Strenuous aerobic exercise or a physically exhaustive work session is known to disrupt innate immune function for up to 24 h(Reference Woods, Davis and Smith1, Reference McFarlin, Flynn and Stewart2). Environmental extremes exacerbate the typical exercise response and may further weaken the immune system(Reference Walsh and Whitham3). Reduction in mucosal immunity, in addition to altered innate immune cells, results in an ‘open window’(Reference Pedersen and Ullum4) where an individual may be more susceptible to infections. Both natural killer cells and monocytes contribute to the innate immune response and are altered during recovery from exercise(Reference McFarlin, Flynn and Stewart2, Reference Gleeson, McFarlin and Flynn5, Reference McFarlin, Hutchison and Kueht6). Becoming ill after exercise can result in lost training days, reductions in performance and lost working days. In the case of physical labourers, police officers, firefighters and soldiers, such sickness may increase the risk of job-related injuries and fatalities due to fatigue. Over the past decade, a number of laboratories including our own have explored the use of many different dietary supplements in mitigating post-exercise immune suppression(Reference Steensberg, Toft and Bruunsgaard7–Reference McFarlin, Flynn and Phillips9). β-Glucans (BG) have repeatedly been (tested) for immune-enhancing/modulatory properties(Reference Allendorf, Yan and Ross10–Reference Wakshull, Brunke-Reese and Lindermuth14). However, there is great variation in these studies with regard to the efficacy of BG to modulate immunity(Reference us and Pharmacopiea15).
A likely reason for this variation is the many sources (and therefore types) of BG tested. BG are carbohydrates composed of glucose molecules linked together by several different types of chemical linkages resulting in either a linear or branched structure. For example, BG from cereal grains (oats and barley) have a linear structure, while BG from fungal sources (mushrooms and yeast) have a 1,3/1,6 linkage pattern with varying degrees of side-chain branches attached to the backbone. The frequency and length of side-chain branches have been shown to have important implications for biological activity; in general, the higher the degree of branching, the more biologically active the BG(Reference Driscoll, Hansen and Ding11). The baker's yeast-derived BG used in the present study is a commercially available preparation that has been structurally characterised in detail and is known to have a high degree of side-chain branching(Reference us and Pharmacopiea15).
Previous studies using baker's yeast BG have reported that supplementation after running a marathon reduces the number of symptomatic upper respiratory tract infection days compared with a placebo (PL) over a 4-week period(Reference Talbott and Talbott16). Similar reductions in upper respiratory tract infection symptoms have been noted in forest firefighters who were supplementing their diet with BG(Reference Harger-Domitrovich, Domitrovich and Ruby17). The purpose of the present study was to determine whether 10 d of supplementation with a particulate form of baker's yeast BG (Wellmune WGP) improved the innate immune system response to a strenuous bout of exercise in a hot, humid environment. In order to evaluate the innate immune system, we focused on monocytes, lipopolysaccharide (LPS)-stimulated cytokine production by whole-blood culture, and plasma cytokine concentrations.
Materials and methods
Approach to the problem
The present study used a double-blind, cross-over design to evaluate the ability of baker's yeast BG to counter post-exercise disruptions in innate immunity. Key outcome measures were changes in monocyte concentration, LPS-stimulated whole-blood cytokine production and plasma cytokine concentration. Each subject completed both conditions (BG or PL) using a 10 d, pre-exercise supplementation period and a 7 d washout period between trials. A given subject completed their two experimental exercise trials at the same time of day, following an overnight fast (>8 h) and abstention from exercise (>24 h). Upon arrival at the laboratory, subjects were asked to complete a 24 h food recall to compare dietary intake. We did not find any significant condition differences for total energy intake or any of the macronutrients. We opted to have the subjects exercise in a hot, humid environment in order to increase physiological stress associated with exercise in a manner that matched our subject population. For the present study, we elected to use ‘recreationally active’ college students who were regularly exposed to stressful situations that may weaken the immune system over time.
Determination of sample size
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of Houston Committee for the Protection of Human Subjects. Written informed consent was obtained from all subjects/patients. Before completing the present study, we performed a pilot study using twelve men and women to evaluate which immune variables (natural killer cells, T-cells, monocytes, cytokines, etc.) were most likely to change with baker's yeast BG (Wellmune WGP) supplementation before exercise (BK McFarlin, unpublished data). From this testing, we found interesting trends for monocytes, but little change for natural killer and T-cells. Of the monocyte responses, the variable with the smallest effect size was monocyte concentration (0·35). Using this effect size and a statistical power of 0·80, we found that we would need a minimum of fifty-five subjects to detect significant differences between the BG and PL conditions. We increased the sample size of the present study to sixty-nine subjects in order to account for any potential dropouts, while adding additional statistical power. Over the course of the study, nine subjects were either excused for failing to follow study protocols or asked to discontinue participation in the study (Fig. 1).
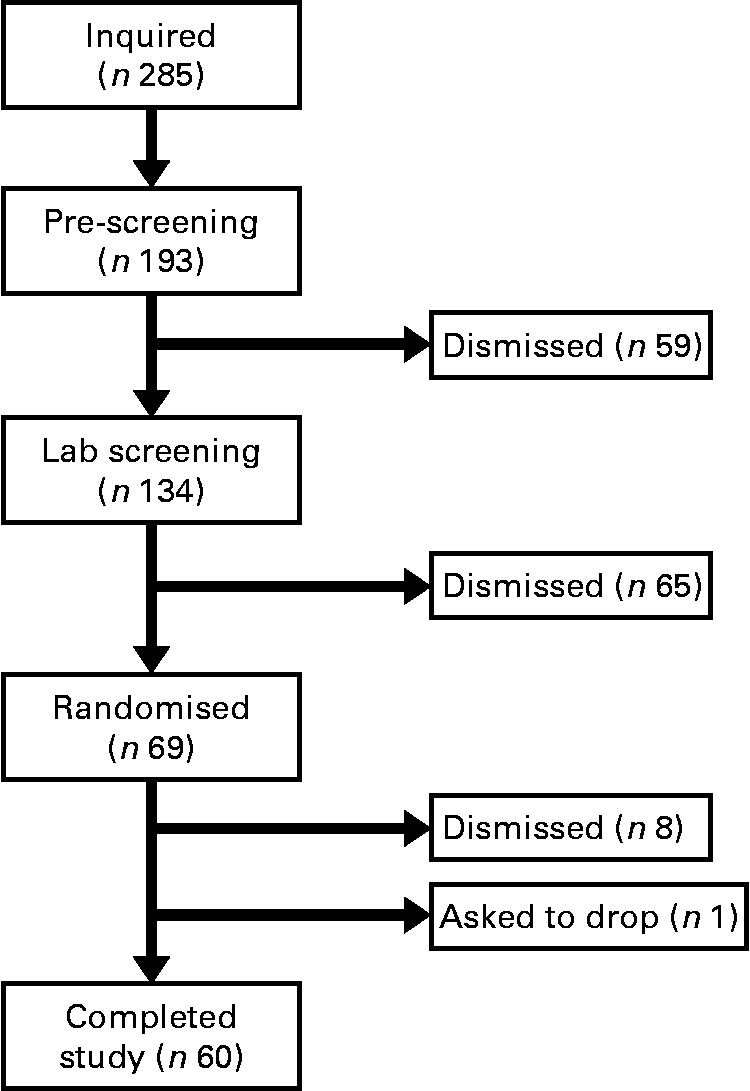
Fig. 1 Consort diagram. This is the consort recruitment diagram for the present study. The subject pool for the study started with 285 interested individuals and was reduced to the final number of sixty based on inclusion/exclusion criteria and subject attrition. Subjects completed two 10 d supplementation periods with either baker's yeast β-glucan (250 mg/d) or placebo (rice flour). After each supplementation period, subjects completed an experimental exercise trial in a hot, humid environment. Blood samples were collected and analysed for markers of immune system function.
Subjects
In the present study, sixty male and female individuals who were recreationally active completed all testing. Fig. 1 demonstrates the recruitment and selection process of subjects in the present study. Subject demographics are presented in Table 1. After expressing interest in the study, subjects reported to the laboratory for a baseline testing session to measure their height, weight and body composition, and determine their maximal aerobic fitness level (VO2peak). Body composition was determined via a whole-body dual-energy X-ray absorptiometry scan (Hologic Discovery W). VO2peak was determined using a graded exercise test on an electronically braked cycle ergometer (Velotron) using a standard graded protocol from our laboratory(Reference McFarlin, Flynn and Stewart2, Reference McFarlin, Flynn and Hampton8, Reference McFarlin, Mitchell and McFarlin18, Reference McFarlin and Mitchell19). When a subject's VO2peak was classified as ‘average’ or ‘good’ for their age/sex group (according to the standards of the American College of Sports Medicine)(Reference Ehrman20), the subject was approved for further participation in the study. Individuals who had ‘excellent’ fitness for their age group were excluded from further participation.
Table 1 Subject characteristics (Mean values and standard deviations)
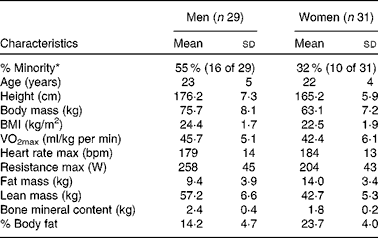
max, Maximal value observed during the graded exercise test; bpm, beats per min.
* % Minority represents the percentage of the subject population that was of self-reported minority status.
Baker's yeast β-glucan supplement
A commercially available, particulate form of baker's yeast BG was used in the present study. BG was prepared by Biothera (the Immune Health Company) and consisted of β-1,3/1,6-glucans derived from baker's yeast (Saccharomyces cerevisiae)(Reference us and Pharmacopiea15). Rice flour was used as a PL because it was similar in colour and appearance to the BG supplement. Both supplements were in powder form and were packed into VegeCap® capsules before packaging in individual coded bottles. Biothera randomly assigned a number to each condition before shipping to the University of Houston. Double-blind protocols were maintained until all study data had been collected in raw form and submitted to Biothera. Upon receipt of the raw data report, Biothera unblinded the University of Houston study staff so that they could interpret the findings of the study based on condition.
In addition to blinding of the study staff, trial conditions were completed in a random, counterbalanced order to minimise possible trial-order effects. Each subject completed two conditions: 10 d of PL (250 mg/d of rice flour) and 10 d of baker's yeast BG (250 mg/d of Wellmune WGP). Supplement conditions were separated by a 7 d washout period. Subjects were instructed to consume the supplement with water, not within 2 h of consuming a meal and at generally the same time each day. Subjects were provided a form to document their daily supplement intake.
Experimental exercise trial
Subjects completed a 10 d supplementation period (PL or BG) before each exercise trial. Exercise consisted of up to 60 min of cycling in a hot (38 ± 2°C), humid (45 ± 2 %) environment. During the exercise trial, physiological stress was monitored by measurement of the core body temperature (rectal, T C), the heart rate (HR) and the rating of perceived exertion(Reference McFarlin and Mitchell19). Rectal T C was measured using a disposable thermistor and HR was measured by telemetry (Polar). Exercise intensity was set to fixed wattage that elicited approximately 65 % of VO2peak based on the HR response. Exercise sessions were stopped if the subject reached a T C>39·2°C. Subjects were allowed ad libitum intake of water during the exercise trials. Hydration status was monitored before and after each exercise trial by measuring Hb and haematocrit using a portable analyser (Stanbio's Hemopoint H2; Stanbio Laboratory). In the event that the subject stopped before 60 min, their exercise duration was matched for their next exercise trial. For example, if a subject reached their T C cut-off at 48 min for trial 1, then in trial 2, they would be asked to stop at 48 min. This was designed to ensure that similar amounts of exercise stress were applied between the trials.
Blood collection
Venous blood was collected from a peripheral arm vein at four time points per supplement condition: before supplementation (baseline); after supplementation period and before exercise (PRE); immediately post-exercise (POST); 2 h post-exercise (2H). With the exception of POST, all blood samples were collected following 15 min of seated rest. Blood was treated with sodium heparin or EDTA in evacuated tubes to prevent clotting (Becton Dickinson). EDTA plasma was separated and frozen at − 80°C for cytokine analysis, while the remaining blood was held at room temperature on an oscillating rocker until used for LPS stimulations or flow cytometry. Samples were processed within 2 h of collection.
Peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using a standard density gradient method involving Histopaque 1077 (Sigma-Aldrich). EDTA-treated whole blood was diluted 1:2 with PBS and 8 ml of diluted blood were layered onto 4 ml Histopaque in a 15 ml conical centrifuge tube. A total of three tubes (24 ml of diluted blood) were separated for each blood sample. After centrifugation (30 min at 1000 g), the floating PBMC layer was removed from the three separation tubes and combined into a single 50 ml conical centrifuge tube. PBS (45 ml) was added to the pooled PBMC. After two additional washes, PBMC were suspended at 4 × 106 cells/ml based on an automated viability count (EMD Millipore ViaCount). PBMC viability was >97 % for all isolations.
Monocyte phenotyping and concentration
All flow cytometry supplies were purchased from eBioscience unless otherwise noted. Aliquots of isolated PBMC (300 000 cells) were added to 1·2 ml library tubes and incubated with pre-titred antibodies against CD14-PerCP-Cy5.5 and CD16-APC. After a 30 min incubation at room temperature in the dark, samples were washed with PBS and re-suspended in a final volume of 300 μl (0·5 % paraformaldehyde). Samples were then transferred to a round bottom polypropylene ninety-six-well plate for acquisition on a Millipore-Guava easyCyte 6HT-2L (EMD Millipore). A minimum of 35 000 monocyte events were acquired for each sample. Monocyte total and subset concentrations were determined by the flow cytometer, which uses a precise volumetric syringe to deliver the sample. Before each set of sample analysis, the easyCyte flow cytometer was checked for calibration and daily variation using standard-sized polystyrene beads (easyCheck; EMD Millipore). Daily variation of fluorescence was < 3 % across all four photomultiplier tubes. Sample flow cytometry standard (FCS) files were acquired uncompensated and analysed offline using FCS Express (version 3.0; De Novo Software). Monocytes were identified based on positive CD14 staining; monocyte subsets were identified based on the differential expression of CD16. Isotype and cells-only tubes were included as negative controls. Classic monocytes were identified as CD14+/CD16−, while pro-inflammatory monocytes were CD14+/CD16+.
Lipopolysaccharide-stimulated cytokine production
Consistent with methods that we have used previously(Reference McFarlin, Flynn and Campbell21, Reference McFarlin, Flynn and Campbell22), we diluted heparinised whole blood 1:10 with PBS and cultured with LPS (15 μg/ml) overnight (24 h). LPS was used as a stimulant in the present study because it has the capacity to act on a variety of leucocytes, but primarily monocytes, because these are the only cells which express the LPS receptor (CD14)(Reference Gleeson, McFarlin and Flynn5). After stimulation, cell-free supernatant was removed and frozen at − 80°C. Supernatants were thawed and measured for the concentration of twelve cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, granulocyte, monocyte colony-stimulating factors (GM-CSF) and TNF-α) using a commercially available flow cytometry-based multiplex bead array (FlowCytomix; eBioscience). The inter- and intra-assay CV reported by the manufacturer was < 8 %.
Plasma cytokine analysis
EDTA plasma was thawed and analysed using a high-sensitivity, multiplex assay for cytokines (Millipore Milliplex). The test protocol included the measurement of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, GM-CSF, IFN-γ and TNF-α. After following the sample processing procedure, labelled magnetic microspheres were acquired on a Luminex MagPix instrument. The low-end detection limit of the assay that we used was 0·13 pg/ml with an inter- and intra-assay CV of < 7 %. Manufacturer-provided controls were utilised to track variability in the analysis and all samples were measured on the same day to minimise variability.
Statistical analysis
All statistical analyses were completed using SPSS (version 19; SPSS, Inc.). Before formal statistical testing, data were analysed for normality and constant error variance. Non-normal data were transformed to stabilise assumptions (noted in the Results section by variable). Exercise response variables were analysed using a two-condition (supplement) × five-time (exercise) ANOVA with repeated measures on both factors. Immune response variables were analysed using a two (supplement) × four (blood collection time) ANOVA with repeated measures on both factors. Significance was set at P< 0·05 and significant P values were adjusted using the Huynh–Feldt method to account for the repeated-measures design. Location of significant effects was determined using a Tukey post hoc test. Data are reported as means with their standard errors.
Results
Exercise-induced physiological stress
Subjects completed an average of 49 ± 6 min of exercise and the majority were stopped because their T C reached our safe cut-off of >39·2°C. Based on the outcome measures that we selected, there was a significant main effect for time for T C (P< 0·001), HR (P< 0·001) and rating of perceived exertion (P< 0·001); however, there was no difference between the conditions (Table 2). The HR response observed in the present study was consistent with an exercise intensity between 60 and 70 % of VO2peak. Based on this analysis, we are confident that the stress associated with the two exercise sessions was not significantly different.
Table 2 Physiological stress in sujects (n 60) who completed 49±6 min of cycling in a hot (38±2°C), humid (45±2 %) environment*(Mean values with their standard errors)
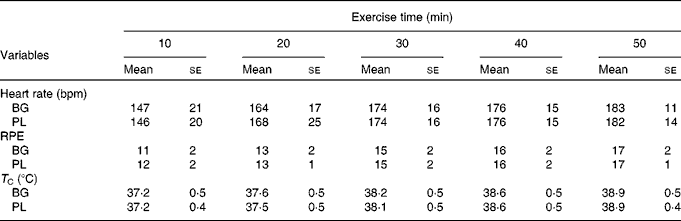
bpm, Beats per min; BG, β-glucan; PL, placebo; RPE, rating of perceived exertion; T C, core body temperature.
* Subjects supplemented with either BG or PL (rice flour) for 10 d before each exercise trial. Heart rate, RPE and T C were measured to track physiological stress in response to exercise. No significant differences between the conditions were noted. These data demonstrate that the quantity of physiological stress was similar between the conditions.
Total and subset monocyte concentrations
We found a significant condition × exercise time effect for total monocyte (CD14+, P= 0·026; Fig. 2(a)) and pro-inflammatory monocyte (CD14+/CD16+, P= 0·033; Fig. 2(b)) concentrations. Total monocyte concentration was significantly greater in BG than in PL at 2H. Pro-inflammatory monocyte concentration was significantly greater in BG than in PL at both POST and 2H. In the PL condition, 2H concentration was significantly less than at PRE for both total and pro-inflammatory monocytes (P< 0·05). While classic monocyte (CD14+/CD16−) concentration demonstrated a similar pattern, there was no statistically significant interaction effect (P= 0·117; Fig. 2(c)). The presented concentrations are based on counts taken from PBMC, which are lower than counts determined from the whole blood.
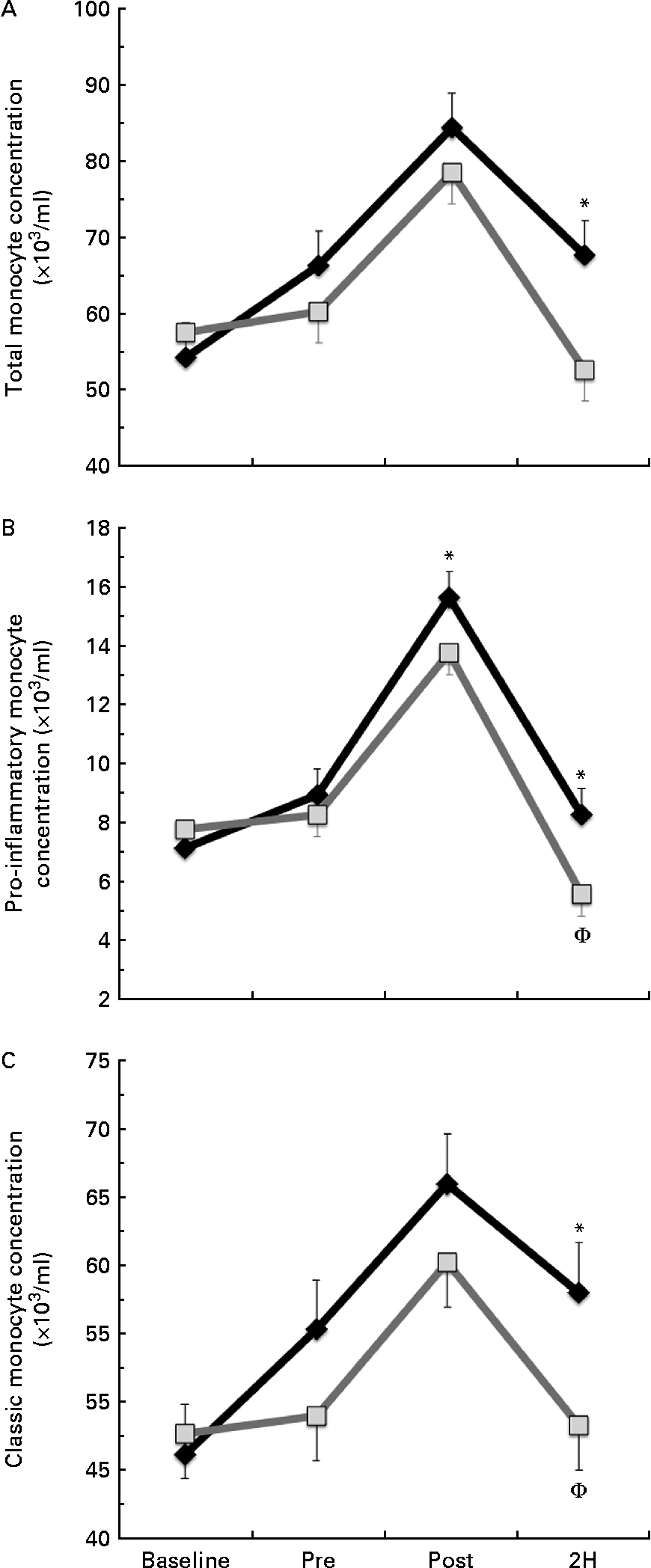
Fig. 2 (a) Total (CD14+), (b) pro-inflammatory (CD14+/16+) and (c) classic (CD14+/16−) monocyte concentration. Venous blood samples were collected before (time point before supplementation (baseline) and after supplementation and before exercise time point (PRE)) and after exercise (immediately post-exercise time point (POST) and 2 h post-exercise time point (2H)) in subjects (n 60) who completed two trial conditions: baker's yeast β-glucan (![]() , 250 mg/d) and placebo (PL;
, 250 mg/d) and placebo (PL;![]() , rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise trial in a hot, humid environment (baseline sample collected before the supplementation period). Monocyte concentrations were determined using flow cytometry (EMD Millipore easyCyte 6HT-2L). Significant interaction effects were found for both total (P= 0·026) and pro-inflammatory monocyte (P= 0·033) concentrations. Classic monocyte concentration showed a similar trend towards an interaction effect as observed in the other two fractions (P= 0·117). Values are means, with standard errors represented by vertical bars. * Mean value was more significantly different from that of PL at the same time point. † Mean value was less significantly different from that at PRE for the same condition.
, rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise trial in a hot, humid environment (baseline sample collected before the supplementation period). Monocyte concentrations were determined using flow cytometry (EMD Millipore easyCyte 6HT-2L). Significant interaction effects were found for both total (P= 0·026) and pro-inflammatory monocyte (P= 0·033) concentrations. Classic monocyte concentration showed a similar trend towards an interaction effect as observed in the other two fractions (P= 0·117). Values are means, with standard errors represented by vertical bars. * Mean value was more significantly different from that of PL at the same time point. † Mean value was less significantly different from that at PRE for the same condition.
Lipopolysaccharide-stimulated cytokines
Significant condition × exercise time interactions were found for the LPS-stimulated cytokines IL-4 (P= 0·025; Fig. 3(a)), IL-5 (P= 0·027; Fig. 3(b)) and IFN-γ (P= 0·011; Fig. 3(c)). For each of these significant cytokines, the location of significance was that the BG condition had significantly greater cytokine production at PRE and POST than PL. It is important to note that changes in stimulated cytokine production preceded the change in plasma cytokines (Fig. 4). All of the apparent BG effects on LPS-stimulated cytokine production occurred before exercise, except for IL-4, which was also significantly increased at 2H for BG. A significant interaction effect was also found for LPS-stimulated IL-2 (P= 0·018); however, this difference occurred at baseline and did not demonstrate any other significant differences between BG and PL (data not shown). No significant differences were found between BG and PL for the LPS-stimulated production of IL-1β, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, GM-CSF or TNF-α.
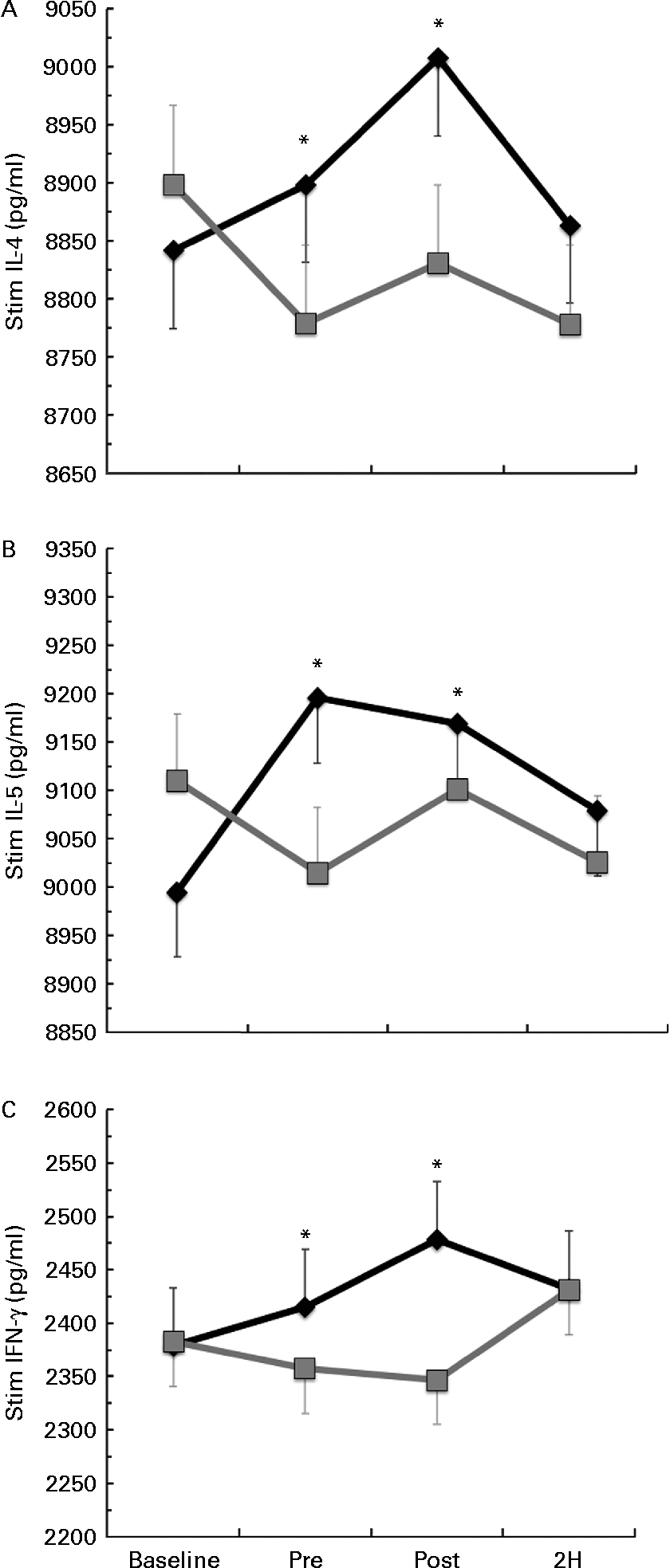
Fig. 3 Lipopolysaccharide (LPS)-stimulated production of (a) IL-4, (b) IL-5 and (c) interferon-γ (IFN-γ). Venous blood samples were collected before (time point before supplementation (baseline) and after supplementation and before exercise time point (PRE)) and after exercise (immediately post-exercise time point (POST) and 2 h post-exercise time point (2H)) in subjects (n 60) who completed two trial conditions: baker's yeast β-glucan (BG, ![]() , 250 mg/d) and placebo (PL,
, 250 mg/d) and placebo (PL, ![]() , rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise in a hot, humid environment (baseline sample collected before the supplementation period). Whole-blood cultures were stimulated for 24 h with LPS. Cell-free supernatants were analysed for cytokine production using a flow cytometry-based multiplex assay. Significant interaction effects were found for BG over PL at specific time points in IL-4 (P= 0·025), IL-5 (P= 0·027) and IFN-γ (P= 0·011). It is important to note that changes in stimulated cytokine production preceded the change in plasma cytokines (Fig. 4). Values are means, with standard errors represented by vertical bars. * Mean value was more significantly different from that of PL (P< 0·05).
, rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise in a hot, humid environment (baseline sample collected before the supplementation period). Whole-blood cultures were stimulated for 24 h with LPS. Cell-free supernatants were analysed for cytokine production using a flow cytometry-based multiplex assay. Significant interaction effects were found for BG over PL at specific time points in IL-4 (P= 0·025), IL-5 (P= 0·027) and IFN-γ (P= 0·011). It is important to note that changes in stimulated cytokine production preceded the change in plasma cytokines (Fig. 4). Values are means, with standard errors represented by vertical bars. * Mean value was more significantly different from that of PL (P< 0·05).

Fig. 4 Plasma concentration of (a) IL-4, (b) IL-5 and (c) interferon-γ (IFN-γ). Venous blood samples were collected before (time point before supplementation (baseline) and after supplementation and before exercise time point (PRE)) and after exercise (immediately post-exercise time point (POST) and 2 h post-exercise time point (2H)) in subjects (n 60) who completed two trial conditions: baker's yeast β-glucan (BG, ![]() , 250 mg/d) and placebo (PL,
, 250 mg/d) and placebo (PL, ![]() , rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise in a hot, humid environment (baseline sample collected before the supplementation period). Plasma was analysed using a high-sensitivity multiplex cytokine kit (EMD Millipore Milliplex kit and Luminex MagPix instrument). Significant interaction effects were found for BG over PL at specific time points in IL-4 (P= 0·042) and IFN-γ (P= 0·047). There was a trend towards significance for this effect in IL-5 (P= 0·053). It is important to note that changes in plasma cytokines followed changes in stimulated cytokine production (Fig. 3). Values are means, with standard errors represented by vertical bars. * Mean value was more significantly different from that of PL (P< 0·05). † Mean value was more significantly different from that of PL (P for trend = 0·053).
, rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise in a hot, humid environment (baseline sample collected before the supplementation period). Plasma was analysed using a high-sensitivity multiplex cytokine kit (EMD Millipore Milliplex kit and Luminex MagPix instrument). Significant interaction effects were found for BG over PL at specific time points in IL-4 (P= 0·042) and IFN-γ (P= 0·047). There was a trend towards significance for this effect in IL-5 (P= 0·053). It is important to note that changes in plasma cytokines followed changes in stimulated cytokine production (Fig. 3). Values are means, with standard errors represented by vertical bars. * Mean value was more significantly different from that of PL (P< 0·05). † Mean value was more significantly different from that of PL (P for trend = 0·053).
Plasma cytokines
Significant condition × exercise time effects were found for plasma concentrations of IL-4 (P= 0·042; Fig. 4(a)), IL-7 (P= 0·047; Fig. 5(a)), IL-8 (P= 0·049; Fig. 5(b)), IL-10 (P= 0·033; Fig. 5(c)) and IFN-γ (P= 0·047; Fig. 4(c)). There was also a trend towards significance for IL-5 (P= 0·053; Fig. 4(b)). For each of these cytokines, the location of significance was the same; the BG condition was significantly greater than PL at 2H. Also, for IL-4 and IL-5, BG was greater than PL at PRE. We found a significant main effect for time (no supplement effect) for IL-1β (P= 0·017), IL-6 (P< 0·0001), TNF-α P< 0·0001) and GM-CSF (P= 0·06) (Table 3). The changes observed in both conditions are consistent with a typical post-exercise pro-inflammatory response (IL-1β, IL-6, TNF-α and GM-CSF). No significant main or interaction effects were found for IL-2, IL-12 (p70) or IL-13.
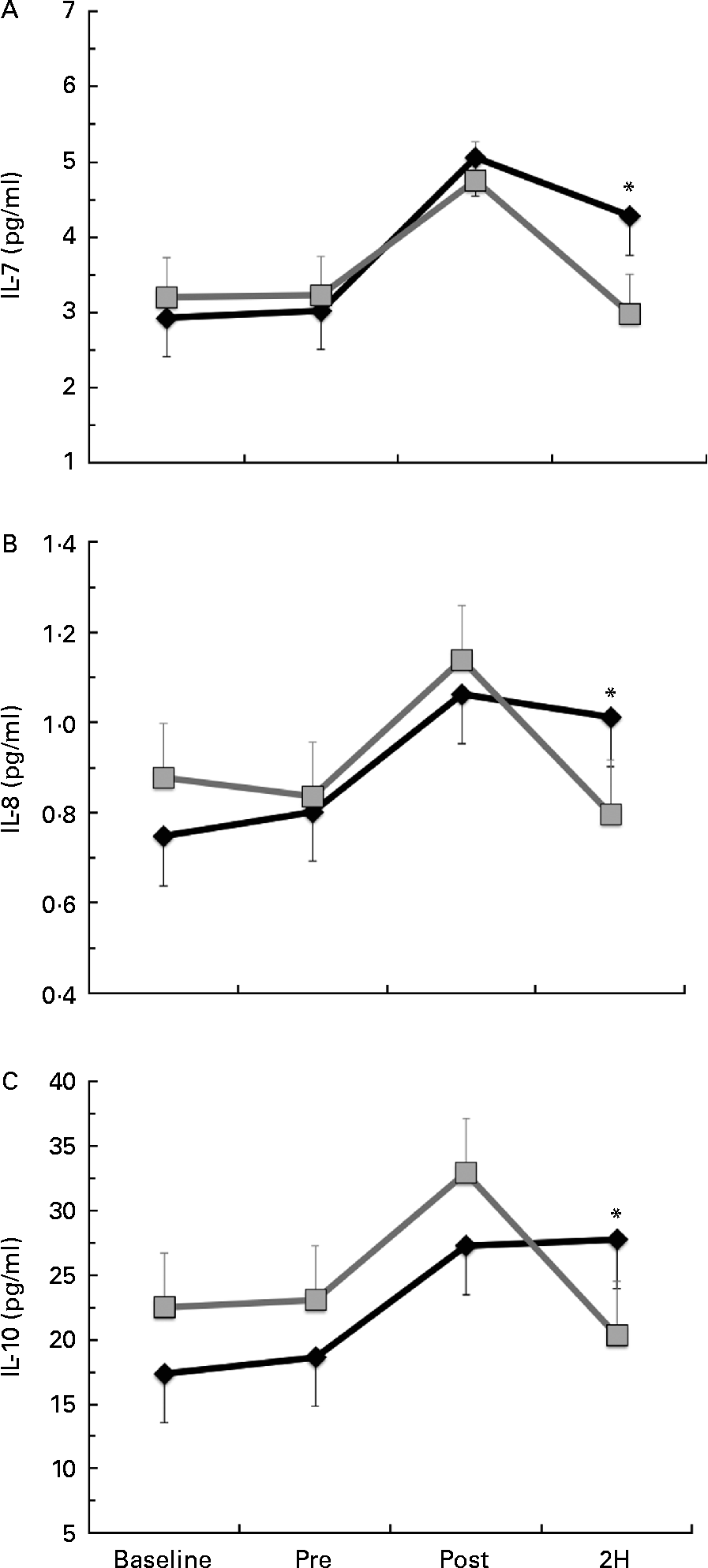
Fig. 5 Plasma concentration of (a) IL-7, (b) IL-8 and (c) IL-10. Venous blood samples were collected before (time point before supplementation (baseline) and after supplementation and before exercise time point (PRE)) and after exercise (immediately post-exercise time point (POST) and 2 h post-exercise time point (2H)) in subjects (n 60) who completed two trial conditions: baker's yeast β-glucan (BG, ![]() , 250 mg/d) and placebo (PL,
, 250 mg/d) and placebo (PL, ![]() , rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise in a hot, humid environment (baseline sample collected before the supplementation period). Plasma was analysed using a high-sensitivity multiplex cytokine kit (EMD Millipore Milliplex kit and Luminex MagPix instrument). Values are means, with their standard errors represented by vertical bars. * Mean value was more significantly different from that of PL (P< 0·05).
, rice flour) in a randomised order. Subjects consumed their supplement for 10 d before the experimental exercise in a hot, humid environment (baseline sample collected before the supplementation period). Plasma was analysed using a high-sensitivity multiplex cytokine kit (EMD Millipore Milliplex kit and Luminex MagPix instrument). Values are means, with their standard errors represented by vertical bars. * Mean value was more significantly different from that of PL (P< 0·05).
Table 3 Plasma cytolcines in subjects (n 60) who completed 49±6 min of cycling in a hot (38±2°C), humid (45±2 %) environment† (Mean values with their standard errors)
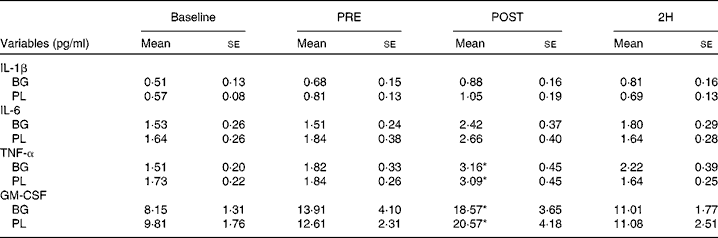
Baseline, time point before supplementation; PRE, after supplementation and before exercise time point; POST, immediately post-exercise time point; 2H, 2 h post-exercise time point; BG, β-glucan; PL, placebo; GM-CSF, granulocyte, monocyte colony-stimulating factors.
* Mean value was significantly different from those at the other time points (P< 0·05).
† Subjects supplemented with either BG or PL (rice flour) for 10 d before each exercise trial. Venous blood samples were collected before supplementation (baseline), PRE, POST and 2H. Plasma IL-1β, IL-6, TNF-α and GM-CSF were measured as part of a Milliplex High-Sensitivity cytokine kit. Other results are presented in Figs. 4 and 5.
Discussion
The key findings of the present study were that supplementation for 10 d with 250 mg of baker's yeast BG per d before a bout of cycling in the heat increased total and pro-inflammatory monocyte concentrations after exercise, increased LPS-stimulated cytokine production before exercise and increased plasma cytokine concentrations after exercise. While other published studies have examined BG supplementation, the results have varied due to the structural differences in BG from different sources(Reference Bergendiova, Tibenska and Majtan23–Reference Murphy, Davis and Brown26). To our knowledge, this is the first published report demonstrating that supplementation with a well-characterised form of baker's yeast BG(Reference us and Pharmacopiea15) alters the post-exercise innate immune system response.
For PL, we observed a 17 % decline in both total and pro-inflammatory monocyte concentrations between PRE and 2H, compared with only a 3 % decline with BG. The 2H decline observed in the PL condition was consistent with previous reports from our laboratory and others of exercise-induced immunosuppression(Reference McFarlin, Flynn and Campbell21, Reference Simpson, McFarlin and McSporran27, Reference Flynn, McFarlin and Phillips28). It appears that BG supplementation maintains the blood monocyte circulation, and counters the monocytopenia that we observed in the PL condition post-exercise. A BG-induced increase in monocytes at 2H may have important implications for the global immune response(Reference Walsh, Gleeson and Shephard29). Monocytes play a role in tissue-specific Th1 and Th2 responses and are know to be activated by Th1 and Th2 cytokines(Reference McFarlin, Flynn and Stewart2, Reference Steensberg, Toft and Bruunsgaard7, Reference Ibfelt, Petersen and Bruunsgaard30). Iijima et al. (Reference Iijima, Mattei and Iwasaki31) reported that an increase in circulating pro-inflammatory monocyte concentration resulted in improved Th1 response in the peripheral tissue. The response that we observed at 2H with BG was very similar to the findings of Iijima et al. For PL, we observed the effects that were completely opposite of BG and consistent with the typical exercise response(Reference Lancaster, Halson and Khan32).
In addition to changes in circulating monocyte concentration, we also measured changes in both plasma cytokine levels and LPS-stimulated cytokine production by peripheral blood cells. Some of the targeted cytokines demonstrated changes in both measures, while others only demonstrated changes in plasma after BG supplementation. We found that BG increased LPS-stimulated production of IFN-γ, IL-4 and IL-5 before exercise and increased in plasma IFN-γ, IL-4 and IL-5 (trend towards significance) concentrations at 2H. Further interpretation of this finding suggests that BG may potentiate the ability of monocytes to increase their cytokine output after exercise, resulting in greater plasma cytokine concentrations. This increased capacity to respond to the LPS challenge is similar to the enhanced response of innate immune cells after exposure to yeast BG reported in animal model systems. Yeast BG has been shown to bind to complement receptor 3 on neutrophils and other innate immune cells(Reference Hong, Yan and Baran33). With BG fragments bound to complement receptor 3, the ‘primed’ neutrophils are more responsive to chemotactic stimuli(Reference Li, Allendorf and Hansen13, Reference Tsikitis, Morin and Harrington34) as well as having enhanced levels of phagocytosis(Reference Wakshull, Brunke-Reese and Lindermuth14) and oxidative burst(Reference Allendorf, Yan and Ross10, Reference Lavigne, Albina and Reichner12, Reference Hong, Yan and Baran33).
Since LPS stimulation mimics a bacterial challenge of the immune system(Reference Wang, Oido-Mori and Fujii35), the increased cytokine production of LPS-stimulated peripheral blood cells after BG supplementation may be indicative of increased monocyte functional capacity at rest. The cytokine response to LPS stimulation observed with PL was the opposite of BG, but consistent with what others have reported in response to exercise-induced stress(Reference Lancaster, Halson and Khan32). Since cytokines are regulators of many immune system processes, based on the data presented here, we speculate that BG supplementation improves monocyte function, which may have downstream effects on other aspects of the innate immune response.
We also observed changes in plasma cytokines that are associated with lymphocyte proliferation (IL-7) and chemotaxis (IL-8), and that exert anti-inflammatory actions (IL-10)(Reference Walsh, Gleeson and Shephard29); however, these cytokines did not have a corresponding change in LPS-stimulated response, suggesting that monocytes may not be the sole plasma cytokine source following exercise with BG supplementation.
This finding is plausible for IL-7 and IL-8, which are not produced by monocytes; however, it fails to explain the source of IL-10, since it is produced by monocytes. IL-7 has been demonstrated to improve T-cell and B-cell proliferation in response to antigenic challenge and IL-8 is an inducer of chemotaxis in neutrophils(Reference Walsh, Gleeson and Shephard29). In the present study, BG supplementation increased IL-7 and IL-8 at 2H, which, based on their respective function, may be associated with improved T-cell and B-cell proliferative capacity and an increased recruitment of neutrophils to the tissue compartment. IL-10 is a potent anti-inflammatory cytokine, which we found to increase at 2H in BG compared with PL. Since we found a similar pro-inflammatory response to exercise in both supplement groups (elevated IL-1β, IL-6, TNF-α and GM-CSF), it is plausible that BG supplementation provided additional anti-inflammatory protection that was not afforded in the PL condition. More research is needed to understand the immunological source of IL-7, IL-8 and IL-10 in response to BG supplementation.
The changes that we observed in the present study demonstrate how BG supplementation before exercise alters the post-exercise innate immune response. Some of the changes that we observed with BG supplementation were small; however, it is important to note that the study was appropriately powered to detect these changes, which did reach statistical significance. Collectively, the observed changes may have implications for post-exercise immune surveillance and subsequent infection risk.
In summary, in the present study, we found that 10 d of supplementation with a defined source of baker's yeast BG increased total and pro-inflammatory monocyte concentrations after exercise, increased LPS-stimulated cytokines (IL-2, IL-4, IL-5 and IFN-γ) before exercise and plasma cytokine (IL-4, IL-5, IL-7, IL-8, IL-10 and IFN-γ) concentrations after exercise. We also observed a post-exercise pro-inflammatory response (IL-1β, IL-6, TNF-α and GM-CSF) regardless of supplement that was consistent with previous reports from our laboratory and others. These findings support the notion that supplementation with the Wellmune WGP form of baker's yeast BG before exercise altered the typical post-exercise innate immune response. More research is needed to understand what the clinical implications of the observed changes are.
Acknowledgements
We would like to thank Kelley Strohacker, Tanya Halliday, Stephanie Collins, Danielle Eagan and Nicole Impero for their various contributions to this study. We would also like to thank all our subjects who participated in the present study, without their commitment the present study would not have been possible. Funding for the present study was provided by Biothera, the Immune Health Company. The authors were not directly paid for the completion of this study and declare that there is no conflict of interest. K. C. C., W. L. B. and B. K. M. participated in the data collection, analysis and manuscript preparation. T. D. and A. A. contributed to the data collection and analysis. B. K. M. designed and directed the overall study.










