Heart failure (HF) is a complex clinical syndrome that affects over 6·5 million adults in the USA(Reference Mozaffarian, Benjamin and Go1). In addition to its detrimental effects on several organ systems, the presence of HF is associated with higher risk of cognitive decline and dementia(Reference Wolters, Segufa and Darweesh2–Reference Elias, Sullivan and Elias5). Similarly, subclinical alterations in cardiac structure and function (i.e. cardiac remodelling) that precede the clinical manifestation of overt HF are associated with poor cognitive function and cerebral health(Reference Jefferson, Himali and Au6–Reference Kresge, Khan and Wagener8). Previous studies have highlighted the importance of diet as a modifiable risk factor for cognitive decline and dementia(Reference Morris9). Whether a dietary pattern that emphasises foods thought to promote the maintenance of neurocognitive health also mitigates cardiac remodelling is unclear.
Lifestyle recommendations for the prevention of HF emphasise the adoption of dietary patterns, such as the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH), that are characterised by high intakes of plant-based foods(Reference Aggarwal, Bozkurt and Panjrath10). Though evidence examining the relations between dietary patterns and subclinical cardiac remodelling is limited, prior work suggests that Mediterranean and DASH dietary patterns are favourably associated with measures of left ventricular (LV) function(Reference Levitan, Ahmed and Arnett11,Reference Nguyen, Bertoni and Nettleton12) . However, the associations between dietary patterns and LV mass (LVM) are less clear(Reference Levitan, Ahmed and Arnett11,Reference Gardener, Rundek and Wright13) . The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet is based on central components from both the Mediterranean and DASH dietary patterns and further emphasises foods that may offer neurocognitive benefit(Reference Morris, Tangney and Wang14). Furthermore, moderate adherence to the MIND diet has been shown to be associated with lower risk of Alzheimer’s disease(Reference Morris, Tangney and Wang15). These observations may suggest that the MIND diet is more efficient compared with other dietary patterns with regard to the maintenance of neurocognitive health. To our knowledge, no studies have examined the relations between the MIND diet and indices of cardiac remodelling. We hypothesised that a greater cumulative MIND diet score would have a favourable association with LV structure and function.
Methods
Study sample
Participants of the Framingham Offspring Study who attended their eighth examination cycle (2005–2008) were eligible for the present community-based cross-sectional investigation. Details of the Framingham Offspring Cohort have been described previously(Reference Kannel, Feinleib and McNamara16). Among the 3021 eligible participants, we excluded 452 who did not have complete dietary data. We further excluded fifty-seven participants who had no echocardiography data. This resulted in a final sample of 2512 for our cross-sectional evaluation of the MIND diet with echocardiographic indices. The detailed sample size for each echocardiographic index is presented in Fig. 1. The Boston University Medical Center Institutional Review Board approved the study protocol and all participants provided written informed consent.
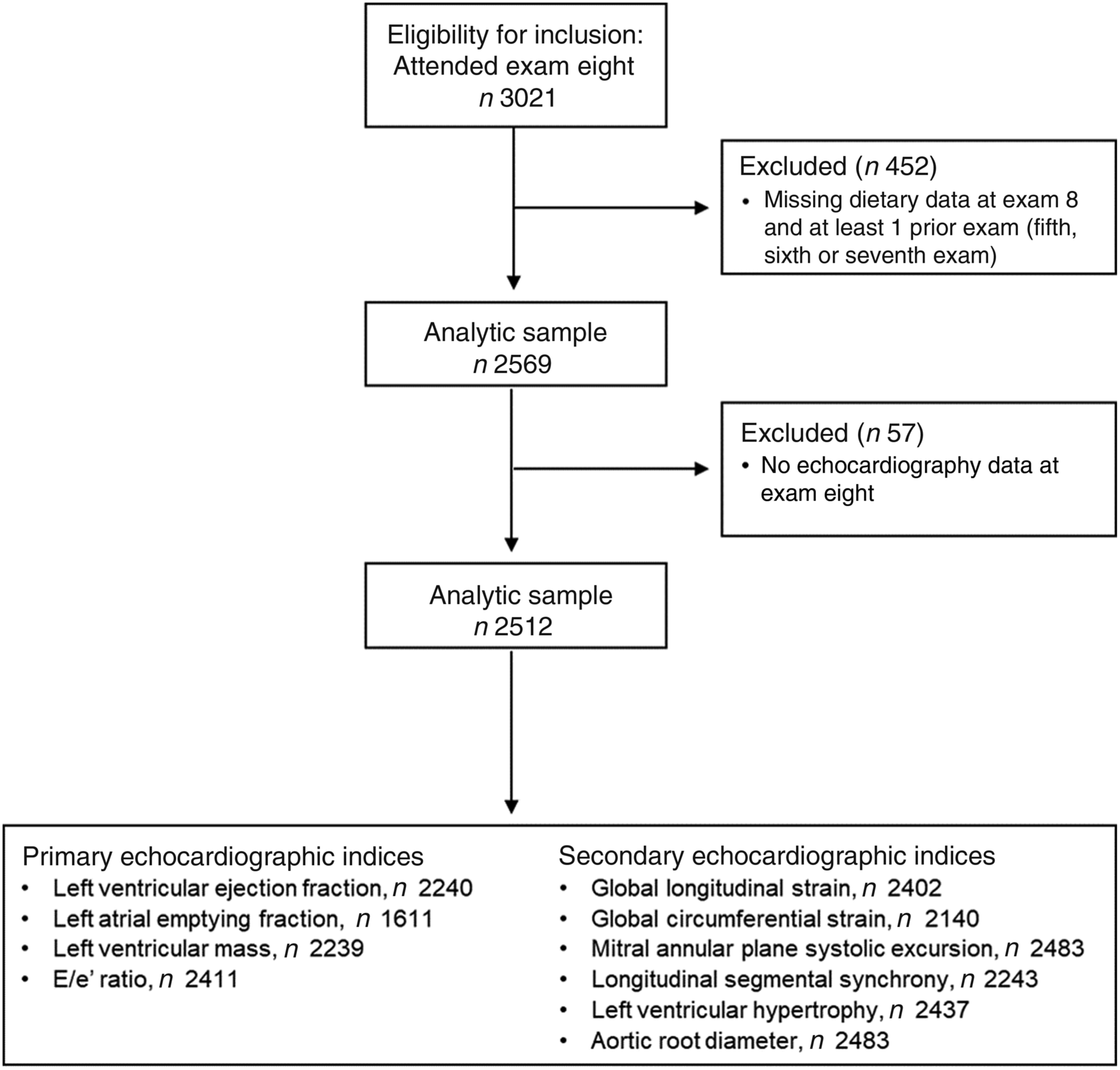
Fig. 1. Participant flow diagram of the study sample.
Dietary assessment and construction of the Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet score
For this investigation, we used the previously created MIND diet score, which was computed based on responses to the Harvard semi-quantitative FFQ.(Reference Morris, Tangney and Wang15) The Harvard FFQ contains 126 food items and nine categories to indicate usual frequency of consumption over the prior 12 months(Reference Rimm, Giovannucci and Stampfer17). Use of this FFQ has previously been validated by comparing with 7-d dietary records(Reference Feskanich, Rimm and Giovannucci18).
Details of the MIND diet score have been previously described(Reference Morris, Tangney and Wang14,Reference Morris, Tangney and Wang15) . In brief, the MIND diet score consists of ten brain healthy components (whole grains, green leafy vegetables, other vegetables, berries, fish, poultry, beans, nuts, alcohol/wine and use of olive oil as the primary cooking oil) and five unhealthy components (red meat and meat products, fast/fried food, butter and margarine, pastries and sweets, and cheese) with specified serving amounts. For each food component, participants were classified into tertiles and assigned a score of either 0, 0·5 or 1 based on concordance with the specified serving frequency (online Supplementary Table S1). Respectively, assigned scores indicate low (0), moderate (0·5) or high (1) concordance with each MIND food component. Olive oil was scored as 1 if used as the primary cooking oil or 0 if not. We calculated the total score by summing all components, with a maximum score of 15 representing the highest adherence to the MIND diet. Cumulative dietary intake better captures the long-term effect of a dietary exposure and reduces potential measurement error(Reference Hu, Stampfer and Rimm19). Hence, for each participant we calculated the cumulative MIND diet score by averaging the MIND diet score from exam eight and all available MIND diet scores at the fifth (1991–1995), sixth (1995–1998) and seventh (1998–2001) examination cycles.
Primary echocardiographic indices
For the present investigation, we defined LVM, LV ejection fraction (LVEF), left atrial emptying fraction (LAEF) and E/e’ as primary echocardiographic indices. Participants underwent routine transthoracic echocardiography using a Sonos 5500 ultrasound machine (Philips Healthcare). Doppler images were obtained in pre-defined two-dimensional views (apical two-chamber, apical four-chamber and mid-ventricular parasternal short axis) using standard protocols(Reference Cheng, Larson and McCabe20). We used the American Society of Echocardiography (ASE)-recommended two-dimensionally guided M-mode tracings and leading edge-to-leading edge technique to measure LV end-diastolic diameter (LVDD), thickness of the septum and posterior wall, and LV end-systolic diameter.(Reference Sahn, DeMaria and Kisslo21) LVM was calculated according to the ASE recommendations using the formula proposed by Devereux et al. (0·8 × 1·04 ((LVDD + septal wall thickness + posterior wall thickness)3 − LVDD3) + 0·6)(Reference Devereux, Alonso and Lutas23). LVEF was calculated using the formula proposed by de Simone et al. ((4·5 × LVDD2) − (3·72 × LV end-systolic diameter2))/(4·5 × LVDD2) × 100(Reference de Simone, Devereux and Ganau24). Left atrial volumes were measured using the area-length method recommended by the ASE(Reference Lang, Badano and Mor-Avi25). Left atrial emptying fraction was calculated as ((left atrial max volume − left atrial min volume)/left atrial max volume) × 100. The E/e’ ratio, a surrogate of LV filling pressure, was used as an assessment of LV diastolic function. E is the maximum early diastolic mitral inflow velocity measured using transmitral Doppler flow velocities(Reference Kaess, Rong and Larson28). e’ is the maximum early diastolic mitral annulus velocity at the lateral mitral annulus measured by tissue Doppler imaging(Reference Kaess, Rong and Larson28).
Secondary echocardiographic indices
Secondary echocardiographic indices included global longitudinal strain (GLS), global circumferential strain (GCS), mitral annular plane systolic excursion (MAPSE), longitudinal segmental synchrony (LSS), LV hypertrophy (LVH) and aortic root diameter (AOR). We used the ASE-recommended two-dimensionally guided M-mode tracings and leading edge-to-leading edge technique to measure aortic root diameter.(Reference Sahn, DeMaria and Kisslo21) LSS was the standard deviation of time-to-peak systolic longitudinal strains measured in twelve regions(Reference Cheng, Larson and McCabe22). ASE recommendations were used to define LVH based on elevated LVM indexed to body surface area (≤115 or >115 gm/m2 for men and ≤95 or >95 gm/m2 for women)(Reference Lang, Badano and Mor-Avi25). Mitral annular plane systolic excursion was measured in the apical four-chamber views at the lateral side of the annulus as systolic excursion of the annulus from its lowest point at end-diastole to its highest point at the time of aortic valve closure(Reference Hu, Liu and Herrmann26). LV cardiac strain was assessed in the pre-defined two-dimensional views by standard protocols(Reference Cheng, Larson and McCabe20). Measurements were obtained using an offline speckle-tracking software package (2D Cardiac Performance Analysis v1.1; TomTec Imaging Systems) with a validated speckle-tracking algorithm(Reference Amundsen, Helle-Valle and Edvardsen27). GLS was computed as the average longitudinal strains from the two- and four-chamber views, and GCS was computed from the short-axis view. We have previously reported the reproducibility of echocardiographic indices measured at the Framingham Heart Study(Reference Cheng, Larson and McCabe20,Reference Kaess, Rong and Larson28,Reference Sundström, Sullivan and Selhub29) .
Covariate measures
For this investigation, we used the following covariates: age, sex, energy intake, smoking status, use of hypertension medication, diabetes status, total cholesterol to HDL-cholesterol ratio (TC/HDL-C), systolic blood pressure (SBP), BMI, physical activity and ventricular rate. Participants underwent a routine physical examination and provided a detailed medical history using standardised protocols at each Framingham Heart Study visit. All covariates were assessed at the eighth examination cycle. We used the average of two blood pressure measurements (taken 5 min apart) obtained by a physician using a mercury column sphygmomanometer on the participant’s left arm. Resting heart rate was measured by 12-lead electrocardiography with the participants lying in a supine position. BMI was calculated as weight divided by height squared (kg/m2). Use of anti-hypertensive medications was based on self-report. We classified participants as having diabetes mellitus if they had fasting blood glucose concentrations ≥126 mg/dl or used hypoglycaemic medications. Energy intake was calculated from the aforementioned FFQ. We classified participants who smoked regularly in the year preceding their eighth examination as current smokers. For each participant, a physical activity index score was calculated based on time and intensity of activities in a day(Reference Kannel and Sorlie30): (basal hours
![]() $\times\, 1\cdot 0$
) + (sedentary hours
$\times\, 1\cdot 0$
) + (sedentary hours
![]() $\times\, 1\cdot 1$
) + (slightly active hours
$\times\, 1\cdot 1$
) + (slightly active hours
![]() $\times\, 1\cdot 5$
) + (moderate active hours
$\times\, 1\cdot 5$
) + (moderate active hours
![]() $ \times\, 2\cdot 4$
) + (heavy activity hour
$ \times\, 2\cdot 4$
) + (heavy activity hour
![]() $\times\, 5\cdot 0$
) = physical activity index.
$\times\, 5\cdot 0$
) = physical activity index.
Statistical analysis
Participant characteristics are presented by sex. Mean and standard deviation are presented for continuous, normally distributed variables, as well as the P-value from a two-sample t test by sex. Median and interquartile range are presented for continuous, skewed variables, as well as the P-value from a Wilcoxon rank sum test by sex. Frequency and percentage are presented for categorical variables, as well as the P-value from a χ 2 test by sex. We first examined the associations between the cumulative MIND diet score and clinical risk factors (smoking status, use of anti-hypertensive medication, prevalent diabetes mellitus, total cholesterol to HDL-cholesterol ratio (TC/HDL-C), SBP, BMI, ventricular rate and physical activity) using Spearman’s partial correlations adjusting for age, sex and energy intake.
Using multivariable linear regression models, we then related the cumulative MIND diet score (independent variable) to echocardiography indices (dependent variables; separate model for each). We modelled the cumulative MIND diet score as a continuous variable to maximise statistical power. Our primary analysis included LVM, LVEF, left atrial emptying fraction and E/e’, which together provide a comprehensive assessment of LV structure and function. Secondary analyses included separate linear regression models for GLS, GCS, MAPSE, LSS and aortic root diameter. Logistic regression was used to examine the association between the cumulative MIND diet score (continuous) and LVH (binary). We completed a visual assessment and considered objective measures of skewness (<|1|) and kurtosis (<|3|) to determine departures from normality. We natural log-transformed BMI, LVM, E/e’ and LSS to normalise their skewed distributions. We adjusted initial models for age, sex and total energy intake (model 1) and then further adjusted for SBP, use of anti-hypertensive medication, smoking status, prevalent diabetes mellitus, TC/HDL-C, BMI, ventricular rate and physical activity (model 2). Results were considered statistically significant based on a Bonferroni correction for primary (P ≤ 0·0125, 0·05/4; four primary echocardiographic traits) and secondary (P ≤ 0·008, 0·05/6; six secondary echocardiographic variables) analyses. We tested for effect modification by age and sex on the associations between the cumulative MIND diet score and echocardiographic indices that were statistically significant in model 1. We used a two-sided 0·05 significance level to define significance of the interaction and stratified results accordingly. We additionally completed a sensitivity analysis to explore the effect of sex on the relations of the MIND diet with all echocardiographic outcomes (model 1). All statistical analyses were completed using SAS statistical software (version 9.4; SAS Institute).
Results
Sample characteristics
Table 1 displays sex-specific participant characteristics. On average, the study sample was metabolically healthy, with a low prevalence of smoking, diabetes mellitus and history of CVD. Approximately 77 % of participants had available data on the MIND score at all four examination cycles evaluated (i.e. exams 5, 6, 7 and 8). The average cumulative MIND diet score was 7·0 (sd 1·7).
Table 1. Sample characteristics by sex
(Numbers and percentages)*
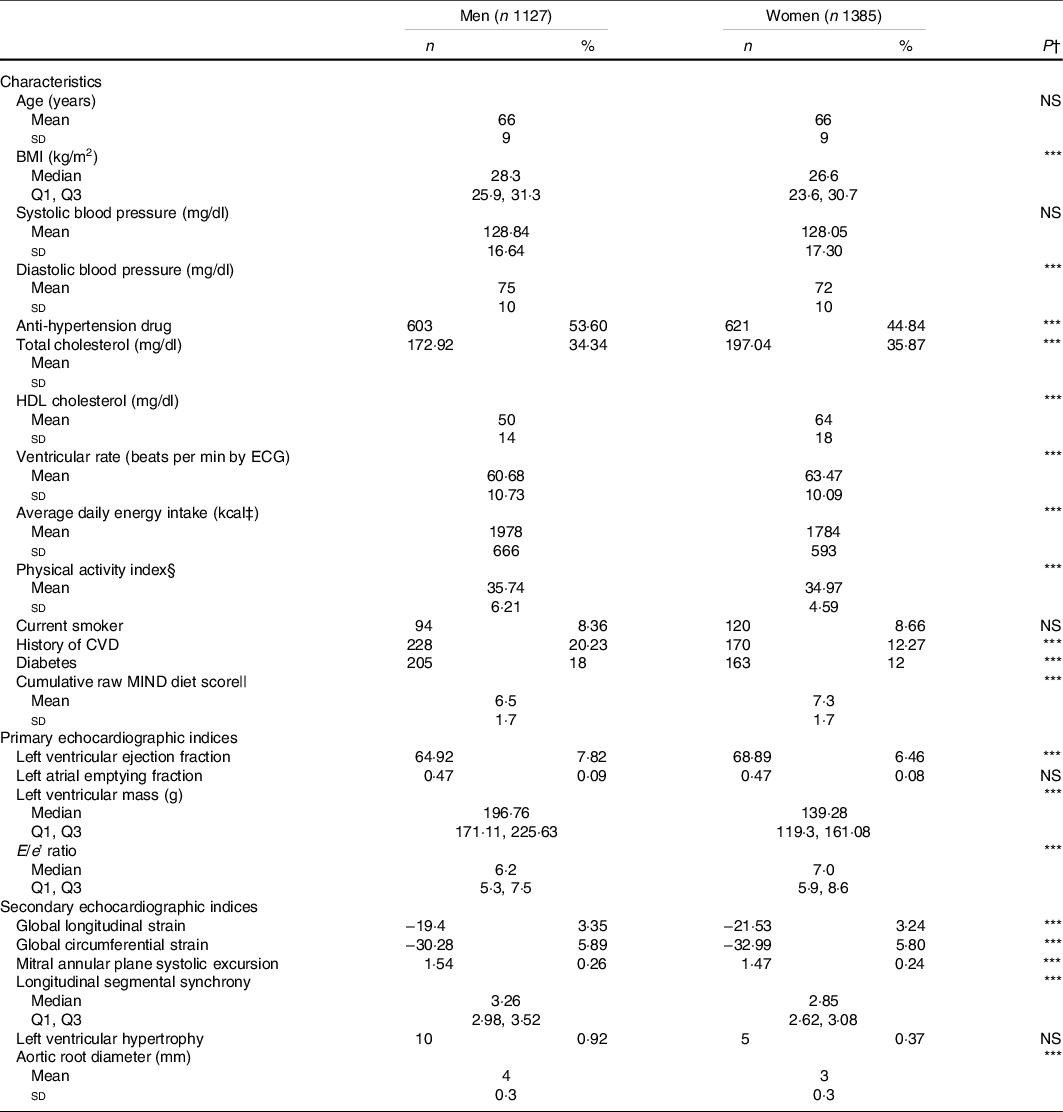
ECG, electrocardiography.
* Values are mean and standard deviation or median and Q1, Q3 for continuous variables and frequency (proportion) for categorical variables.
† P-value assessing difference in characteristic between sexes. NS indicates a non-significant P-value. *** indicates P < 0·01.
‡ To convert kcal to kJ multiply by 4·184.
§ Physical activity index = (basal hours × 1·0) + (sedentary hours × 1·1) + (slightly active hours × 1·5) + (moderate active hours × 2·4) + (heavy activity hours × 5·0).
|| Cumulative MIND diet score is the average diet score of exam eight and at least one of exams five, six or seven.
Associations between the cumulative Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet score and primary echocardiographic indices
Adjusting for age, sex and total energy intake, we observed inverse correlations of the cumulative MIND diet score with ventricular rate, TC/HDL-C, BMI, use of anti-hypertensive medication, prevalent diabetes and current smoking (Table 2). The cumulative MIND diet score had a positive correlation with physical activity. In a model adjusting for age, sex and total energy intake, the cumulative MIND diet score was directly associated with LVM and inversely associated with E/e’ (model 1, Table 3). Upon further adjustment for clinical risk factors (smoking status, use of anti-hypertensive medications, prevalent diabetes, TC/HDL-C, SBP, BMI, ventricular rate and physical activity; model 2), only the association between the cumulative MIND diet score and LVM was maintained. In a sensitivity analysis stratified by sex, we observed that the positive association between the cumulative MIND diet score and LVM only appeared in women.
Table 2. Correlations between the cumulative Mediterranean-Dietary Approaches to Systolic Hypertension Intervention for Neurodegenerative Delay diet score and clinical risk factors*
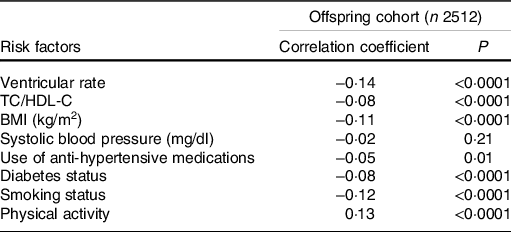
TC/HDL-C, total cholesterol to HDL-cholesterol ratio.
* Spearman partial correlation coefficients adjusted for age, sex and total energy intake.
Table 3. Associations between the cumulative Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet score and echocardiographic indices
(Numbers; β-coefficients and standard errors)
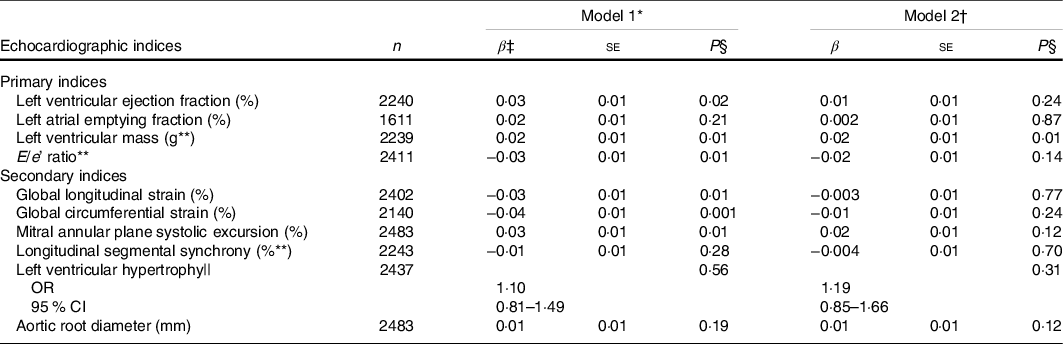
* Model 1 adjusted for sex, age and total energy intake.
† Model 2 additionally adjusted for systolic blood pressure, anti-hypertensive medication, smoking status, diabetes mellitus, total to HDL-cholesterol ratio, BMI*, ventricular rate and physical activity.
‡ β estimates represent the change in standardised echocardiographic index per 1 unit increase in the cumulative MIND diet score.
§ Bonferroni-corrected significance level is 0·01 for primary indices and 0·008 for secondary indices.
|| Odds ratios and corresponding 95 % CI. Indicates change in the odds of LV hypertrophy.
** Log-transformed.
Associations between the cumulative Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet score and secondary echocardiographic indices
We observed inverse associations of the cumulative MIND diet score with GCS (Table 3). We also observed effect modification in the association between the cumulative MIND diet score and GCS by age (P interaction = 0·03, median = 66 years). In the analyses stratified by age, the cumulative MIND diet score had the strongest inverse association with GCS in participants above the median age of 66 years (Table 4).
Table 4. Associations between cumulative Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet score and global circumferential strain (GCS) stratified by age
(Numbers and percentages; β-coefficients and standard errors)

* Model 1 adjusted for sex, age and total energy intake.
† Model 2 additionally adjusted for systolic blood pressure, anti-hypertensive medication, smoking status, diabetes mellitus, total cholesterol to HDL-cholesterol ratio, BMI, ventricular rate and physical activity.
‡ β estimates represent the change in each echocardiographic index per 1 unit increase in the cumulative MIND diet score.
Sensitivity analysis
In a sensitivity analysis stratified by sex, we observed a positive association between the cumulative MIND diet score and LVM in women (P = 0·003) but not in men (P = 0·40; model 1; online Supplementary Table S2). Relations of the cumulative MIND diet score with other primary (LVEF, LAEF and E/e’) and secondary (GLS, GCS, MAPSE, LSS, LVH and AOR) echocardiographic indices were not significant following the appropriate Bonferroni adjustments.
Discussion
In contrast to our initial hypothesis, we observed that the cumulative MIND diet score had a direct association with LVM, which persisted in models adjusted for clinical risk factors and with further adjustment for physical activity. In agreement with our initial hypotheses, we observed favourable associations of the cumulative MIND diet score with echocardiographic indices of both LV diastolic (E/e’) and systolic function (GCS). However, following adjustment for clinical risk factors (smoking status, use of anti-hypertensive medications, prevalent diabetes, TC/HDL-C, SBP, BMI, ventricular rate and physical activity) only the association with GCS in participants >66 years old remained. Our results suggest that relations between the cumulative MIND diet score and cardiac remodelling are not uniform and differ among indices of LV structure and function.
The positive association between the cumulative MIND diet score and LVM was unexpected, as a higher LVM has been shown to be associated with adverse cardiac remodelling and future incident of cardiovascular events(Reference Levy, Garrison and Savage31,Reference Bikkina, Levy and Evans32) . However, we did not observe an association between the cumulative MIND diet score and LVH, and initial (model 1) associations of the cumulative MIND diet score with indices of LV systolic and diastolic function suggested favourable relations. Hence, the clinical significance of the observed positive association between the cumulative MIND diet score and LVM needs to be further evaluated.
Few studies have examined relations between dietary patterns and LVM. A clinical trial reported a favourable effect of a DASH diet on LV diastolic function but no effect on LVM in individuals who had HF with preserved ejection fraction and hypertension(Reference Hummel, Seymour and Brook33). Another trial reported that a DASH diet only in combination with weight loss reduced LVM in individuals with pre-hypertension(Reference Blumenthal, Babyak and Hinderliter34). Previous population-based studies examining dietary patterns and LVM have varying results. Prior work in the Northern Manhattan study noted that a Mediterranean-style diet was inversely associated with LVM.(Reference Gardener, Rundek and Wright13) In the Multi-ethnic Study of Atherosclerosis cohort, an empirically derived Western-type dietary pattern was positively associated with LVM(Reference Liu, Nettleton and Bertoni35). In contrast with these studies, and more similar to our results, additional work in the Multi-ethnic Study of Atherosclerosis reported that greater conformity to a Mediterranean-style diet was associated with LVM in a quadratic manner but was not associated with LVH(Reference Levitan, Ahmed and Arnett11). In the Multi-ethnic Study of Atherosclerosis, moderate alcohol consumption (5–25 g/d for women and 10–50 g/d for men), relative to light or heavy, was the Mediterranean diet component that had the strongest direct association with LVM(Reference Levitan, Ahmed and Arnett11). Investigators from the Suivi Temporaire Annuel Non-Invasif de la Santé des Lorrains Assurés Sociaux study reported positive associations of dietary patterns, derived by reduced-rank regression including ‘alcohol’ and ‘fast food and alcohol’, with LVM in men(Reference Wagner, Lioret and Girerd36). Moderate alcohol consumption was also reported to be directly associated with LVM in the Trøndelag Health Study(Reference Gémes, Janszky and Strand37). Thus, it is plausible that moderate alcohol intake associated with adherence to the MIND diet is not related to LVM in a favourable manner.
We did not observe significant interactions by sex in the relation of the MIND diet with echocardiographic indices. However, there are known differences in LVM among men and women, with men typically having a higher LVM(Reference Bella, Palmieri and Wachtell38). We explored the effect of sex in a stratified analysis, which suggested the association of the MIND diet and LVM was limited to women. Prior assessments of dietary patterns and LVM have not observed significant effect modification by sex(Reference Levitan, Ahmed and Arnett11,Reference Gardener, Rundek and Wright13,Reference Liu, Nettleton and Bertoni35) . The aforementioned Suivi Temporaire Annuel Non-Invasif de la Santé des Lorrains Assurés Sociaux study observed distinct associations of derived unhealthy dietary patterns and LVM in an analysis stratified by sex(Reference Wagner, Lioret and Girerd36). Future well-powered studies should seek to determine if relations between healthy dietary patterns and LVM differ by sex.
The favourable associations we observed between the cumulative MIND diet score and indices of LV function in initial models are in agreement with prior work. Dietary patterns similar to the MIND diet (DASH and Mediterranean) have favourable associations with indices of LV systolic function such as stroke volume, end-diastolic volume and LVEF(Reference Nguyen, Bertoni and Nettleton12,Reference Gardener, Rundek and Wright13,Reference Chrysohoou, Panagiotakos and Aggelopoulos39,Reference Chrysohoou, Pitsavos and Metallinos40) . Conformity to a Mediterranean-style diet has additionally been noted to be favourably associated with E/A ratio, a measure of LV filling pressure(Reference Chrysohoou, Pitsavos and Metallinos40). Similarly, we observed a favourable association of the cumulative MIND diet with E/e’ ratio, an index of diastolic function which serves as a sensitive surrogate of LV filling pressure. The exact indices used to measure LV function differ across studies making direct comparisons of different dietary patterns on specific indices difficult. Our investigation adds to the literature by identifying favourable associations of the cumulative MIND diet score with E/e’ ratio and GCS, an indicator of circumferential strain.
Mechanisms mediating the association between the cumulative MIND diet score and LV function may include multiple clinical risk factors. We observed that the cumulative MIND diet score had inverse associations with the use of hypertension medication, prevalent diabetes, blood lipids, ventricular rate, BMI and smoking. Further, the favourable associations we observed between the cumulative MIND diet score and indices of LV function were attenuated following adjustment for the aforementioned clinical risk factors. The MIND diet puts individual foods that have the most convincing evidence for neurocognitive benefit into context of a complete dietary pattern(Reference Morris9,Reference Morris, Tangney and Wang14) . In particular, the MIND diet emphasises consumption of berries and green leafy vegetables while limiting intakes of foods high in saturated fat and animal products but omits specific recommendations on total fruit, total dairy and a high (≥ 6 servings) weekly fish consumption. Antioxidant and anti-inflammatory effects are proposed mechanisms thought to mediate associations of healthy dietary patterns, including the MIND diet, with cognitive decline and dementia(Reference van de Rest, Berendsen and Haveman-Nies41). Prior studies indicate that oxidative stress and systemic inflammation may contribute to LV remodelling(Reference Münzel, Gori and Keaney42–Reference Velagaleti, Gona and Levy44). Hence, it is plausible that favourable associations of the MIND diet with LV function and cognitive decline are through shared mechanisms. Lastly, the results from our primary analysis suggest the MIND diet is associated with higher LVM, which may be potentially deleterious. Reasoning for this observation is unclear, and discordance in literature concerning associations between dietary patterns and LVM warrants further investigation.
Strengths and limitations
The strengths of the present investigation include the large sample size and collection of both detailed dietary data and echocardiographic indices in the Framingham Offspring Cohort. In particular, we had measurements on ten unique echocardiographic indices to provide comprehensive assessment of a dietary pattern and cardiac remodelling. Further, we accounted for multiple testing using a Bonferroni correction to control the rate of type 1 error. However, the present investigation is not without limitations. Notably, our study is cross-sectional and cannot establish causality between the MIND diet and echocardiographic indices of cardiac remodelling. It is possible that, for some echocardiographic indices, we lacked statistical power to observe true associations with the MIND diet. Self-reported dietary data are subject to measurement error and recall bias. We used repeated measures of dietary intake over 8–14 years to reduce potential biases. The Harvard semi-quantitative FFQ used does not measure all components of the MIND diet. Hence, our calculation of the MIND diet may omit individual food components that are associated with echocardiographic indices. Additionally, while the MIND diet score has been associated with slowed cognitive decline and reduced incidence of Alzheimer’s disease in prior studies(Reference Morris, Tangney and Wang14,Reference Morris, Tangney and Wang15) , the efficacy of the MIND diet has yet to be established in clinical trials. Our study sample is predominantly white and of European descent and may not be generalisable to other ethnic populations. However, our results pertaining to LV function were generally in agreement with prior work in more ethnically diverse populations. Lastly, although the models we used adjusted for many lifestyle and clinical risk factors, we cannot rule out the possibility of uncontrolled or residual confounding.
Conclusion
In this cross-sectional sample of middle-aged adults, we observed that a higher cumulative MIND diet score differentially associated with echocardiographic indices of LV structure and function. Clinical risk factors may in part modulate favourable associations of a higher cumulative MIND diet score with indices of LV function (E/e’ ratio and GCS). Future studies should seek to examine the longitudinal relation between dietary patterns, including the MIND diet, and LVM.
Acknowledgements
The authors thank and acknowledge employees and participants of the Framingham Heart Study, without whom this research would not be possible.
This work was supported by the NHLBI Multidisciplinary Training Program in Cardiovascular Epidemiology (5T32HL125232) and the PRIMER (Promoting Research In MEdical Residency program) (1R38HL143584), the American Heart Association (20CDA35310237), the NIH National Heart Lung and Blood Institute (NHLBI) Framingham Heart Study (contract nos. NO1-HC-25195, HHSN268201500001I and 75N92019D00031; and P20 HL113444 and P30 DK020579). Dr. Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the NIH or the ARS.
M. E. W., V. X. and R. S. V.: designed the research; A. A. O. and A. S. B.: analysed the data; M. E. W.: wrote the manuscript; V. X., A. A. O., J. J. H., I. R., D. M. v. L., F. A., P. F. J., A. S. B., S. S. and R. S. V.: critically revised the manuscript; M. E. W. and V. X.: had primary responsibility for the final content and all authors: read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000660








