Table 2).
In the sub-group analysis, cholesterol absorption and FSR for cholesterol remained unchanged for all genotypes of CYP7A1 SNP rs3808607. Although genotype×diet effect was not statistically significant for 7α-HC concentrations (Table 2), the three genotypes of CYP7A1 showed inconsistent results of 7α-HC concentrations (Table 3). G/G group responded to 3 g /d HMW β-glucan in increasing 7α-HC concentrations compared with control (P=0·033); yet serum 7α-HC levels were not affected by β-glucan consumption in G/T and T/T groups. This observation is consistent with the changes in circulating cholesterol levels that individuals carrying homozygous G allels responded to 3 g HMWβ-glucan in reducing TC and LDL-cholesterol (Table 3).
Linear relationship between serum 7α-hydroxy-4-cholesten-3-one concentrations and viscosity of β-glucan
Viscosity of β-glucan is believed to be the determinant for its cholesterol-lowering effect. Our previous study has shown a linear relationship between viscosity of β-glucan and cholesterol changes in participants that responded to β-glucan in reducing their circulating cholesterol levels( Reference Wang, Harding and Eck 2 ). In this study, results consistently support the critical role of viscosity of β-glucan in mediating cholesterol metabolism. A linear relationship between viscosity of β-glucan and 7α-HC concentrations was observed in homozygous G allele carriers (P=0·025, r 2 0·95, Fig. 1).
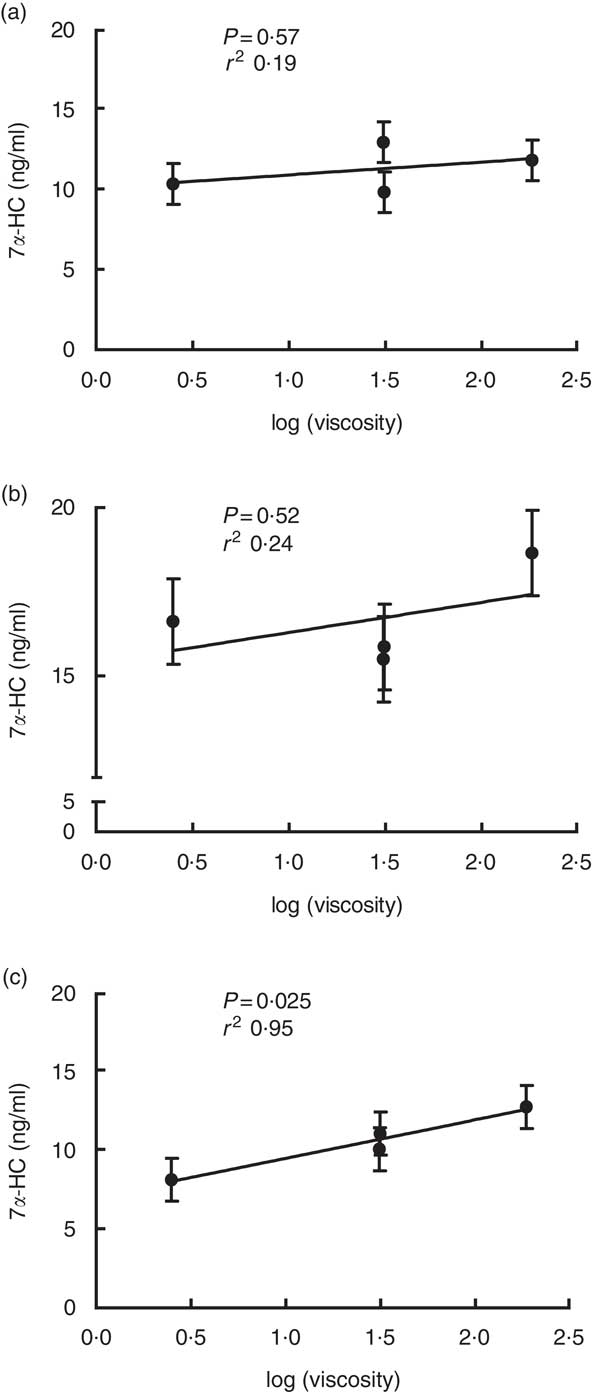
Fig. 1 Linear relationship between 7α-hydroxy-4-cholesten-3-one (7 α-HC) concentration and log (viscosity) for the three genotype carriers of cholesterol 7α hydroxylase (CYP7A1) SNP rs3808607. (a) T/T carriers; (b) G/T carriers; (c) G/G carriers. Values are least squares means with their standard errors for the four treatments following the order of wheat and rice control, 3 g low-molecular weight (LMW), 5 g LMW and 3 g high-molecular weight from left to right.
Correlation between cholesterol and bile acid kinetic parameters
In the non-parametric correlation (Table 4), AUC and FSR were negatively related with each other, although the correlation strength is weak (P=0·0008, Spearman’s ρ=−0·30). Serum 7α-HC concentrations were not related to either AUC of 13C enrichment in erythrocytes or FSR for cholesterol.
Table 4 Non-parametric correlation between cholesterol absorption, synthesis and bile acid synthesis
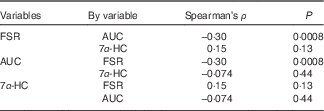
Spearman’s ρ, Spearman’s correlation coefficient; FSR, fractional rate of synthesis for cholesterol; 7α-HC, 7α-hydroxy-4-cholesten-3-one.
Discussion
Consistent with the previous findings that high-viscosity β-glucan with HMW is effective in reducing circulating cholesterol levels( Reference Wang, Harding and Eck 2 ), we found that consumption of 3 g/d HMW β-glucan resulted in higher serum 7α-HC levels compared with the control, but not either of the LMW β-glucan diets. However, cholesterol absorption or cholesterol synthesis was not changed by any of the β-glucan diets. These results indicate the importance of the physiochemical characteristics of β-glucan in its cholesterol-lowering action and suggest that increasing bile acid synthesis rather than inhibition of cholesterol synthesis or absorption is the mechanism responsible for the cholesterol-lowering effect of β-glucan.
Inhibition of cholesterol absorption has been speculated widely as one of the mechanisms responsible for the cholesterol-lowering effect of β-glucan( Reference Chen and Huang 13 ). Hypothetically, with the viscous property, β-glucan might impact lipid absorption by altering the luminal environment such as slowing the transit time and increasing the thickness of UWL, or affecting activities of digestive enzymes and the formation of micelles( Reference Eastwood and Morris 4 , Reference Rideout, Harding and Jones 26 ). Naumann et al. reported that sitosterol as a cholesterol absorption marker was decreased in a fruit drink containing β-glucan( Reference Naumann, van Rees and Onning 27 ); however, in an ileostomy study, Lia et al.( Reference Lia, Andersson and Mekki 28 ) found no changes in the absorption of dietary cholesterol after an oat bran meal containing β-glucan using a radioisotope method with [β-4-14C]-sitosterol and [1α, 2α-n-3H]-cholesterol as tracers. This discrepancy may be due to the different methodologies used for measuring cholesterol absorption. As an inhibitor of intestinal cholesterol uptake, the use of sitosterol may lead to an underestimation of the actual capacity of absorption( Reference Mackay and Jones 29 ). In agreement with Lia et al.’s finding, our study using a single isotope method showed that cholesterol absorption after consuming a β-glucan diet did not differ from the control diet (P=0·25). Therefore, our results provide more evidence suggesting that the cholesterol-lowering effect of β-glucan is not mediated through the inhibition of cholesterol absorption but through alternative mechanisms.
The 2H incorporation method utilised in this study is a reliable means to assess cholesterogenesis( Reference Jones, Ausman and Croll 30 ). Ellegard et al.( Reference Ellegard and Andersson 31 ) reported in ileostomy patients that within 24 h consumption of native β-glucan increased bile acid excretion and also increased serum lathosterol concentration, a marker for cholesterol synthesis. Cholesterol homoeostasis is regulated by both cholesterol input and output. The loss of bile acid in excretion and demand for cholesterol to replenish the bile acid pool can possibly up-regulate the synthesis of cholesterol( Reference Chen and Huang 13 ). However, in our study, changes in cholesterol FSR did not reach statistical significance (P=0·14). Despite the discrepancy in increasing and not changing cholesterol synthesis, results of both Ellegard et al.’s and our present study do not support the hypothesis that consumption of β-glucan inhibits the cholesterol synthesis.
Our results demonstrated that consumption of HMW β-glucan is able to increase bile acid synthesis. Moreover, our data also revealed that the high viscosity resulting from HMW of β-glucan is the factor that drove this mechanism: serum 7α-HC concentrations showed a linear relationship with the viscosity of the treatment diets (Fig. 1). After ingestion, high-viscosity β-glucan enters the lumen of the small intestine, where it may act similarly as a bile acid sequestrating agent to interact with bile acids and lead to bile acid being entrapped instead of being reabsorbed. The expected consequences of these actions would increase faecal bile acid loss and enhance bile acid synthesis from cholesterol to replenish the bile acid pool( Reference Chen and Huang 13 ). The interruption of enterohepatic circulation of bile acid caused by β-glucan has been reported in ileostomy patients( Reference Lia, Andersson and Mekki 28 , Reference Ellegard and Andersson 31 – Reference Lia, Hallmans and Sandberg 34 ). In a short-term interventional cross-over study, nine participants with conventional ileostomies received a diet containing either native or hydrolysed β-glucan (11·6 g), each for 3 d. The native β-glucan consumption resulted in 40 % more ileal bile acid excretion compared with consuming the hydrolysed β-glucan diet. Moreover, native β-glucan in this study also increasedserum 7α-HC levels by 57 % within 24 h of consumption( Reference Ellegard and Andersson 31 ). A study from Marlett et al. investigated whether oat β-glucan lowers cholesterol levels by decreasing bile acid and fat absorption and increasing bile acid synthesis in nine normolipidaemic men. Participants consumed a low-fibre diet for 28 d followed by an oat bran diet containing 5·4 g of soluble β-glucan for another 28 d without an interval( Reference Marlett, Hosig and Vollendorf 14 ). The oat bran diet resulted in increased synthesis and fractional turnover rates of two primary bile acid cholic acid and chenodeoxycholic acid, along with decreased serum cholesterol levels compared with a low-fibre diet without β-glucan( Reference Marlett, Hosig and Vollendorf 14 ). These previous studies observed bile acid metabolism being interrupted by β-glucan but possess limitations such as small sample size (n<10). Moreover, the ileostomy studies used short intervention periods of just a few days. Marlett et al. had a longer term for intervention but lacked a washout period. Therefore, results from the present study using thirty healthy participants with a controlled, randomised cross-over design provide stronger evidence to support that β-glucan reduces circulating cholesterol concentration through increasing bile acid synthesis.
The cholesterol-lowering effect of β-glucan was associated with the genetic variation of CYP7A1 rs3808607. Specifically, individuals carrying SNP rs3808607-G allele CYP7A1 were more responsive to HMW β-glucan in lowering LDL-cholesterol levels. In the present study, the participants were sub-grouped based on genotypes of CYP7A1 SNP rs3808607, and we found that individuals carrying homozygote for G allele (G/G) showed significant response to 3 g/d HMW β-glucan in increasing 7α-HC concentrations compared with control, but not for participants who carry T/T and G/T genotype of CYP7A1 SNP rs3808607. This result reveals the important role of CYP7A1 in the bile acid interruption mechanism and the influence of the genetic variation in this action. As reported previously, G-allele mediates greater gene expression than homozygote T based on increased transcriptional activity, which was suggested to increase bile acid synthesis in the liver as CYP7A1 is the key enzyme in the classical pathway( Reference De Castro-Oros, Pampin and Cofan 18 ). These results confirm that rs3808607-G allele carriers are more responsive to elimination of bile acids from the enterohepatic circulation caused by β-glucan( Reference Marlett, Hosig and Vollendorf 14 , Reference Lia, Andersson and Mekki 28 , Reference Ellegard and Andersson 31 – Reference Andersson, Ellegard and Andersson 33 ). As a consequence, under the stimulation of 3 g HMW β-glucan that ‘bound’ or ‘entrapped’ the greatest amount of bile acid, G/G carriers responded more to the ‘elimination signal’ given by the decreased level of primary bile acid in the liver, to produce more bile acid to restore the bile acid pool.
A negative correlation between AUC of 13C enrichment and FSR was observed, which agrees with the theory that when more free cholesterol enters the liver from the diet, synthesis of cholesterol will be suppressed, and vice versa. Yet, neither of these pathways was influenced by β-glucan intervention. The concentration of 7α-HC increased by 3 g/d HMW β-glucan but was corrected with neither cholesterol absorption nor cholesterol synthesis. Accordingly, it is reasonable to speculate that interrupting bile acid enterohepatic circulation might be the sole mechanism for the cholesterol-lowering effect of β-glucan. This notion is further supported by the observation that log(viscosity) of β-glucan has a linear relationship with 7α-HC levels but not with cholesterol absorption or synthesis parameters.
This study is an extension of a previous published study with serum cholesterol changes as the primary outcome( Reference Wang, Harding and Eck 2 ). It should be noted that sample size of this study was not determined for detecting changes in cholesterol and bile acid metabolism following β-glucan consumption or the interaction between genetic variation of CYP7A1 SNP rs3808607 and β-glucan intervention. Although the distribution of genoptype of CYP7A1 SNP rs3808607 among the participants in this study is similar to the minor genotype frequency reported in the general population( Reference Flicek, Ahmed and Amode 25 ), we suggest a future study with appropriate sample size to confirm our current findings.
Overall, results in the current study showed that consumption of 3 g HMW β-glucan increased serum 7α-HC but did not change the AUC of 13C-cholesterol enrichment in erythrocytes and FSR for cholesterol. Secondarily, aligning with our previous findings, homozygous rs3808607-G allele carriers resulted in greater responses in lowering cholesterol levels and a higher level of bile acid synthesis after ingesting 3 g HMWβ-glucan. In conclusion, our results suggest that interrupting enterohepatic circulation of bile acids rather than inhibiting cholesterol absorption and synthesis might be the mechanism responsible for the cholesterol-lowering effect of β-glucan; the different responsiveness of individuals to the cholesterol-lowering effect of β-glucan might be attributed to the different levels of bile acid synthesis of the three genotypes, G/G, G/T and T/T following β-glucan consumption.
Acknowledgements
The authors thank Tracy Exley and Camille Rhymer for their technical support as well as Parrheim Foods (Saskatoon, SK Canada) and barley breeder Brian Rossnagel from the University of Saskatchewan for providing the barley raw materials used in the treatments.
The research was funded by Agriculture and Agri-Food Canada’s Growing Forward.
S. M. T. and N. P. A. designed the research; Y. W. and S. V. H. conducted the research; P. J. H. J. provided facilities, technical support and supervisions for stable isotope analysis; S. J. T. helped with the sample extractions and manuscript preparation; Y. W. analysed the data and performed the statistical analysis; Y. W. and N. P. A. wrote the paper. N. P. A. had primary responsibility for final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.








