An important randomised controlled trial was reported from the UK( 1 ) in 1991. The periconceptional administration of folic acid (FA) 4 mg/d to women who had previously had pregnancies that were affected with a neural tube defect (NTD) demonstrated a 72 % risk reduction of recurrence. An intervention study from China( Reference Berry, Li and Erickson 2 ) in 1999 giving FA supplements 400 μg/d demonstrated that the first occurrence of NTD was prevented by 41 % in the southern and 79 % in the northern regions. These studies have confirmed that FA can be effectively used to prevent NTD, although not all the cases could be prevented.
In 1992 the United States Public Health Service( 3 ) recommended that all women of childbearing age who are capable of becoming pregnant should consume 400 μg of FA/d for the purpose of reducing their risk of having a pregnancy affected by NTD. The US Food and Drug Administration( 4 ) mandated in 1996 that food fortification must involve fortification with 140 μg of FA/100 g of enriched cereal-grain products by January 1998 to further decrease the incidence of NTD.
The Ministry of Health and Welfare of Japan( 5 ) in 2000 recommended that those women planning pregnancy should take a well-balanced diet and FA supplements of 400 μg daily from 1 month before to the first 3 months of pregnancy. According to the Japan Association of Obstetricians and Gynaecologists( 6 ), the incidence of spina bifida, encephalocele and anencephaly was 5·2, 0·8 and 0·4 per 10 000 total births (live births and stillbirths) in 2012, respectively. They were ranked as the 16th, 42nd and 61st most frequent anomalies in the Japanese population, respectively, while an unknown number of foetuses affected with NTD were aborted. If the number of terminations were counted, they would be ranked in much higher positions. Based on domestic( 6 ) and international( 7 ) data (Fig. 1), the mean prevalence of spina bifida in Japan for each 5-year period over the past three decades has not shown any declining tendency, whereas that of encephalocele plus anencephaly declined steeply in accordance with clinical application of ultrasonography followed by induced abortions. Since efficacy of FA supplements for the prevention of spina bifida has not been ascertained in Japan, we aimed to evaluate the OR of having a pregnancy affected by spina bifida for women who periconceptionally took supplements and examine the association between an increase in supplement use and possible promoting factors for the increase.

Fig. 1 The mean prevalence of spina bifida (–■–) and encephalocele plus anencephaly (–●–) per 10 000 births (live births+stillbirths) is illustrated for each 5-year period since 1978.
Subjects and methods
Data were gathered from a case–control study which identified four risk factors for spina bifida among Japanese women( Reference Kondo, Morota and Ihara 8 ). Recruitment of subjects was conducted during the period between June 2011 and January 2013. Women who were considered eligible for the study were those who gave birth to live-born offspring afflicted with spina bifida (case women), and those who gave birth to healthy live-born offspring (control women) during the period from 2001 to 2012. Judging by the recent prevalence of spina bifida (Fig. 1), we estimate that approximately 500 to 600 neonates are born every year with this anomaly. Recruitment of these patients, however, was not easy because the patients have not been officially registered in Japan. We had to rely on a membership list of the Spina Bifida Society of Japan which comprised approximately 1400 patients together with their family members. The Society sent questionnaires to 402 eligible case women, and twenty medical colleagues handed at their outpatient clinic a questionnaire over to sixty case women who were not members of the Spina Bifida Society of Japan. A total of 177 obstetricians and gynaecologists residing in various parts of Japan sent the questionnaire to 4200 women who, based on the birth record, had delivered live-born babies without spina bifida at their hospitals. The questionnaire consists of fourteen questions regarding FA supplement use, awareness of the role of FA, diet and so forth.
Initially 364 case women and 2337 control women completed the questionnaire and were later compensated with a ¥500 coupon. Of the 364 women, four were excluded – one because of lipomyelomeningocele and three for providing incomplete data. Of the 2337 controls, four were similarly excluded – two because of stillbirth and two for providing incomplete data. Subsequently 360 case women and 2333 control women were divided into two 6-year periods, i.e., the first period from 2001 to 2006 and the second period from 2007 to 2012. Birth places were grouped into two regions: the northern region comprising the Hokkaido, Tohoku, Kanto and Chubu areas, and the southern region comprising the Kansai, Chugoku, Shikoku and Kyushu areas. The frequencies of demographic characteristics among women and their newborns are depicted in Table 1. Distributions of women's BMI and age, and sex of offspring were not significantly different between cases and controls. Body weight of neonates, the year of birth, place of birth and the rate of FA supplement use were significantly different between the groups (P< 0·01).
Table 1 Demographic data of 360 cases and 2333 controls are depicted and compared with χ2 tests* (Number of subjects and percentages)

FA, folic acid.
* The rate of FA supplement use, when all women of both groups were put together, significantly increased from 10 % (119/1193) to 30 % (454/1500) in the 2nd period (P< 0·0001).
We chose six of fourteen parameters (Table 2) as possible promoters which seemed to be closely associated with an increase in the rate of maternal supplement use, and deserved to be statistically assessed, namely (1) knowledge about the preventive role of FA before pregnancy in relation to the occurrence of spina bifida; (2) planned pregnancy; (3) well-balanced diet consuming any amount of fruits, green-yellow vegetables, or consuming any amount of cooked poultry/animal liver during the period between 0 and 15 weeks' gestation; (4) daily smoking during a period between 0 and 15 weeks' gestation; (5) treatment of infertility with assisted reproductive technologies such as fertility drugs, in vitro fertilisation, or intracytoplasmic sperm injection prior to pregnancy; (6) family history of spina bifida in first-, second-, or third-degree relatives. If any parameters of both groups were found increased or decreased simultaneously in the second period with regard to those in the first period, they were determined as promoting factors for an increase in supplement use. Seven other parameters which were not evaluated as they had nothing to do with an increase in maternal supplement use were: (1) intakes of antiepileptic drugs without FA; (2) treatment of diabetes mellitus before pregnancy; (3) febrile episodes above 39°C lasting more than 24 h during the period between 0 and 15 weeks' gestation; (4) very hot bathing/sauna bathing for 15 min or more during the period between 0 and 15 weeks' gestation; (5) pre-pregnancy BMI of the mother; (6) maternal age at birth; (7) birth weight of the baby. The last parameter, namely intake of FA supplements, was an independent variable (Table 3).
Table 2 Six parameters were evaluated to find out possible promoters which increased the rate of maternal folic acid (FA) supplement use† (Number of subjects and percentages)
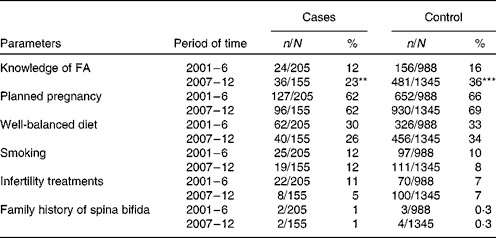
** P= 0·0037.
*** P< 0·0001
† Knowledge of FA of both groups significantly increased in the 2nd period relative to the 1st period (χ2 test), which was suggested to be a possible promoter for an increase in supplement use.
Table 3 Supplement use* in case and control women (Number of subjects and percentages; odds ratios and 95 % confidence intervals)
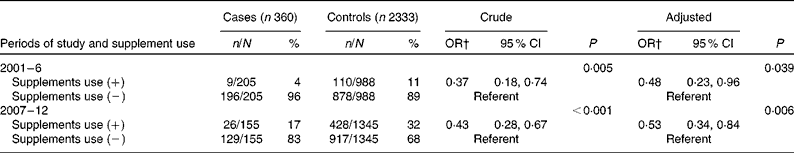
* Supplement use significantly increased in the 2nd period in both groups (P< 0·0001; χ2 test).
† The adjusted OR was 0·48 and 0·53 in the 1st and 2nd periods, respectively. OR were adjusted for the year and place of birth.
Ethical approval and statistical analyses
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical committee of Tsushima Rehabilitation Hospital. Our clinical trial was registered as ‘Recommendation on prevention of spina bifida: Investigation of risk factors related to spina bifida and studies to transmit important information of a role of folic acid’ at the Japan Pharmaceutical Information Center with the registration identification number of 1011 1850 9739 (http://www.japic.or.jp/index.html). Statistical difference of distributions of Table 1 was assessed with χ2 tests. Changes in the values of six parameters were evaluated by χ2 or Fisher's exact tests (Table 2). OR with 95 % CI were estimated (Table 3) using logistic regression analyses (IBM SPSS Statistics 20) where an independent variable of FA supplement use was adjusted for the year and place of birth.
Results
The proportion of case and control women who periconceptionally took FA supplements significantly increased in the second period, i.e., the rate increased fourfold in the cases from 4 to 17 % and threefold in the controls, from 11 to 32 % (P< 0·001) (Table 1). When all women of both the groups were put together, the rate of supplement use significantly increased from 10 % (119/1193) to 30 % (454/1500) (P< 0·0001). Of the six possible promoting parameters (Table 2), knowledge about the preventive role of FA significantly increased in the second period, from 12 to 23 % in the cases (P= 0·0037) and from 16 to 36 % in the controls (P< 0·0001), suggesting that this parameter was associated with an increase in maternal supplement use. The estimated adjusted OR of having a baby with spina bifida for periconceptional FA users relative to non-users was 0·48 (95 % CI 0·23, 0·96) in the first period and 0·53 (95 % CI 0·34, 0·84) in the second period, respectively (Table 3). Periconceptional supplement use reduced the risk of having an infant afflicted with spina bifida by approximately 50 %.
Discussion
The recommendation of the Ministry of Health and Labour of Japan in 2000 significantly increased the proportion of women who periconceptionally took FA supplements (Table 1) and who were aware of the important role of FA (Table 2). The real problem, however, is that the prevalence of spina bifida has not decreased over the past 30 years (Fig. 1). It can be said, therefore, that the recommendation is a public health failure, and mandatory food fortification with FA is urgently required and long overdue in Japan.
Folic acid use in the era of food fortification
Mosley et al. ( Reference Mosley, Cleves and Siega-Riz 9 ) reported a case–control study from the US in 2009 with 285 case women who gave birth to newborns with spina bifida and 2743 control women during a period from 1998 through 2003; their study background was different from that of ours because mandatory food fortification with FA in the former had been implemented( 4 ). It is not surprising that these authors failed to confirm any risk reduction due to FA supplementation with an adjusted OR of 1·4 (95 % CI 1·0, 1·8), because food fortification had significantly improved blood constituents. For instance, serum folate concentration significantly increased from 12·6 to 18·7 ng/ml( Reference Laurence, Petitti and Watkins 10 ), and the proportion of people with either low serum folate ( < 3 ng/ml) or with high homocysteine concentration (>13 μmol/l) declined from 22·0 to 1·7 % in the former (P< 0·001), and from 18·7 to 9·8 % in the latter (P< 0·001)( Reference Jacques, Selhub and Bostom 11 ), respectively. It is possible that the higher serum folate and lower homocysteine concentrations decreased the occurrence of FA-preventable NTD; thus, an add-on effect of FA supplements was not obtainable. It is possible that the risk reduction of supplement use was considerably influenced by the presence of mandatory food fortification.
Knowledge promotes supplement use
Among the six possible parameters (Table 2), knowledge of the preventive role of FA was considered the sole promoting factor for an increase in supplement use, whereas the rest of the parameters had neither increased nor decreased in either group between periods 1 and 2, and had no relationship with supplement use. Matsuo( Reference Matsuo 12 ) evaluated awareness of FA use among 836 young female college students in Japan. He observed that although 42 % of them had some knowledge of FA, only 9 % had a detailed understanding of the preventive role of FA. The data suggest that women of reproductive age or women planning to conceive in Japan are mostly unaware of the preventive role of FA and this crucial awareness should be systematically disseminated by the government, mass media and medical societies. If we take advantage of the high rate of students' enrolment to junior high school, 99·9 %, or to the senior high school, 98·1 %( 13 ), transmitting this information to female students as a part of health education or sex education components of school curricula would seem to be quite rational and effective. In 2005, Botto et al. ( Reference Botto, Lisi and Robert-Gnansia 14 ) studied the efficacy of transmitting information by exploring thirteen birth defect registries in European countries from 1988 to 1998. They concluded that governmental recommendations alone were followed by no detectable improvement in the trend of incidence of NTD. We fully agree with their emphasis on the great importance of FA supplement and prompt implementation of food fortification and guidelines.
Planned pregnancy and supplement use
Although the rate of planned pregnancy among our subjects was fairly high – 62 % in case women and 68 % in control women (Table 2), the proportion of women who periconceptionally took FA supplements was surprisingly low – 10 % (35/360) in the former and 23 % (538/2333) in the latter (Table 3). In order to establish an association between the two parameters, we searched for articles published after 1991, when the classic Medical Research Council study( 1 ) was reported. Figures available in other reports are: Werler et al. ( Reference Werler, Shapiro and Mitchell 15 ), 60 % of planned pregnancies v. 8 % of supplement use in case mothers and 13 % in control mothers from the US and Canada; Knudsen et al. ( Reference Knudsen, Orozova-Bekkevold and Rasmussen 16 ), 76 % of planned pregnancies v. 14 % supplement use from Denmark; Inskip et al. ( Reference Inskip, Crozier and Godfrey 17 ), 77 v. 3 % from the UK; Nilsen et al. ( Reference Nilsen, Mastroiacovo and Gunnes 18 ), 80 v. 31 % from Norway. On the other hand, among 212 women serving as US military soldiers( Reference Custer, Waller and Vernon 19 ), the rate of planned pregnancy was considerably lower, 35 %. These data obtained from women living in the conventional environment of developed countries suggest that planned pregnancy is prevalent in a range of 60 to 80 %; however, it appears not to be necessarily associated with an increased use of FA supplements, as its actual range of use is 3 to 31 %. Consequently, providing information on FA effectiveness to women planning pregnancy and the careful inclusive designing of health education programs are very effective strategies to encourage greater use of FA supplements and to decrease the prevalence of NTD.
How far is mandatory food fortification effective?
Youngblood et al. ( Reference Youngblood, Williamson and Bell 20 ) estimated that 328 300 infants are born with NTD globally each year, and 75 % of them, 246 200, are FA-preventable. They reported that sixty-nine countries fortified wheat or maize flour with FA to varying extents in 2012, which prevented an estimated 38 400 (15·6 %) to 62 800 (25·5 %) cases of the potential 246 200 FA-preventable NTD cases every year, based on their own models. These authors stressed that world-wide FA fortification is necessary for global prevention of FA-preventable spina bifida and anencephaly.
Heseker et al. ( Reference Heseker, Mason and Selhub 21 ) based on their systematic review observed that (1) food fortification or supplement use decreased the prevalence of NTD at birth or abortions by five to eight cases per 10 000, irrespective of countries, ethnicities, and the amount of FA administered, suggesting the presence of a floor effect which restricts the effect of food fortification (the lower threshold); they observed further that (2) the magnitude of decline of the NTD prevalence depends on the initial NTD rate. These authors also mentioned that counting NTD cases among foetuses at birth and from abortions will avoid underestimating the preventive effect of fortification and provide a more realistic analysis. The mean prevalence of NTD in Japan was reported to be 7·2 (5·7+1·5) per 10 000 total births (live births and stillbirths) for a 5-year period from 2008 to 2012 (Fig. 1), while there is no information on the number of induced abortions due to NTD. Based on a prediction of our clinically active colleagues (obstetricians and gynaecologists), the proportions of induced abortions were deemed to be 30 % for fetuses afflicted with spina bifida and 90 % with encephalocele or anencephaly. When using these figures, the baseline incidence of current NTD would increase from 7·2 to 23·1 (i.e. 8·1+15·0) per 10 000 total births and abortions. If we then let the value 23·1 equal X in the formula (Y= 0·77X− 4·6) reported by De Wals et al. ( Reference De Wals, Tairou and Van Allen 22 ), we would get Y= 13·2 as the difference in prevalence rates before and after fortification. In other words, the post-fortification prevalence of NTD would decrease from 23·1 to 9·9 (i.e. 23·1 − 13·2), which is a 57 % reduction. We believe that our case–control study together with the epidemiological analysis of De Wals et al. ( Reference De Wals, Tairou and Van Allen 22 ) suggest that the present prevalence of spina bifida will be halved by a food fortification policy in Japan, and that the floor effect theory( Reference Heseker, Mason and Selhub 21 ) will not be applicable to the Japanese prevalence. It should be noted that three times as many NTD have occurred in Japan as have been officially reported( 6 ), and that two-thirds have been insidiously aborted, creating a health hazard for the pregnant women involved and increasing healthcare expenditure.
Study limitations
First, we had difficulty in collecting responses to questionnaires from the control women because of selection bias. Initially, we planned to recruit six control women matched for age, sex, place and calendar year of birth for each case woman. However, very few control women responded to the questionnaire, because the number of women who had delivered were not enough in the majority of hospitals to obtain a sufficient number of controls, and because the women chosen as controls shifted residence and hence we could not contact them. Subsequently we changed the study plan and sent a questionnaire to two random control women who had delivered a healthy baby for each year between 2001 and 2012. This is the reason why place and year of birth were different between the groups (Table 1). Second, the bearing of recall bias on data collected needs to be considered: we expected more than half of the case and control women to recall anthropometric variables and life style factors for 6 to 12 years after delivery. It is likely that intakes of supplements, knowledge of FA, diet and other information were difficult to recall accurately as time went by; analysis of inherently weak data could lead to inherently weak interpretation and weak findings. Despite these limitations we believe that the present analysis offers valuable information for clinicians and policy makers to prevent the occurrence of NTD.
In conclusion, our case–control study evidenced that knowledge about the preventive role of FA was a promoter of increased FA supplement use, which was found to be associated with a reduction in the risk of spina bifida births by 50 % approximately. We strongly urge the Japanese Government to introduce a mandatory FA food fortification programme for Japanese women, and launch a sustained awareness campaign on the preventive role of FA at the earliest. This will surely reduce the number of neonates born with avoidable serious birth defects, which in turn will lead to considerable economic benefits( Reference Jentink, van de Vrie-Hoekstra and de Jong-van den Berg 23 ), and significant reduction in the cost burden on the healthcare system and healthcare payers( Reference Grosse, Waitzman and Romano 24 ).
Acknowledgements
We thank K. Jonin, S. Matsumoto, T. Morioka, A. Usui and K. Yoshino who cooperated with us in recruiting case women. We are grateful to Godfrey P. Oakley, Jr for his helpful comments.
The study was supported by a grant from the Ministry of Health, Labour and Welfare of Japan, 2011: H23 Nanchi-Japan 050.
The authors declare no conflict of interest.
The authors' contributions are as follows: A. K. and N. M. designed the study, collected literature, wrote the manuscript and had primary responsibility for the final content. All authors except T. W. formulated the questionnaire, interpreted raw data, and discussed the study outcome. T. W. did all statistical analyses and interpreted the study outcome. All authors read and approved the final version of the report.







