The rapid increase in obesity worldwide has inevitably been accompanied by an increasing interest in identifying modifiable lifestyle factors of excess body fatness, including dietary glycaemic index (GI) and glycaemic load (GL). A diet with a low GI or GL, due to the slower blood glucose and insulin response following consumption, is hypothesised to stimulate increased satiety and reduce voluntary energy intake (EI)(Reference Ludwig1), reduce fat storage by regulating fuel partitioning(Reference Stevenson, Williams and Mash2), limit the decrease of resting energy expenditure in the fasting state(Reference Pereira, Swain and Goldfine3) and in turn prevent the accumulation of body fat(Reference Augustin, Franceschi and Jenkins4). However, epidemiological studies on dietary GI and GL in relation to measures of body fatness in free-living adults have provided a mixture of positive(Reference Lau, Toft and Tetens5–Reference Hare-Bruun, Flint and Heitmann12), null(Reference Murakami, Sasaki and Takahashi7, Reference Ma, Olendzki and Chiriboga10–Reference Liese, Schulz and Fang16) and inverse(Reference Youn, Woo and Cho8, Reference Finley, Barlow and Halton9, Reference Du, van der and van Bakel11, Reference Mendez, Covas and Marrugat14, Reference Rossi, Bosetti and Talamini17, Reference Sahyoun, Anderson and Kanaya18) associations. These heterogeneous results may reflect cultural differences in food and nutrient intake patterns associated with dietary GI and GL. Previous studies have generally shown that dietary GL is associated with many carbohydrate-rich foods and hence strongly with carbohydrate intake, while dietary GI is associated with not only a higher intake of major carbohydrate-rich foods (e.g. breads and potatoes) but also a lower intake of other foods with low-carbohydrate and non-carbohydrate nutrients (e.g. fruit and dairy products)(Reference Du, van der and van Bakel13, Reference Mendez, Covas and Marrugat14, Reference Schulz, Liese and Mayer-Davis19, Reference van Bakel, Kaaks and Feskens20). However, the strength and direction of the associations of dietary GI and GL with each of the food groups and nutrients vary considerably in different contexts of food cultures(Reference Du, van der and van Bakel13, Reference Mendez, Covas and Marrugat14, Reference Schulz, Liese and Mayer-Davis19, Reference van Bakel, Kaaks and Feskens20).
Alternatively, the diversity of the associations between dietary GI and GL and measures of body fatness may be due to methodological problems in dietary assessment. For example, while most studies in this area have used FFQ, with some exceptions(Reference Finley, Barlow and Halton9, Reference Hare-Bruun, Flint and Heitmann12, Reference Milton, Briche and Brown15), these instruments have not been developed specifically to measure dietary GI and GL, possibly having little utility relative to the original concept of GI(Reference Mayer-Davis, Dhawan and Liese21). In addition, with some exceptions(Reference Du, van der and van Bakel11, Reference Du, van der and van Bakel13), most studies have calculated dietary GI and GL mainly based on an earlier GI table, which contains a limited number of food items (about 750) with a mixture of GI values obtained from standard and non-standard protocols in both healthy and diabetic subjects(Reference Foster-Powell, Holt and Brand-Miller22). The application of GI based on diabetic subjects to the general (healthy) population has been questioned because of the established inconsistencies in the values obtained(Reference Atkinson, Foster-Powell and Brand-Miller23, Reference Louie, Flood and Turner24). More importantly, associations between dietary GI and GL and measures of body fatness may be confounded by misreporting of dietary intake, a serious problem in all dietary surveys(Reference Livingstone and Black25–Reference Nielsen, Nielsen and Toubro32). For example, a Danish study has shown a positive association of dietary GI and GL with BMI in non-low-energy reporters (ratio of EI:BMR ≥ 1·14), but there was no association in low-energy reporters(Reference Lau, Toft and Tetens5). Furthermore, in Spanish adults, an inverse relationship between dietary GL (but not GI) and BMI has been observed in non-low-energy reporters (ratio of EI:BMR ≥ 1·20) but not in low-energy reporters(Reference Mendez, Covas and Marrugat14). However, these studies have used a fixed cut-off value to identify low-energy reporters, without taking into account the physical activity level of each subject, which may result in misclassification of up to 50 % of low-energy reporters(Reference Black33, Reference Tooze, Krebs-Smith and Troiano34).
The aim of the present cross-sectional study in British adults was to examine the association of dietary GI and GL with food and nutrient intake and general and central obesity, taking into account energy under-reporting. Dietary GI and GL were estimated using a 7 d weighed dietary record and an updated GI table including about 2500 food items(Reference Atkinson, Foster-Powell and Brand-Miller23). Energy under-reporting was assessed based on EI against individualised measure of estimated energy requirement (EER).
Subjects and methods
Survey design and analytical sample
The present cross-sectional study was based on the National Diet and Nutrition Survey (NDNS): Adults Aged 19 to 64 Years. Data from the NDNS were obtained from the UK Data Archive, University of Essex. Complete details of the rationale, design and methods of the survey have been described elsewhere(35). Briefly, the sample was randomly selected from 152 randomly selected postal sectors within mainland Great Britain. Eligibility was defined as being aged 19–64 years and not pregnant or breast-feeding. Only one eligible adult per private household was selected at random. Data collection was conducted during a 12-month period (July 2000 to June 2001).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by a Multi-centre Research Ethics Committee and the National Health Service Local Research Ethics Committee covering each of the postal sectors. Written informed consent was obtained from all subjects.
Of 3704 potentially eligible people identified for the study, 2251 (61 % of the eligible sample) participated in the survey. For the present analysis, we excluded 736 subjects with missing information on the variables used (n 468 for anthropometric data; n 527 for dietary data; n 56 for social class; n 3 for smoking status; and n 593 for physical activity; some subjects had more than one missing value) and twenty-eight underweight subjects (BMI < 18·5 kg/m2)(36). The final analysis sample comprised 1487 adults aged 19–64 years (678 men and 809 women; 40 % of the eligible sample).
Anthropometric measurements
All anthropometric measurements were performed in duplicate by trained fieldworkers, and the mean value of two measurements was used in the analysis. Height (to the nearest 0·1 cm) and weight (to the nearest 0·1 kg) were measured while the subjects were barefoot and wearing light clothes only. BMI (kg/m2) was calculated as weight (kg) divided by height (m) squared. Waist circumference (WC) was measured at the midpoint between the iliac crest and the lower rib, and hip circumference was measured at the point of maximum circumference over the buttocks and below the iliac crest (to the nearest 0·1 cm). The waist:hip ratio (WHR) was calculated as WC divided by hip circumference, and the waist:height ratio (WHtR) was calculated as WC divided by height. General obesity was defined as BMI ≥ 30 kg/m2(36). Central obesity was defined as WC ≥ 102 cm in men and ≥ 88 cm in women(36). Additionally, greater WHR was defined as WHR ≥ 1·00 in men and ≥ 0·85 in women(36), and greater WHtR was defined as WHtR ≥ 0·5(Reference Ashwell, Gunn and Gibson37).
Dietary assessment and calculation of dietary glycaemic index and glycaemic load
Dietary data were collected using a 7 d weighed dietary record. A detailed description of the procedure has been published elsewhere(35). Briefly, each subject was supplied with a set of digital food scales and recording diaries. The subject (and the main record-keeper if appropriate) was given both written and verbal instructions by trained interviewers on how to weigh and record items in the diary, with an example of a recording diary. When weighing was not possible (e.g. eating out), the subject was asked to record as much information as possible, including the brand name of the food item, the portion size consumed and details of any leftovers. Trained interviewers, who were responsible for coding the diaries, visited the subject's household at least twice during the recording period and checked the completeness of food recording, and if necessary, additional information was added. All the collected diaries were checked by trained nutritionists, who were responsible for converting descriptions of portion sizes to weights and all aspects of the diary, including coding, recorded weights and descriptions of items consumed. Estimates of daily intake for foods, energy and selected nutrients were calculated from the records of food consumption based on the Food Standards Agency nutrient data bank(Reference Smithers38), which is based on the McCance and Widdowson's composition of foods series(39) and manufactures' data where applicable.
To calculate dietary GI and GL, GI values were assigned to individual food items (n 4612) in the dietary record, according to the following strategy developed based on previous studies(Reference Louie, Flood and Turner24, Reference van Bakel, Slimani and Feskens40, Reference Flood, Subar and Hull41). GI values were obtained from the latest international table of GI(Reference Atkinson, Foster-Powell and Brand-Miller23). Glucose was used as the reference (GI for glucose 100).
Step 1. Determine whether the item has < 0·5 g carbohydrate (sum of sugars and starch) per 100 g. If yes, assign a GI value of 0 (n 707; 15·3 %). If no, go to step 2.
Step 2. Determine whether there is a direct link to a food in the international GI table. If yes, assign that value (n 736; 16·0 %). If no, go to step 3.
Step 3. Determine whether there is a closely related food (based on macronutrient and fibre contents) in the international GI table. If yes, assign that GI value (n 2569; 55·7 %). If no, go to step 4.
Step 4. Determine whether the median GI value of the food subgroup is available. If yes, assign the median GI value of the subgroup (n 81; 1·8 %). If no, go to step 5.
Step 5. Determine whether the item is categorised to one of the following: vegetables; dairy products; sauce; dressing; alcoholic beverages; flour. If yes, assign the following nominal GI values(Reference Atkinson, Foster-Powell and Brand-Miller23, Reference van Bakel, Slimani and Feskens40): 40 for vegetables; 30 for dairy products; 60 for sauce; 30 for dressing; 65 (GI of sucrose) for alcoholic beverages; the GI value of bread made from the same flour for flour (n 449; 9·7 %). If no, go to step 6.
Step 6. Determine whether the item is categorised to one of the following: fats; egg; fish; meat; tea; coffee; spice; sugar-free foods or beverages. If yes, assign the nominal value of 0 (n 62; 1·3 %). If no, go to step 7.
Step 7. Assign a nominal GI value of 50 (n 8; 0·2 %).
Where possible, foods were given GI values that were derived from groups of eight or more healthy subjects with an appropriate methodology (i.e. Table A1 in the international GI table) (n 4582; 99·3 %). If the only relevant value was available from studies in subjects with diabetes or impaired glucose metabolism, or from studies using too few subjects, or showing wide variability (i.e. Table A2 in the international GI table), this value was used. If more than one eligible GI value was available for a given food, we assigned the mean of the GI values.
Dietary GL was calculated by multiplying the GI value of each individual food item by the amount (g) of carbohydrate consumed from that food item, and then summing the products divided by 100. Dietary GI was calculated by dividing dietary GL by the total amount (g) of carbohydrate consumed, and then multiplying this value by 100. For all dietary variables, mean daily values over 7 d were used in the analysis.
Assessment of non-dietary variables
The socio-economic status of each respondent (i.e. occupational social class) was self-reported and categorised as manual (i.e. skilled manual, partly skilled and unskilled occupations: social classes III (manual), IV and V) or non-manual (i.e. professional, managerial, technical and skilled non-manual occupations: social classes I, II and III (non-manual)). Smoking status (never, former or current) was also self-reported.
A 7 d physical activity diary was used concurrently with the dietary record. A detailed description of the procedure has been published elsewhere(35). Briefly, the subjects were shown by trained interviewers as to how to record the information and were asked to record, to the nearest 10 min, how long they spent performing various activities on that day. Trained interviewers checked the completeness of records at least twice during the recording period, and if necessary, additional information was added. Subsequently, time spent daily on sleep and light-, moderate- and vigorous-intensity activities was computed for each day of recording. Each type of activity was assigned a metabolic equivalent (MET) value from a published table: 1·0 for sleep; 2·0 for light-; 3·5 for moderate-; 7·5 for vigorous-intensity activities(Reference Ainsworth, Haskell and Herrmann42). The number of hours spent per d on each activity was multiplied by the MET value of that activity, and all MET-h products were summed to produce a total MET-h score for the day. A mean daily value over 7 d was used in the analysis.
Evaluation of energy intake reporting
We calculated each subject's EER, based on the information on age, weight, height and physical activity, with the use of equations published from the US Dietary Reference Intakes(43). The equations were developed from a meta-analysis of methodologically sound studies using doubly labelled water as the criterion measure of total energy expenditure (TEE)(43). Based on the physical activity level value, which was calculated as total MET-h/d (from the 7 d physical activity diary) divided by 24 h, physical activity category was determined for each subject. The sex- and age-specific equations for use in populations with a range of weight statuses(43) were used.
The subjects were identified as acceptable reporters (AR), under-reporters (UR) or over-reporters of EI based on their ratio of EI:EER, according to whether the individual's ratio was within, below or above the 95 % confidence limits of the expected ratio of 1·0. The 95 % confidence limits ( ± 2 sd cut-offs) were calculated according to the following equation:
where CVEI is the within-person CV in the reported EI, d is the number of days of dietary assessment, CVEER is the error in the EER equations and CVTEE is the day-to-day variation in TEE(Reference Huang, Roberts and Howarth44). The values used were 22·8–32·8 % for CVEI (calculated from the present NDNS data; values varied depending on the sex–age–BMI stratum), 7 for d, 7·9–11·5 % for CVEER (based on the US Dietary Reference Intakes data(43, Reference Huang, Roberts and Howarth44); values varied depending on the sex–age–BMI stratum) and 8·2 % for CVTEE (as reported previously from doubly labelled water studies(Reference Black and Cole45)). As the 95 % confidence limits were found to be similar across the sex–age–BMI strata (32·1–36·3 %), an average of 33·5 % was used in the present analysis. Thus, AR were defined as having the EI:EER ratio in the range of 0·665–1·335, UR as EI:EER < 0·665 and over-reporters as EI:EER >1·335.
Statistical analyses
All statistical analyses were performed for men and women separately, using SAS statistical software (version 9.2, SAS Institute, Inc.). Differences between AR and UR (but not for over-reporters because of there being only a few of them) were tested by the independent-samples t test (for continuous variables) and by the χ2 test (for categorical variables). Stepwise regression analyses were carried out to investigate the contribution of the selected nineteen food groups to the inter-individual variation in dietary GI and GL. For those food groups contributing at least 1 % variation, multiple regression analyses were performed with only those predictive food groups plus EI as explanatory variables and dietary GI or GL as the response variable. The associations of intakes of energy and selected nutrients with dietary GI and GL were investigated by Spearman's correlation analyses.
Multiple linear regression analyses were performed to explore the associations of dietary GI and GL (independent variables) with measures of body fatness (dependent variables). With the use of the PROC REG procedure, we calculated the adjusted regression coefficients (with se) of variation of BMI, WC, WHR and WHtR by a 10-unit GI increase and a 50-unit GL increase. Furthermore, multiple logistic regression analyses were performed to explore the associations of dietary GI and GL with general and central obesity in addition to greater WHR and WHtR. Using the PROC LOGISTIC procedure, adjusted OR (and 95 % CI) for these statuses per 10-unit GI increase and 50-unit GL increase were calculated. Potential confounding factors included in the multivariate models were age, social class, smoking status, physical activity, total fat intake, alcohol intake, dietary fibre intake and EI:EER. The analyses were conducted not only for the entire population but also for only AR or only UR.
The values of nutrient intake were energy adjusted using the density method (i.e. percentage of energy for energy-providing nutrients and amount per 10 MJ of energy for dietary fibre). We used crude values for dietary GI and energy-adjusted values in the density method (per 10 MJ) for dietary GL because dietary GI is by definition a measure of carbohydrate quality, not of quantity, whereas dietary GL is a measure of the combination of carbohydrate quality and quantity(Reference Murakami, Sasaki and Okubo6, Reference Murakami, Sasaki and Takahashi7). Use of energy-adjusted values (including dietary GI) by the residual method(Reference Willett, Howe and Kushi46) did not change the results materially (data not shown).
Data were not weighted to take into account known sociodemographic differences between responders and non-responders, not only because the impact of this adjustment, applied as a weighting factor, for nutritional variables was extremely small(35) but also because we were only interested in the relationships between variables, rather than in the estimates of prevalence. All reported P values are two tailed, and P values < 0·05 were considered statistically significant.
Results
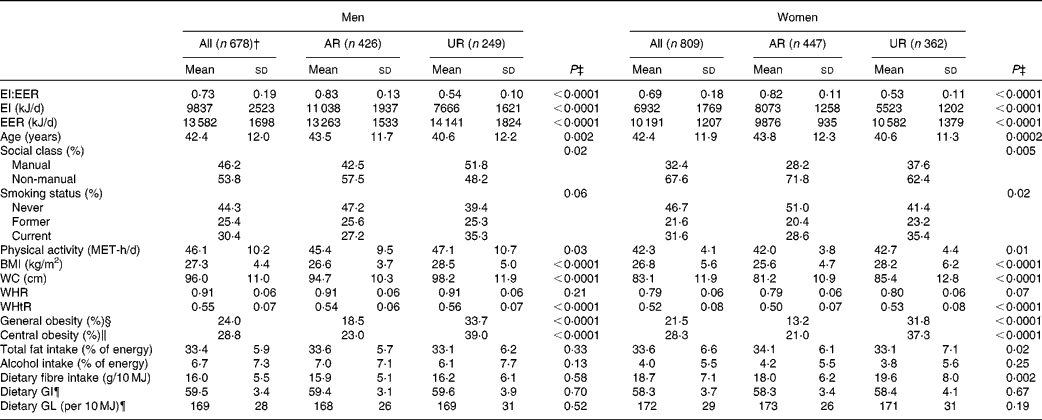
Under-reporting of EI compared with that of EER was, on average, 27 % in men and 31 % in women (Table 1). The prevalence of general and central obesity was 24 and 29 % in men and 22 and 28 % in women, respectively. The mean values of dietary GI and GL were 60 (sd 3) and 169 (sd 28) per 10 MJ in men and 58 (sd 4) and 172 (sd 29) per 10 MJ in women, respectively.
Table 1 Characteristics of the subjects* (Mean values and standard deviations or percentages)

AR, acceptable reporters; UR, under-reporters; EI:EER, ratio of energy intake:estimated energy requirement; EI, energy intake; EER, estimated energy requirement; MET, metabolic equivalents; WC, waist circumference; WHR, waist:hip ratio; WHtR, waist:height ratio; GI, glycaemic index; GL, glycaemic load.
* AR were defined as subjects with EI:EER 0·665–1·335; UR were defined as subjects with EI:EER < 0·665.
† Including over-reporters of EI (n 3), defined as subjects with EI:EER >1·335.
‡ P values for differences between AR and UR based on the independent-samples t test for continuous variables and the χ2 test for categorical variables.
§ BMI ≥ 30 kg/m2.
∥ WC >102 cm for men and ≥ 88 cm for women.
¶ Based on the GI of glucose (100).
The percentages of AR and UR were 63 and 37 % in men and 55 and 45 % in women, respectively (only three men (0·4 %) were classified as over-reporters). In both men and women, compared with AR, UR had a lower mean value of EI and age and a higher mean value of EER, physical activity and all measures of body fatness (except WHR), and were more likely to be employed in manual occupations, be current smokers and be obese. In only women, UR had a lower mean intake of total fat and a higher mean intake of dietary fibre. In terms of dietary GI and GL, there were no differences between UR and AR in both sexes. In both men and women, UR had lower mean intakes of all food groups examined (g/d) compared with AR, except for no differences in soft drinks (both sexes), nuts and seeds (only men), and fish and fish dishes (only women) (data not shown).
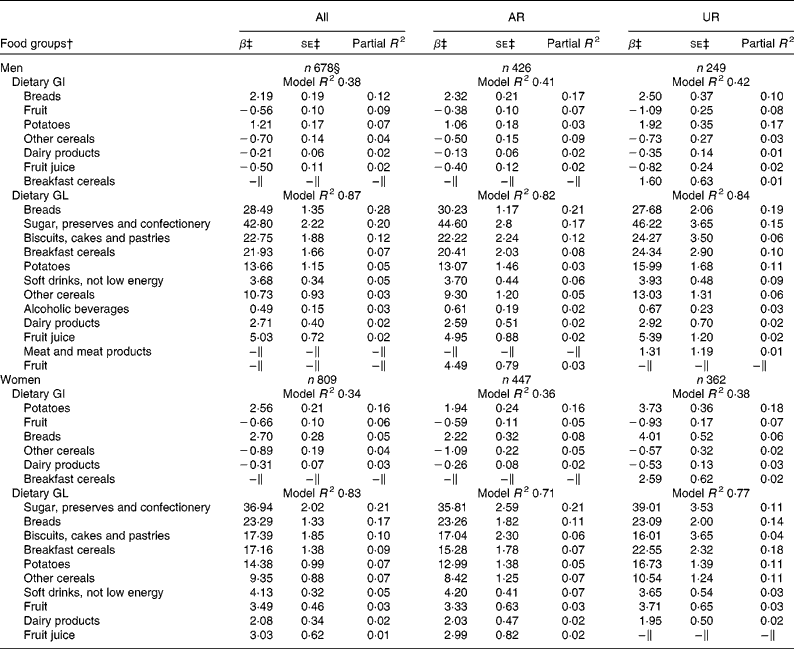
In both men and women, breads and potatoes were the positive predictive food groups for dietary GI, while fruit, other cereals and dairy products (and fruit juice in only men) were the negative predictors (Table 2). In total, these food groups accounted for 38 % (men) and 34 % (women) of the variation in dietary GI. For dietary GL, 87 % (men) and 83 % (women) of the variation was explained by ten carbohydrate-rich food groups, all of which showed positive associations. Food groups identified as the predictors of dietary GI and GL as well as the variation explained were relatively similar in the analysis of only AR or only UR for both men and women, although the ranking of the most predictive food groups somewhat differed in some cases. For example, in men, the most contributing food group of GI was breads in AR but potatoes in UR; in women, the most contributing food group of GL was sugar, preserves and confectionery in AR but breakfast cereals in UR.
Table 2 Food groups contributing to the inter-individual variation in dietary glycaemic index (GI) and glycaemic load (GL)* (Regression coefficients with their standard errors and partial determination coefficients)

AR, acceptable reporters; UR, under-reporters; β, Regression coefficient.
* AR were defined as subjects with the ratio of energy intake:estimated energy requirement (EI:EER) 0·665–1·335; UR were defined as subjects with EI:EER < 0·665.
† Food groups listed are those contributing at least 1 % of the variation of dietary GI or GL based on the stepwise regression analysis with nineteen food groups (i.e. breads; breakfast cereals; biscuits, cakes and pastries; other cereals; dairy products; egg and egg dishes; butter and spreads; meat and meat products; fish and fish dishes; vegetables; potatoes; fruit; sugar, preserves and confectionery; fruit juice; alcoholic beverages; tea, coffee and water; nuts and seeds; soft drinks, not low energy; and soft drinks, low energy) as explanatory variables and dietary GI or GL as the response variable.
‡ Models with the listed variables and energy intake as the explanatory variables and dietary GI or GL as the response variable; regression coefficients mean the change of dietary GI or GL with a 100 g increase of each food group.
§ Including over-reporters of energy intake (n 3), defined as subjects with EI:EER >1·335.
∥ Not contributing at least 1 % of the variation of dietary GI or GL.
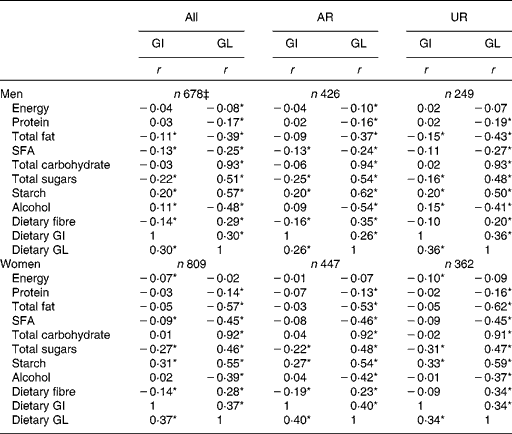
There was no association between dietary GI and EI in men, while a weak inverse association was found in women (Table 3). Dietary GI was positively associated with starch and dietary GL (and alcohol in only men) and inversely associated with SFA, total sugars and dietary fibre (and total fat in only men). Dietary GI was not associated with total carbohydrate. There was a weak inverse association between dietary GL and EI in only men. Dietary GL was positively associated with total carbohydrate, total sugars, starch and dietary fibre and inversely associated with protein, total fat, SFA and alcohol. Relatively similar correlation coefficient values were obtained when AR and UR were analysed separately.
Table 3 Correlation of energy and nutrient intake with dietary glycaemic index (GI) and glycaemic load (GL)† (Spearman's correlation coefficients)

AR, acceptable reporters; UR, under-reporters.
*P< 0·05.
† Values are Spearman's correlation coefficients calculated using energy-adjusted values (i.e. percentage of energy for energy-providing nutrients and amount per 10 MJ of energy for dietary fibre and GL) except for energy and dietary GI (for which crude values were used). Dietary GI and GL were calculated based on the GI of glucose (100). AR were defined as subjects with the ratio of energy intake:estimated energy requirement (EI:EER) 0·665–1·335; UR were defined as subjects with EI:EER < 0·665.
‡ Including over-reporters of energy intake (n 3), defined as subjects with EI:EER >1·335.
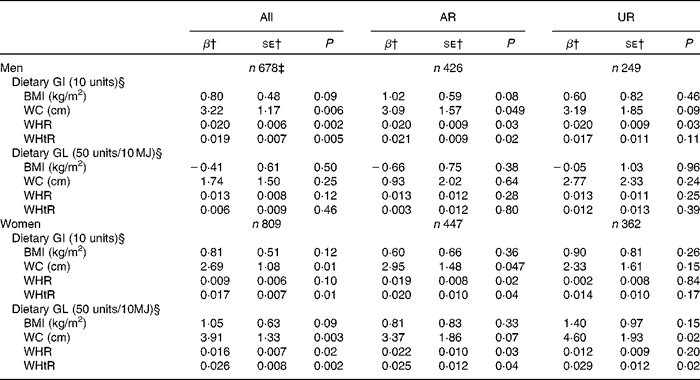
While dietary GI showed no association with BMI in both men and women, dietary GI was independently positively associated with all the other measures of body fatness (WC, WHR and WHtR), except with WHR in women (Table 4). Dietary GL was not associated with any measure of body fatness in men. Conversely, in women, although there was no association between dietary GL and BMI, dietary GL showed an independent positive association with all the other measures of body fatness. Similarly, in the analysis including AR, dietary GI showed positive associations with all the measures of body fatness except with BMI in both sexes. Additionally, dietary GL similarly showed no associations in men, but positive associations with all the measures of body fatness except with BMI in women. Conversely, in the analysis of UR, the associations were generally weaker, with significant associations only being observed between GI and WHR in men and GL and WC and WHtR in women.
Table 4 Associations of dietary glycaemic index (GI) and glycaemic load (GL) with the measures of body fatness* (Regression coefficients with their standard errors)

AR, acceptable reporters; UR, under-reporters; β, regression coefficient; WC, waist circumference; WHR, waist:hip ratio; WHtR, waist:height ratio.
* Adjustment was made for age (years, continuous), social class (manual or non-manual), smoking status (never, former or current), physical activity (metabolic equivalents-h/d, continuous), total fat intake (% of energy, continuous), alcohol intake (% of energy, continuous), dietary fibre intake (g/10 MJ, continuous) and ratio of energy intake:estimated energy requirement (EI:EER, continuous). AR were defined as subjects with EI:EER 0·665–1·335; UR were defined as subjects with EI:EER < 0·665.
† Regression coefficients mean the change of body fatness measures with a 10-unit GI increase and a 50-unit GL (per 10 MJ) increase.
‡ Including over-reporters of energy intake (n 3), defined as subjects with EI:EER >1·335.
§ Based on the GI of glucose (100).
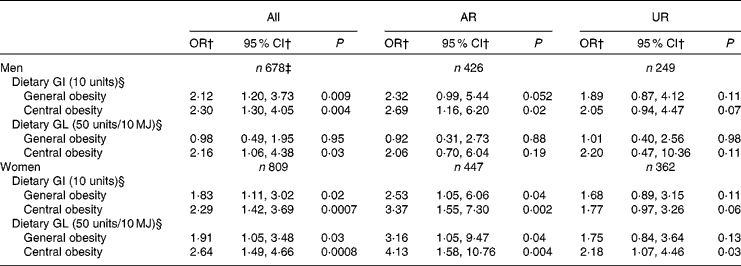
Table 5 presents the independent association between dietary GI and GL and general and central obesity. Dietary GI was associated with a higher risk of both general and central obesity in both men (P= 0·009 and 0·004, respectively) and women (P= 0·02 and 0·0007, respectively). Dietary GL was associated with a higher risk of general obesity in only women (P= 0·03) and central obesity in both men and women (P= 0·03 and 0·0008, respectively). Similarly, in the analysis of AR, dietary GI showed positive associations with both general and central obesity in both men (P= 0·052 and 0·02, respectively) and women (P= 0·04 and 0·002, respectively). Dietary GL also showed positive associations with both general and central obesity in women (P= 0·04 and 0·004, respectively), although there was no association with central obesity in men. Conversely, in the analysis of UR, although the direction of the associations was similar, the associations were generally weaker and many of them failed to reach statistical significance (P= 0·06–0·13), except that between dietary GL and central obesity in women (P= 0·03). Excluding EI:EER from the models did not change the results materially (data not shown). When greater WHR and WHtR were used as dependent variables, only a few significant positive associations were observed (data not shown): those between dietary GL and greater WHtR in all women (P= 0·002) and female AR (P= 0·03) and those between dietary GI and greater WHR in all men (P= 0·03), male AR (P= 0·03) and female AR (P= 0·04).
Table 5 Associations of dietary glycaemic index (GI) and glycaemic load (GL) with general and central obesity* (Odds ratios and 95 % confidence intervals)

AR, acceptable reporters; UR, under-reporters.
* Adjustment was made for age (years, continuous), social class (manual or non-manual), smoking status (never, former or current), physical activity (metabolic equivalents-h/d, continuous), total fat intake (% of energy, continuous), alcohol intake (% of energy, continuous), dietary fibre intake (g/10 MJ, continuous) and ratio of energy intake:estimated energy requirement (EI:EER, continuous). AR were defined as subjects with EI:EER 0·665–1·335; UR were defined as subjects with EI:EER < 0·665.
† OR for general and central obesity per 10-unit increase of GI and 50-unit increase of GL (per 10 MJ).
‡ Including over-reporters of energy intake (n 3), defined as subjects with EI:EER >1·335.
§ Based on the GI of glucose (100).
Excluding 397 subjects who reported dieting or that illness had affected their eating during the diet-recording period did not alter the findings. The exactly same food groups presented in Table 2 were identified as the predictors of dietary GI and GL in both men (model R 2 0·39 and 0·88, respectively) and women (model R 2 0·37 and 0·82, respectively), in addition to fruit for GL in men and vegetables for GI in women. Dietary GL was closely correlated with carbohydrate intake (Spearman's r 0·92 in both sexes). Dietary GI was associated with a higher risk of both general and central obesity in both men (adjusted OR 2·87, 95 % CI 1·46, 5·66; P= 0·002 and adjusted OR 3·57, 95 % CI 1·80, 7·07; P= 0·003, respectively) and women (adjusted OR 1·94, 95 % CI 0·95, 3·95; P= 0·07 and adjusted OR 2·59, 95 % CI 1·37, 4·91; P= 0·003, respectively). Dietary GL was associated with a higher risk of central obesity in both men and women (adjusted OR 4·29, 95 % CI 1·76, 10·42; P= 0·001 and adjusted OR 2·49, 95 % CI 1·18, 5·22; P= 0·02, respectively).
Discussion
The present cross-sectional study in British adults showed that a high-GI diet, characterised by a high intake of breads and potatoes and a low intake of fruit, other cereals and dairy products, was independently associated with an increasing risk of general and central obesity in both men and women. Dietary GL, which was closely correlated with carbohydrate intake, was independently associated with a higher risk of central obesity in both sexes and general obesity only in women. Generally, similar associations were observed in the analysis of AR, while the associations were generally weaker and many of them did not reach statistical significance in the analysis of UR. Thus, the associations between dietary GI and GL and general and central obesity seemed to be confounded by energy under-reporting. To our knowledge, this is the first study to examine dietary GI and GL in relation to food and nutrient intake and measures of body fatness, taking into account EI misreporting assessed by individualised measures of EER.
Misreporting of dietary intake is a common phenomenon and appears to occur non-randomly(Reference Livingstone and Black25–Reference Nielsen, Nielsen and Toubro32) and to be selective for different kinds of foods and hence nutrients(Reference Subar, Kipnis and Troiano28–Reference Murakami, Sasaki and Takahashi31). The resulting potential for differential errors in dietary data complicates the interpretation of studies on diet and health and, at worst, might produce spurious diet–health relationships(Reference Livingstone and Black25, Reference Mattisson, Wirfalt and Aronsson26, Reference Rosell, Hellenius and De Faire29). In the present study, the prevalence of EI misreporters (i.e. UR and over-reporters) was 37 % in men and 45 % in women, which was relatively similar to that observed in previous studies using similar dietary assessment methodologies (37 %(Reference Nielsen, Nielsen and Toubro32) and 38 %(Reference Black33)). The results are also consistent with numerous previous studies(Reference Livingstone and Black25–Reference Nielsen, Nielsen and Toubro32, Reference Okubo, Sasaki and Hirota47), which have shown that UR had considerably different characteristics compared with AR, including greater body fatness, younger age, lower socio-economic status and current smoking. Particularly, higher physical activity and EER were associated with EI under-reporting. This appears reasonable, given that active subjects with a greater energy requirement can fall into the category of under-reporting(Reference Barnard, Tapsell and Davies48). Alternatively, this may be due to over-reporting of physical activity. Despite these differences, the associations of dietary GI and GL with food and nutrient intake observed in UR were relatively similar to those observed in AR. It is reasonable to assume that the observed associations in UR are not artefacts produced by energy under-reporting. Thus, the exclusion of UR in the present analysis was not warranted. The exact reason for this observation is unknown, and we are unaware of previous studies where the associations among dietary variables have been compared according to the categories of dietary misreporting status. However, it may be because the calculation of dietary GI and GL is based on only carbohydrate intake, independent of fat (which seems to be more prone to misreporting than other macronutrients)(Reference Subar, Kipnis and Troiano28–Reference Murakami, Sasaki and Takahashi31) as well as energy. In addition, the influence of energy under-reporting may be minimised by energy adjustment, as misreporting of any food and nutrient should be correlated with EI misreporting, at least to some extent(Reference Subar, Kipnis and Troiano28, Reference Murakami, Sasaki and Takahashi31).
A limited number of studies have suggested that the influence of dietary misreporting on the association between diet and health depends on dietary variables(Reference Mattisson, Wirfalt and Aronsson26, Reference Rosell, Hellenius and De Faire29, Reference Huang, Roberts and Howarth44, Reference Mendez, Popkin and Buckland49) or dietary assessment methods(Reference Subar, Kipnis and Troiano28, Reference Bingham, Luben and Welch50, Reference George, Thompson and Midthune51). In the present study, the positive association between dietary GI and general and central obesity observed in the total male and female populations and the positive association between dietary GL and central obesity observed in the total male and female populations and general obesity in the total female population were also observed in AR. Conversely, in the analysis of UR, many of the associations failed to reach statistical significance. Thus, although the associations seemed to be confounded by energy under-reporting, there was no evidence that the association between dietary GI and GL and obesity was distorted by energy under-reporting in the present study. In line with this observation, previous studies have shown that the associations of carbohydrate intake (expressed as percentage of energy) with BMI(Reference Huang, Roberts and Howarth44, Reference Mendez, Popkin and Buckland49) and fasting plasma insulin(Reference Rosell, Hellenius and De Faire29) were not affected by EI misreporting.
While we found the positive association of dietary GI with general and central obesity in both men and women, dietary GL was associated with central obesity in both sexes but with general obesity only in women. As dietary GL was strongly correlated with carbohydrate intake, as has been shown in several previous studies(Reference Du, van der and van Bakel11, Reference Du, van der and van Bakel13), dietary GL might have provided little information beyond the carbohydrate content of the diet compared with dietary GI(Reference Du, van der and van Bakel11, Reference Mayer-Davis, Dhawan and Liese21). Furthermore, central obesity may be more susceptible to dietary GI and GL than general obesity given that visceral fat is more vulnerable to the influence of high insulin responses stimulated by high-GI foods compared with subcutaneous fat(Reference Du, van der and van Bakel11). This may explain why we did find significant associations of dietary GI and GL with measures of central fatness (WC, WHR and WHtR), but not with a measure of general fatness (BMI) as well as somewhat stronger associations with central obesity than with general obesity. Men may also be less susceptible than women to the proposed adverse effects of high dietary GI and GL on body fatness(Reference Hare-Bruun, Flint and Heitmann12), which may account for the generally weaker associations observed in men than in women. It is not clear why we did find the association for central obesity (defined by WC) but not for greater WHR or WHtR in this population. The WC cut-offs may be more sensitive than the WHR and WHtR cut-offs, at least in this population, as the associations were similar when WC, WHR and WHtR were treated as continuous variables.
The advantages of the present study include the use of a 7 d weighed dietary record, a systematic assignment of GI values based on an updated and more representative GI table, measured anthropometric data and the use of individualised measure of EER to identify EI misreporters. However, there are also several limitations. First, the cross-sectional nature of the study does not permit the assessment of causality, owing to the uncertain temporality of the association. Only a prospective study taking into account dietary misreporting would provide a better understanding of the relationship between dietary GI and GL and measures of body fatness.
At present, the only way to obtain unbiased information on energy requirements in free-living settings is to use doubly labelled water as a biomarker(Reference Livingstone and Black25). This technique is expensive and impractical for application to large-scale epidemiological studies. Instead, we calculated EER with the use of equations from the US Dietary Reference Intakes(43). In the absence of measured TEE, these equations with high R 2 values (0·82 for men and 0·79 for women)(43) should serve as the best proxy, although the selection of physical activity category was based on self-report (i.e. 7 d physical activity diary), which may be susceptible to reporting bias. Additionally, we do not know the sensitivity and specificity of the procedure for identifying EI misreporters used. If misclassification between AR and UR is big, the real differences in the associations of GI and GL with food and nutrient intake and obesity would be attenuated to be not significant. Nonetheless, we are confident of our conclusions, because the associations observed in the entire populations were similarly observed in AR.
Another limitation of the present study is the relatively low response rate (61 %), and only 40 % of the eligible sample was included in the present study. The subjects included in the present analysis (n 1487) differed somewhat from those excluded from the analysis (n 705–758 depending on the variables). The excluded subjects were more likely to be younger, be in manual occupations and be current smokers (all P< 0·05). However, a previous analysis has concluded that there is no evidence to suggest serious non-response bias in the NDNS(35). Although GI values were carefully determined with, for example, consideration of cooking methods (e.g. boiled, fried, mashed and canned potatoes) as much as possible, general limitations to assigning appropriate GI values should be acknowledged, including the still restricted number of items in the GI table, the inclusion of mainly American or Australian food items, the lack of values of mixed dishes and the lack of information on differences in variety (e.g. potato and rice), degree of ripeness (e.g. banana), composition (e.g. more or less fat), cooking methods and product formulations of the same brand(Reference van Bakel, Slimani and Feskens40, Reference Henry, Lightowler and Strik52). Finally, although we adjusted for a variety of potential confounding variables, residual confounding could not be ruled out.
In conclusion, in the present cross-sectional study in British adults, a high-GI diet, which was high in breads and potatoes but low in fruit, other cereals and dairy products, showed an independent positive association with the risk of general and central obesity in both men and women. Dietary GL, which was strongly correlated with carbohydrate intake, showed an independent positive association with the risk of central obesity in both sexes and general obesity in only women. These associations were similarly observed in the subjects with a plausible EI. Conversely, in the subjects with an implausibly low EI, the associations were generally weaker and many of them failed to reach statistical significance. Thus, under-reporting of EI seemed to confound the positive associations of dietary GI and GL with general and central obesity by reducing them. Further research (particularly with a prospective design) in diverse populations is needed, taking into account dietary misreporting as well as using different dietary assessment methods so that any firm conclusions can be drawn with regard to the effect of dietary GI and GL on excess body fatness. More generally, as misreporting of dietary intake is a well-known phenomenon in nutritional epidemiology(Reference Livingstone and Black25–Reference Nielsen, Nielsen and Toubro32) and there is not enough knowledge of differential misreporting among foods and nutrients(Reference Subar, Kipnis and Troiano28–Reference Murakami, Sasaki and Takahashi31), the routine application of some procedures to identify and separately treat those who report data of poor validity(Reference Black33, Reference Huang, Roberts and Howarth44, Reference Mendez, Popkin and Buckland49) would improve the precision and accuracy of results in studies of diet and health.
Acknowledgements
The present study was supported in part by the JSPS Postdoctoral Fellowships for Research Abroad, Japan Society for the Promotion of Science, Japan. K. M. designed the study, analysed and interpreted the data, and wrote the manuscript. T. A. M. and M. B. E. L. designed the study and helped to write the manuscript. All authors read and approved the final manuscript. None of the authors has a conflict of interest.