Recent studies are raising awareness about humans’ circadian patterns of hormones, enzymes and digestive system, and how predisposition to weight gain and metabolic disorders can be affected by the timing of meals(Reference Gill and Panda1–Reference Bandín, Scheer and Luque3). This clock–nutrition interplay, called chrono-nutrition, calls for attention to the timing and frequency of meals(Reference Almoosawi, Vingeliene and Karagounis4). However, whether the composition of each meal influences non-communicable disease (NCD) risk (for instance, whether the response to vegetable intake is different at breakfast than at dinner) has not yet been studied comprehensively.
On a global scale, a low intake of fruits and vegetables, whole grains and fibre, and a high intake of red and processed meats are risk factors of premature death and disability associated with NCD(Reference Gakidou, Afshin and Abajobir5). The risk of NCD is often associated with intermediate cardiometabolic and inflammatory biomarkers such as LDL-cholesterol, HDL-cholesterol, glycated Hb (HbA1c) and C-reactive protein (CRP)(6).
Associations between diet and biomarkers of cardiometabolic risk have been established mostly with data on usual food intake. For instance, an increase in fruit and vegetable consumption has been associated with lower levels of the inflammatory marker CRP(Reference Oliveira, Rodriguez-Artalejo and Lopes7, Reference Sutliffe, Wilson and de Heer8), a diet high in fruit, vegetables and cereal fibre has been associated with lower CRP, and high starch and low fibre intake related to higher HbA1c levels(Reference AlEssa, Ley and Rosner9). Furthermore, a diet rich in whole-grain foods has been shown to decrease LDL-cholesterol(Reference Hollaender, Ross and Kristensen10, Reference Schwingshackl, Hoffmann and Iqbal11), and greater intake of red and unprocessed meats has been associated with higher plasma CRP and HbA1c(Reference Ley, Sun and Willett12). Such averaged consumption data, however, do not consider the contexts of meals, the different times of the day and the corresponding circadian response to the specific foods consumed, which could further help understand diet–disease relationships(Reference Leech, Worsley and Timperio13). To our knowledge, only few studies have reported on meal-specific diet–disease associations. Almoosawi et al. (Reference Almoosawi, Prynne and Hardy14) investigated macronutrient intakes at meals and their association with the risk of metabolic syndrome in a large prospective cohort study and found lower risk with higher carbohydrate and lower fat intakes in the morning period. A smaller, intervention study in healthy overweight subjects found no influence on blood lipids, body weight or glucose metabolism but reduced markers of inflammation after a 3-month prudent breakfast(Reference Adamsson, Reumark and Marklund15).
The aim of the present study is to investigate whether the same foods (vegetables, fruits, whole grains, red and processed meats and refined grains) eaten at different meals (breakfast, lunch or dinner) show different associations with cardiometabolic and inflammatory biomarkers (i.e. LDL-cholesterol, HDL-cholesterol, HbA1c, and CRP).
Methods
Study sample
The eligible study sample consisted of active study participants from the EPIC study (European Prospective Investigation into Cancer and Nutrition) who were selected based on sex- and age-stratified random sampling and invited to take part in a validation sub-study. Out of 1447 invitations sent, 815 adults provided socio-demographic, dietary and anthropometric data, and blood samples between August 2010 and December 2012. Details on the validation sub-study are available elsewhere(Reference Neamat-Allah, Wald and Hüsing16). Ethical approval of the study was obtained from The Ethics Committee of the Medical Association of the State of Brandenburg, and the written informed consent was obtained from all study participants.
Dietary assessment
Dietary intake data were collected with three 24-h dietary recalls (24hDR). All 24hDR were collected by trained interviewers using the EPIC-Soft software(Reference Voss, Charrondiere and Slimani17). The first 24hDR data were collected in the study centre during the participants’ first visit and the following 24hDR data were collected over the telephone on randomly selected days. The mean time between the first and final 24hDR was 7 months and most recalls were recorded within 1 year (99·4 % within 1 year). For every recalled day, food consumption was recorded in grams over eleven different eating occasions, including the three main (participant-identified) meals breakfast, lunch, and dinner. Skipping of the main meals was very low. Only four participants skipped breakfast on 1 d, four participants skipped lunch on 2 d and thirty-eight on 1 d, and thirty-two participants skipped dinner on 1 d (online Supplementary Table S1). Consumption of the following foods at the main meals was selected: vegetables, fruits, whole grains, refined grains and red and processed meats. These food groups were selected for analysis because a high intake (vegetables, fruits and whole grains) or low intake (refined grains, red and processed meats) of these foods are characteristics of diets that have been associated with lower risk of chronic disease(Reference Bechthold, Boeing and Schwedhelm18–Reference Schwingshackl, Schwedhelm and Hoffmann20) and such characteristics are important components of diets recommended by dietary guidelines(Reference Lichtenstein, Appel and Brands21–Reference Schwingshackl, Schlesinger and Devleesschauwer23). Whole grains included cereal products and bread made with/containing the whole unprocessed grain. Refined grains consisted of grains or grain flours that were modified to remove bran and germ. Red meat was considered as meat from beef, veal, pork, mutton/lamb and rabbit, and processed meats included all processed meat products, including those from poultry.
Assessment of biomarkers
Blood was drawn from each study participant on the first visit to the study centre (on the same day as the first 24hDR) and a second time within a time range of 9 months to 3 years later. At each time period, the blood was centrifuged and aliquoted immediately after drawing into seven tubes, containing either plasma (heparin-containing drawing tubes) or serum, and one tube was sent to a local laboratory accredited and specialised for clinical chemical measurements for routine parameters to analyse the samples.
Assessment of socio-demographic and lifestyle information
Socio-demographic and lifestyle information such as age, smoking status, education level, current occupation and hours of physical activity/week was obtained using questionnaires. The physical activity questionnaire consisted of questions on physical activity during the past 12 months. In the present study, physical activity included sports, gardening, physical work, housework and cycling. Anthropometric data, such as height and weight, were measured in the study centre during the first visit. BMI was calculated as weight (in kg) divided by height squared (m2).
Statistical analysis
Analyses are based on a final study sample of 806 participants, after excluding one participant with dementia, and eight participants who missed one of the main meals of interest (lunch) on all 24hDR (see flow chart in the online Supplementary Figure S1). For each food group, we calculated usual intakes at breakfast, lunch, dinner, as well as total (non meal-specific) usual intakes based on the three replicates of the 24hDR per participant and using the National Cancer Institute (NCI) method(Reference Tooze, Kipnis and Buckman24, Reference Kipnis, Midthune and Buckman25). Usual food intakes consisted of two-part regression models representing consumption probability and consumed amount, respectively. Models with correlated random effects were used if there was a positive correlation between probability of consumption and amount consumed and models with uncorrelated random effects were used if there was negative or no correlation (see online Supplementary Table S2). In the case of whole grains at lunch, the uncorrelated model was used despite a positive correlation due to sub-optimal convergence because of participants’ infrequent consumption on two or more occasions. Estimated usual food intakes by the NCI method differ from the observed intakes in that they account for random day-to-day variation (within-person variation); as a result, the pseudo-individual intakes described by the NCI method have a narrower distribution than the observed intakes as the mean of each individual shrinks towards the overall mean(Reference Tooze, Kipnis and Buckman24, Reference Dodd, Guenther and Freedman26). Usual food intakes were adjusted for sex, age, BMI, education level, current occupation, physical activity, smoking status and usual energy intake; usual energy intake was calculated with the NCI method and adjusted for sex, age, BMI, education level, current occupation, physical activity and smoking status.
The first measurement of biomarkers was used for analyses. If the first blood sample was unavailable for participants, the second sample was used (n 4 for LDL-cholesterol, HDL-cholesterol, and HbA1c; n 13 for CRP). Participants with missing data for a particular biomarker were excluded from the analysis (see online Supplementary Figure S1).
The fitted linear regression models parameterised as:
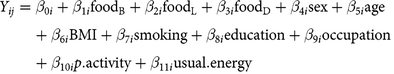 $$\eqalign{{Y_{ij}}& = {\beta _{0i}} + {\beta _{1i}}{\text{foo}}{{\text{d}}_{\text{B}}} + {\beta _{2i}}{\text{foo}}{{\text{d}}_{\text{L}}} + {\beta _{3i}}{\text{foo}}{{\text{d}}_{\text{D}}} + {\beta _{4i}}{\text{sex}} + {\beta _{5i}}{\text{age}}\cr & \quad + {\beta _{6i}}{\text{BMI}} + {\beta _{7i}}{\text{smoking}} + {\beta _{8i}}{\text{education}} + {\beta _{9i}}{\text{occupation}} \cr & \quad + {\beta _{10i}}p.{\text{activity}} + {\beta _{11i}}{\text{usual}}.{\text{energy}}}$$
$$\eqalign{{Y_{ij}}& = {\beta _{0i}} + {\beta _{1i}}{\text{foo}}{{\text{d}}_{\text{B}}} + {\beta _{2i}}{\text{foo}}{{\text{d}}_{\text{L}}} + {\beta _{3i}}{\text{foo}}{{\text{d}}_{\text{D}}} + {\beta _{4i}}{\text{sex}} + {\beta _{5i}}{\text{age}}\cr & \quad + {\beta _{6i}}{\text{BMI}} + {\beta _{7i}}{\text{smoking}} + {\beta _{8i}}{\text{education}} + {\beta _{9i}}{\text{occupation}} \cr & \quad + {\beta _{10i}}p.{\text{activity}} + {\beta _{11i}}{\text{usual}}.{\text{energy}}}$$
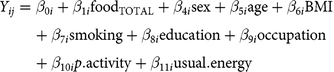 $$\eqalign{{Y_{ij}}& = {\beta _{0i}} + {\beta _{1i}}{\text{foo}}{{\text{d}}_{{\text{TOTAL}}}} + {\beta _{4i}}{\text{sex}} + {\beta _{5i}}{\text{age}} + {\beta _{6i}}{\text{BMI}}\cr & \quad + {\beta _{7i}}{\text{smoking}} + {\beta _{8i}}{\text{education}} + {\beta _{9i}}{\text{occupation}}\cr & \quad + {\beta _{10i}}p.{\text{activity}} + {\beta _{11i}}{\text{usual}}.{\text{energy}}}$$
$$\eqalign{{Y_{ij}}& = {\beta _{0i}} + {\beta _{1i}}{\text{foo}}{{\text{d}}_{{\text{TOTAL}}}} + {\beta _{4i}}{\text{sex}} + {\beta _{5i}}{\text{age}} + {\beta _{6i}}{\text{BMI}}\cr & \quad + {\beta _{7i}}{\text{smoking}} + {\beta _{8i}}{\text{education}} + {\beta _{9i}}{\text{occupation}}\cr & \quad + {\beta _{10i}}p.{\text{activity}} + {\beta _{11i}}{\text{usual}}.{\text{energy}}}$$
where i refers to the ith study participant, j refers to the biomarker type, foodB is usual food intake at breakfast, foodL is usual food intake at lunch, foodD is usual food intake at dinner, and foodTOTAL is total usual food intake (non meal-specific); we assumed independence among the three meals (see partial correlations across meals in online Supplementary Table S3). Dependent variables (Yij) were circulating blood biomarkers: CRP (mg/l), HbA1c (%), and LDL- and HDL-cholesterol (mmol/l). Models were adjusted for sex (men, women), age (continuous, in years), BMI (continuous, in kg/m2), smoking status (never, former, current), education level (no vocational training/current training, technical college, university), current occupation (full-time, part-time/hourly, no job/retired), physical activity (continuous, in h/week), and usual energy intake (continuous, kilocalories/d). Equation (1) was used for the main analyses, which are meal-specific. Equation (2) for total (non meal-specific) food intake was analysed for comparison and interpretation support of meal-specific analyses. Results are expressed per 50 g of the respective food group. Because CRP concentration was not normally distributed, we used quantile regression based on the median, as this approach is robust for skewed distributions without the need to transform the data(Reference Beyerlein27, Reference Koenker28). We further estimated Spearman partial correlations (rho) for usual meal-specific food intakes and biomarkers, adjusting for sex, age, BMI, smoking status, education level, current occupation, physical activity and usual energy intake. In sensitivity analyses, we applied mutually adjusted models (each model adjusted for the total usual intakes of the other four food groups) and models stratified by sex and BMI categories (under- and normal-weight for BMI <25 kg/m2, overweight for BMI 25 to <30 kg/m2 and obesity for BMI > 30 kg/m2).
SAS version 9.4 statistical software, and SAS Enterprise Guide, version 6.1 (SAS Institute) were used for statistical analysis.
Results
Participants’ characteristics are presented in Table 1. In the present study, 50·5 % were men and 49·5 % were women. Participants were between 47 and 81 years old. Men were on average 66·4 years old with an average BMI of 27·6 kg/m2 and women were on average 64·6 years old with an average BMI of 27·4 kg/m2. On average, participants did 22·6 h of physical activity per week (including sports, gardening, physical work, housework and cycling) and were mostly non-smokers (89·6 % were never or former smokers); 54·3 % of men and 33·8 % of women had a university degree. The majority of the participants were not working (62·2 % unemployed/retired). The mean usual energy intake was 2058 kilocalories (kcal) per day. Women consumed on average more vegetables and less refined grains and red and processed meats at meals than men did. Participants consumed on average fewer vegetables and red and processed meats at breakfast and more of them at lunch. Less refined grains were consumed at dinner and less whole grains at lunch. The main analyses were based on 782 participants with LDL-cholesterol and HDL-cholesterol concentrations, 781 participants with HbA1c, and 779 participants with CRP.
Table 1. Participants’ characteristics at the time of the first visit
(Numbers of participants and percentages; mean values and standard deviations)
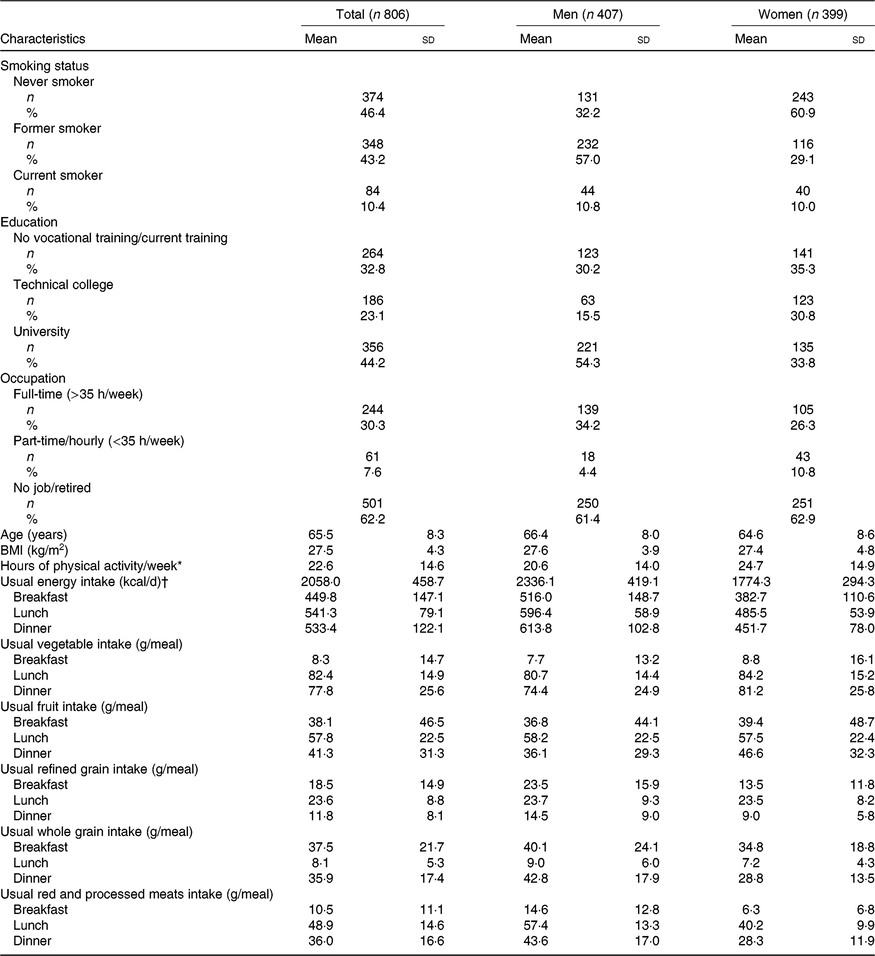
* Self-reported. Includes the following activities done in the past 12 months: sports, gardening, physical work, housework, cycling.
† To convert energy in kcal to kJ, multiply by 4·184.
For vegetables, fruits and whole grains, there was a positive correlation between the frequency of consumption and amount consumed (the higher the frequency of consumption, frequently the higher the consumed amount). For refined grains as well as red and processed meats, the frequency of consumption and amount consumed were positively correlated at breakfast, but negatively correlated at lunch and dinner, but in the total intakes, all correlations were positive (see online Supplementary Table S1).
Vegetable intake at meals
At breakfast, vegetable intake (in 50 g per meal) was associated with lower LDL-cholesterol concentrations by 0·37 mmol/l (95 % CI −0·61, −0·12), suggesting lower cardiometabolic risk; however, there was no association with lunch intake, dinner intake or total intake. There could be a weak association between vegetable intake and HDL-cholesterol concentrations by 0·05 mmol/l for every 50-g increase in intake both at dinner (95 % CI 0·00, 0·10) and in total intake (95 % CI 0·02, 0·09). As for CRP, vegetable intake at dinner could be associated with lower CRP concentrations by −0·24 mg/l for every 50g of vegetable intake (95 % CI −0·50, 0·02). There were no associations between vegetable intake and HbA1c. Total vegetable intake was associated with CRP (−0·22 mg/l; 95 % CI −0·37, −0·07). As we did not observe remarkable associations between vegetable intake at breakfast or lunch and CRP, the association observed for total vegetable intake could have its origin, at least partially, at dinner (Table 2).
Table 2. Associations of foods consumed at meals with cardiometabolic and inflammatory biomarkers among participants in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam validation sub-study*
(β Coefficients and 95 % confidence intervals; Spearman partial correlations)
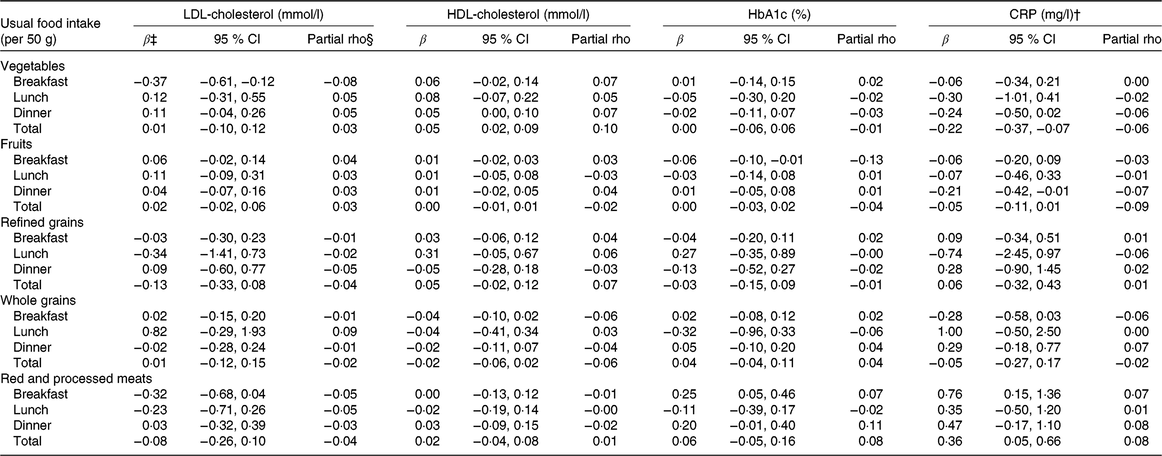
HbA1c, glycated Hb; CRP, C-reactive protein.
* Biomarker sample sizes vary: LDL-cholesterol (n 782), HDL-cholesterol (n 782), HbA1c (n 781), CRP (n 779).
† CRP results based on quantile regression using the median due to a skewed distribution.
‡ Effect estimates (β) and corresponding 95 % CI expressed as per 50 g usual meal food intake. Values were determined by use of linear regression models. All models are adjusted for sex, age (continuous), BMI, (continuous), smoking status (never, former and current), education level (no vocational training/current training, technical college, university), current occupation (full-time, part-time/hourly, no job/retired), physical activity (continuous) and usual energy intake (continuous).
§ Spearman partial correlations, adjusted for sex, age (continuous), BMI, (continuous), smoking status (never, former and current), education level (no vocational training/current training, technical college, university), current occupation (full-time, part-time/hourly, no job/retired), physical activity (continuous) and usual energy intake (continuous).
Fruit intake at meals
Fruit intake at different meals was not associated with LDL-cholesterol or HDL-cholesterol concentrations. Intake of fruits at breakfast was inversely associated with HbA1c (−0·06 % per 50 g of fruit; 95 % CI −0·10, −0·01), but this was not reflected in the model using total fruit intake. Intake of fruits at dinner was inversely associated with CRP (−0·21 %; 95 % CI −0·42, −0·01), which was attenuated for total fruit intake (−0·05 %; 95 % CI −0·11, 0·01) (Table 2).
Refined grain intake at meals
There were no clear remarkable associations with any of the investigated biomarkers. However, refined grains at lunch show strong estimates for all biomarkers, although the precision of such associations was low and included the scenario of no association: LDL-cholesterol (−0·34 mmol/l; 95 % CI −1·41, 0·73), HDL-cholesterol (0·31 mmol/l; 95 % CI −0·05, 0·67) and HbA1c (0·27 %; 95 % CI −0·35, 0·89), and towards lower CRP concentrations (−0·74 mg/l; 95 % CI −2·45, 0·97) (Table 2).
Whole grain intake at meals
Similar to refined grains, whole-grain intake did not show clear associations with any of the investigated biomarkers but included larger estimates, for instance, between whole-grain intake at lunch and LDL-cholesterol (0·82 mmol/l; 95 % CI −29, 1·93), HbA1c (−0·32 %; 95 % CI −0·96, 0·33) and CRP (1·00 mg/l; 95 % CI −0·50, 2·50), as well as whole-grain intake at breakfast with CRP (−28 mg/l; 95 % CI −0·58, 0·03) (Table 2).
Red and processed meat intake at meals
Intake of red and processed meat could be inversely associated with LDL-cholesterol when consumed at breakfast; the effect estimate was −0·32 mmol/l (95 % CI −0·68, 0·04). Red and processed meat was positively associated with HbA1c when consumed at breakfast (0·25 %; 95 % CI 0·05, 0·46) and at dinner (0·20 %; 95 % CI −0·01, 0·40). However, the positive association was not reflected in the total intake of red and processed meat. With CRP, red and processed meat intake was positively associated for intake at breakfast (0·76 mg/l; 95 % CI 0·15, 1·36) and for total intake (0·36 mg/l; 95 % CI 0·05, 0·66). There were no associations with HDL-cholesterol (Table 2).
Food–biomarker correlations
Covariate–adjusted meal-specific intakes were mildly correlated with all of the investigated biomarkers. The strongest correlations were seen for HbA1c with fruit intake at breakfast (partial rho = −0·13) and with red and processed meat intake at dinner (partial rho = 0·11) (Table 2).
Sensitivity analyses
Mutually adjusted models for total usual intakes of the other four food groups were mostly compatible with the main results, with the exception of weakened associations for CRP and dinner vegetable (−0·18 mg/l; 95 % CI −0·44, 0·08) and total vegetable intake (−0·15 mg/l; 95 % CI −0·31, 0·01) and for CRP and breakfast red and processed meat (0·48 mg/l; 95 % CI −0·12, 1·08) and total red and processed meat intake (0·21 mg/l; 95 % CI −0·08, 0·50) (see online Supplementary Table S4).
Analyses stratified by sex confirmed most of the results in the main analyses except for the associations with CRP among men; the effect estimates for CRP among men that were highly compatible with our data, given our model, now comprised the scenario of no association and were in general less precise. Some other differences included the weakening of the association between dinner vegetable intake and HDL-cholesterol among men (0·02 mmol/l; 95 % CI −0·05, 0·09) and red and processed meats and HbA1c among women: 0·15 % (95 % CI −0·25, 0·55) for breakfast, and 0·17 % (95 % CI −0·14, 0·48) for dinner. Among men, fruit intake at lunch seemed to be positively associated with LDL-cholesterol (0·26 mmol/l; 95 % CI 0·01, 0·52) and refined grains at lunch seemed to be associated with HDL-cholesterol (0·58 mmol/l; 95 % CI 0·14, 1·02); this was not observed among women. The associations with CRP among women remained, but interestingly, associations previously observed for red and processed meat intake at breakfast seemed to be present for dinner instead. Finally, the effect estimate seemed to go in opposite directions for dinner red and processed meat intake and LDL-cholesterol for men and women, but especially in men, precision was low and both for men and women compatible estimates with our model included the scenario of no association (see online Supplementary Tables S5 and S6).
Results for stratified analyses by BMI status are shown in online Supplementary Tables S7–S9. There were some differences according to BMI status and some similarities with the main results; in general, however, estimates were less precise. For example, the association in the main results (Table 2) between breakfast vegetable intake and LDL-cholesterol was only precise among obese participants, where compatible estimates with our model ranged from −0·77 to −0·12 mmol/l. Associations between dinner and total vegetable intake and CRP were only observed among participants with under- and normal-weight and were stronger than in the main results (−0·42 mg/l; 95 % CI −0·71, −0·12, and −0·34 mg/l; 95CI −0·53, −0·15, respectively for dinner and total vegetable intake), and the positive association between red and processed meat at breakfast and CRP was observed only among obese participants (1·80 mg/l; 95 % CI 0·26, 3·34). Other differences with the main results were inverse associations between lunch and total vegetable intake and HbA1c among participants with under- and normal-weight. Also, effect estimates for fruit intake and LDL-cholesterol suggest positive associations among participants with under- and normal-weight for breakfast and dinner, as well as total fruit intake. Similarly, for total fruit intake among participants with under- and normal-weight, the compatible estimates for HDL-cholesterol and HbA1c were more precise. Finally, in obese participants, there was a positive association between lunch vegetable intake and HDL (0·32 mmol/l; 95 % CI 0·05, 0·58) as well as between breakfast refined grain intake and HDL-cholesterol (0·23 mmol/l; 95 % CI 0·06, 0·40).
Discussion
In the present study, we investigated possible associations between meal-specific usual intake of foods considered beneficial to health (fruits, vegetables and whole grains) and those considered detrimental to health (red and processed meats, refined grains) and cardiometabolic and inflammatory biomarkers (LDL-cholesterol, HDL-cholesterol, HbA1c, CRP). Such associations have been previously investigated using total (non meal-specific) intakes. The study showed that food intake in the morning (breakfast) and the late afternoon/evening (dinner) seem to be convincing associations (more precise) for health outcomes (NCD markers), whereas the noon time (lunch) seems less important. Specifically, results suggest a beneficial association between breakfast and dinner intake of fruits and vegetables and the cardiometabolic profile. Intake of red and processed meat at breakfast showed associations with HbA1c and CRP, affecting the cardiometabolic profile negatively. When evaluating total food intakes (non meal-specific), not all meal-specific associations were reflected, but the associations for vegetables and HDL-cholesterol, vegetables and CRP, fruits and CRP, as well as red and processed meats and CRP remained, suggesting the association observed with total intake is attributable, at least partly, to meal-specific intakes suggestive of an association. In general, correlations between food groups and biomarkers were weak, reflecting a low variance explained by the regression models taking one sample and 3 d of consumption. This was not surprising due to the multifactorial causation regarding biomarkers and NCD risk(Reference Rutter29).
Few studies have been carried out on meal-specific food–biomarker associations(Reference Adamsson, Reumark and Marklund15, Reference Nilsson, Ostman and Holst30, Reference Iqbal, Schwingshackl and Gottschald31). Studies on meal patterns often focus on skipping and frequency of meals(Reference Park, Freisling and Huseinovic32–Reference Mekary, Giovannucci and Willett34). There have also been studies delineating an association between diet and sleep disorders but meal-based studies are still scarce(Reference Pot35). Because of the high heterogeneity across meal-based studies, the comparison of our results with available evidence is limited. An intervention study in subjects with hypercholesterolaemia resulted in no effect on LDL-cholesterol but found lower plasma CRP after advising a prudent breakfast including oat bran porridge, fruits and a sandwich with whole-grain bread and turkey meat/pickled fish for 3 months(Reference Adamsson, Reumark and Marklund15). Although, the comparison of this intervention with our study is not straightforward, we also did not find any association for breakfast fruit, refined grains, whole grains or red and processed meat intake (which were components of the intervention breakfast) with LDL-cholesterol and our results suggested there could be an inverse association between breakfast whole-grain intake and CRP concentrations. A recent meta-analysis of randomised controlled trials on the effects between total whole-grain intake and inflammation markers found a beneficial effect(Reference Hajihashemi and Haghighatdoost36); our results suggested an inverse association with CRP only when whole grains were consumed for breakfast. As for associations relating to HbA1c, a cross-sectional study on a sample partly overlapping with the study sample in the present study looked at breakfast quality and cardiometabolic risk and found that a breakfast rich in vegetables, fruit, whole grains, nuts and legumes, n-3 long-chain fatty acids and PUFA was associated with lower HbA1c and that a processed foods breakfast pattern (including processed meat, fruiting vegetables and bread) was associated with higher HbA1c, though these associations were only observed in men but not in women(Reference Iqbal, Schwingshackl and Gottschald31). In terms of the foods investigated in our study, only fruit intake at breakfast showed an inverse (beneficial) association with HbA1c, but neither vegetables nor whole grains showed this. Red and processed meat has been extensively investigated in the context of cardiometabolic profile and NCD risk, but not in a meal-specific context. Results on an association between red meat and CRP have been mixed(Reference Ley, Sun and Willett12, Reference Azadbakht and Esmaillzadeh37, Reference Schwedhelm, Pischon and Rohrmann38) but a risk-increasing association with type 2 diabetes has been consistent(Reference Pan, Sun and Bernstein39, Reference Bendinelli, Palli and Masala40). In this respect, our finding of a positive association between red and processed meat and HbA1c is in line with literature. In terms of the surprising possible inverse association between red and processed meat at breakfast and LDL-cholesterol, we could not find other meal-specific observational studies with the same exposure and outcome for comparison. But non meal-specific evidence from randomised trials and observational studies on meat intake (as well as our model for total red and processed meat intake) have not observed such an association(Reference Schwingshackl, Hoffmann and Iqbal11, Reference Wagemakers, Prynne and Stephen41, Reference Lenighan, Nugent and Li42). Most of the findings in the main results were replicated in sensitivity analyses. Differences observed, however, could be due to chance or multiple testing, as for some of these differences we do not find any biological plausibility, such as the positive association seen in men but not in women, as well as in overweight but not other BMI status categories between refined grain intake at lunch and HDL-cholesterol, the association among women between dinner whole grains and CRP, and the inverse association between red and processed meats at lunch and HbA1c among obese participants. However, there were other sex-specific or BMI-specific differences which require further investigation in terms of their biological plausibility, such as the weakening of the positive associations between meal-specific red and processed meats intake and CRP concentrations among men, the strong inverse association between vegetable intake at dinner and CRP only among participants with under- and normal-weight and the strong positive association between red and processed meat and CRP only among obese participants. The field of meal-based and chrono-nutrition research is still in an early stage and findings cannot yet be consistently summarised, as large differences across studies, such as study design, eating behaviour across different populations, foods and biomarkers tested and discord in meal comparisons limit comparability.
There are potential explanations for the apparent greater influence of foods consumed at breakfast and dinner rather than at lunch on biomarker concentrations. Enzymes and hormones involved in metabolism subject to circadian variations may modulate how foods are processed(Reference Froy43). For example, insulin production is increased during the day and slows down at night, resulting in lower glucose tolerance and insulin responses at night(Reference Boden, Ruiz and Urbain44). Breakfast is often referred to as the most important meal of the day and it has been shown that early meals are typically very stable, consisting more or less of the same foods across the days(Reference Vainik, Dube and Lu45, Reference Schwedhelm, Iqbal and Knuppel46). Although the importance of breakfast could be overstated, it could play an especially important role because of being (usually) the first meal of the day, when the circadian-influenced metabolic processes are most responsive. Similarly, in the case of dinner, because of being a later and typically large meal, slower metabolic processes corresponding to our evening circadian rhythm might have a bigger impact that could be unfavourable for health. Recent literature points to metabolic benefits when all foods in a day are consumed in a short time window, for instance, within 8 or 10 h(Reference Gill and Panda1, Reference Moro, Tinsley and Bianco47), and especially stresses the benefits of early rather than late meals(Reference Bandín, Scheer and Luque3, Reference Sakuma, Noda and Morimoto48). What the present study adds is not only the concept that the time-of-day/meal is important, but also which foods are consumed. Our results suggest that consuming fruits and vegetables for breakfast and dinner might have greater benefits for the cardiometabolic profile than consuming them for lunch, and that avoiding red and processed meats might be important at breakfast and dinner.
Because inferential statistics are based on simplified models relative to the complex reality being studied and should be therefore regarded as ‘unstable local descriptions of relations between assumptions and data’(Reference Amrhein, Trafimow and Greenland49), it is difficult to distinguish between spurious associations and true effects. This is why it is important to present and discuss all results. In the present study, we present and interpret results based on 95 % CI rather than showing the P-value, as it is prone to misinterpretations(Reference Wasserstein and Lazar50, Reference Greenland, Senn and Rothman51). Rather than categorising and concluding that a result is statistically significant (or non-significant), we interpret CI by describing all the values inside the interval and the limits as compatible with our data. These compatible estimates can include the scenario of strong associations, even if the null is included. As an example, the association of dinner vegetable intake with CRP concentrations was −0·24 mg/l (95 % CI −0·50, 0·02). This estimate was considerably large, although the CI showed compatible values from −0·50 (relatively strong inverse association) to 0·02 (virtually no association).
The strength of the present study pertains to use of meal-specific usual intakes. Methodological difficulties might be a limiting factor as to why there are so few studies on meal composition in terms of foods, as there is a high frequency of non-consumption (zero) occasions since not all food groups are consumed in each meal. Due to the relatively large sample size and the three repeated 24hDR, we were able to calculate meal-specific usual intakes, which adjusted out day-to-day variation (within-person variation) in intake and represent food consumption over a longer period of time(Reference Tooze, Kipnis and Buckman24). Because the biomarkers studied are assumed to reflect the longer-term cardiometabolic risk of participants, our research question should be approached by using an exposure which also reflects the longer-term meal-specific intake of foods. The present study also has a few limitations. First, we are aware of an interdependency of meals and also that meals consist of different combinations of foods, complexity that is not captured in our analyses. Some studies have tried to encompass this complexity(Reference Schwedhelm, Iqbal and Knuppel46, Reference Woolhead, Gibney and Walsh52, Reference Murakami, Livingstone and Sasaki53) but with dozens of meal patterns being derived and thousands to over a million possible combinations, these results are at often difficult to interpret. In our study, we attempted to get a simplified and easier to interpret overview of specific foods consumed at meals and approached the problem of interdependency between foods by adjusting for the other food groups in sensitivity analyses. Nevertheless, residual confounding cannot be discarded and the interdependency between meals was not addressed. Another limitation is that only one measurement of biomarkers was available for this analysis; LDL-cholesterol, HDL-cholesterol and HbA1c are relatively stable over time, however, CRP is less so(Reference Selvin, Coresh and Zhu54, Reference Al-Delaimy, Jansen and Peeters55). Furthermore, CRP can reflect acute inflammation with short-term changes and thus cannot be easily identifiable with one measurement per study participant. Nevertheless, the blood was drawn close in time from the collection of the 24hDR (on the day of the first recall), so that a reflection of the food intake at the time on the measured biomarkers is feasible. Thus, it might be important to look at other short-term biomarkers such as blood glucose. Also, it should be kept in mind for interpretation purposes that out of the selected biomarkers, only LDL-cholesterol is considered a causal marker of NCD risk(Reference Holmes, Ala-Korpela and Smith56, Reference Holmes, Asselbergs and Palmer57) but HDL-cholesterol, HbA1c and CRP were included as they are associated with NCD risk and are influenced by lifestyle changes(6, Reference Schwingshackl, Hoffmann and Iqbal11, Reference Waugh, Shyangdan and Taylor-Phillips58, Reference Ko, Park and Shin59). Finally, an important factor in chrono-nutrition is the sleep–wake cycle, which could not be considered in our analyses but could influence how food is metabolised and impact cardiometabolic and inflammatory biomarkers(Reference Pot35). Future research integrating this information could provide further insight into the mechanisms behind meal-specific food–biomarkers associations.
In summary, intake of fruits and vegetables at breakfast and at dinner was beneficially associated with cardiometabolic and inflammatory biomarkers, while intake of red and processed meat at breakfast was detrimentally associated. Attention should be paid to timing of food intake and the consumed amount. Our results suggest that preferring and avoiding certain foods at specific meals might modulate cardiometabolic profiles and ultimately chronic disease risk. Further research is needed to confirm these findings.
Acknowledgements
We thank the Human Study Center (HSC) of the German Institute of Human Nutrition Potsdam-Rehbruecke, namely the trustee and the examination unit for the collection, the data hub for the processing, and the participants for the provision of the data, and the head of the HSC, Manuela Bergmann, for the contribution to the study design and leading the underlying processes of data generation.
This work was supported by the German Federal Ministry of Education and Research (BMBF) (grant numbers FKZ-01ER0808, FKZ-01EA1408A).
The authors’ responsibilities were as follows: all authors contributed to the conception and design of the research; C. S. and S. K. analysed data; S. K. provided essential methodological advice; C. S., G. O. A. and S. K. wrote the paper; H. B. was responsible for the conduct of the EPIC-Potsdam sub-study and the general concept of the research, C. S. had primary responsibility for the final content of the manuscript; all authors revised the manuscript and approved the final version.
There were no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451900151X





