In parallel with the increasing trend of gestational diabetes mellitus (GDM)( Reference Correa, Bardenheier and Elixhauser 1 ), the prevalence of childhood obesity has been increasing at an alarming rate of up to 18 % worldwide( 2 ). Mounting evidence indicates that children born to mothers with diabetes have a higher risk of high birth weight( Reference Catalano, McIntyre and Cruickshank 3 ), amplified adiposity in infancy( Reference Logan, Emsley and Jeffries 4 ), later obesity( Reference Gillman, Rifas-Shiman and Berkey 5 ) and type 2 diabetes( Reference Sellers, Dean and Shafer 6 ). The growth of these children could be ‘programed’ during pregnancy by intra-uterine over-nutrition and follow adverse trajectories characterised by larger size and accelerated velocity of weight gain during infancy and childhood. Nutrition during infancy is an important modulator for energy balance throughout life( Reference Cripps, Archer and Mercer 7 ). Breast milk (BM) as the natural food for infant has been recommended widely in general population for its protective effects against obesity( Reference Armstrong and Reilly 8 ) and type 2 diabetes( Reference Owen, Martin and Whincup 9 ) in later life. However, the effect of breast-feeding on the future obesity of the children born to mothers with GDM remains uncertain. Studies of mothers with diabetes showed contradictory results, including increasing the risk( Reference Plagemann, Harder and Franke 10 ), no effects on the risk( Reference Mayer-Davis, Rifas-Shiman and Zhou 11 ), or lowering increased adiposity associated with exposure to diabetes in utero ( Reference Crume, Ogden and Maligie 12 ).
The favourable energy balance and growth of breastfed infants have been suggested to be derived in part from the hormones in BM( Reference Savino, Benetti and Liguori 13 ). Hormones are stable in BM and could be absorbed through the receptors in human gastrointestinal tract( Reference Bronsky, Mitrova and Nevoral 14 – Reference Mitrović, Čokić and Đikić 17 ). Some bioactive BM hormones are reported to link maternal metabolic status with metabolic health of offspring. For instance, adiponectin and leptin concentrations in BM are associated with maternal BMI( Reference Schuster, Hechler and Gebauer 18 – Reference Dundar, Dundar and Cesur 21 ) and could be altered by GDM( Reference Aydin 22 , Reference Ley, Hanley and Sermer 23 ). Breastfed infants exposed to higher concentration of adiponectin in BM have a lower weight gain during the first 6 months( Reference Woo, Guerrero and Altaye 24 ). However, the effects of maternal factors on adiponectin and leptin were inconsistent among studies, and the inconclusive results regarding insulin and ghrelin render further confirmation( Reference Andreas, Hyde and Gale 25 ). The association of BM hormone with infant growth was also inconclusive in previous studies using different anthropometric measurements, such as weight gain( Reference Schuster, Hechler and Gebauer 18 ), body composition( Reference Fields and Demerath 20 ), BMI( Reference Dundar, Dundar and Cesur 21 , Reference Weyermann, Brenner and Rothenbacher 26 – Reference Chan, Goruk and Becker 29 ) or weight-for-height( Reference Woo, Guerrero and Altaye 24 , Reference Chan, Goruk and Becker 29 , Reference Woo, Guerrero and Guo 30 ). Moreover, few studies examined the links in women with GDM and their infants.
In this study, we recruited women with GDM( Reference van Beusekom, Zeegers and Martini 31 ), healthy women and their exclusively breastfed infants for longitudinal follow-ups on days 3, 42 and 90. We aimed to evaluate the adiponectin, leptin, insulin and ghrelin concentrations in BM of women with GDM and the relationship between maternal factors, BM hormones and early infant growth.
Methods
The study protocol was approved by the institutional review boards at Peking Union Medical College Hospital and Beijing Obstetrics and Gynecology Hospital. Signed informed consent was obtained from all the participating families. Trial identification number and URL: NCT03145649 https://clinicaltrials.gov/show/NCT03145649.
Subjects
Nulliparous women with GDM and healthy women who intended to exclusively breastfeed their singletons were recruited consecutively from the obstetric wards at Peking Union Medical College Hospital and Beijing Obstetrics and Gynecology Hospital during the 37th gestational week. The exclusion criteria were: pre-pregnancy diabetes, fetal anomaly, gestational hypertension, pre-eclampsia, fetal growth restriction, ruptured membranes, postpartum glucose abnormalities (see below) and introduction of formula feeding during the follow-ups. Women with plasma glucose >7·8 mmol/l in the 1 h 50 g glucose load test (GLT) during the 24th to 28th gestational week underwent a 3 h 100 g diagnostic oral glucose tolerance test (OGTT) following a 12 h overnight fast. GDM was diagnosed if two or more plasma glucose reads equaled or exceeded the threshold according to Carpenter/Coustan diagnostic criteria( 32 ). All subjects diagnosed with GDM initially received dietary therapies to ensure euglycaemia, adequate nutrition and appropriate weight gain. Those who did not achieve glycaemic targets (3·3–5·6 mmol/l at fasting, 3·3–5·8 mmol/l pre-prandially, 4·4–6·7 mmol/l 2 h post-prandially and 4·4–6·7 mmol/l at night) in 2 weeks were given insulin via injection. As the close link between BM macronutrients and the maternal glucose metabolic status could potentially bias our evaluation of the association between BM hormones and infant growth, we excluded women with postpartum glucose abnormalities, that is, impaired glucose tolerance (IGT) with the 2 h plasma glucose between 7·8 and 11·0 mmol/l and type 2 diabetes with the 2 h plasma glucose ≥11·1 mmol/l in 75 g OGTT on day 42 (Fig. 1).
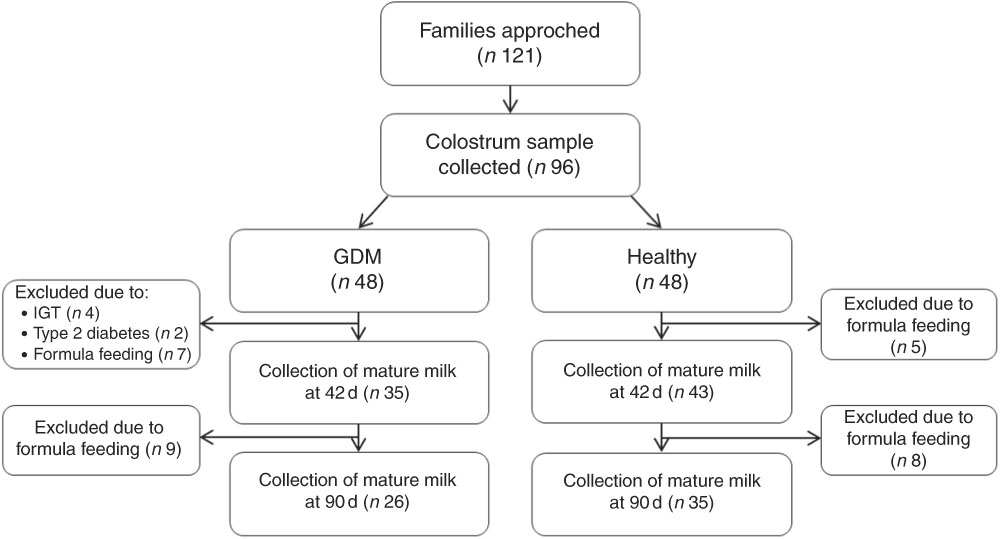
Fig. 1 Diagram of participant and follow-up flow. GDM, gestational diabetes mellitus; IGT, impaired glucose tolerance.
Anthropometric measurements
Obstetric data including glycaemic tests, gestational age and mode of delivery were collected from medical records. Pre-pregnancy weight was self-reported. Height was measured twice to the nearest 0·1 cm with a wall-mounted stadiometer. Weight was measured twice to the nearest 0·1 kg with a medical balance scale before delivery, on postpartum days 42 and 90. Infant weight, length and head circumference were measured at birth, on days 42 and 90. The infants were weighed twice in nude using a precision scale (Seca). Body length and head circumference were measured twice to the nearest 0·1 cm with a length board and non-stretchable measuring tapes (Seca). We averaged the two readings for data analysis.
Milk sample collection, processing and laboratory tests
Colostrum samples were collected between 08.00 and 09.00 hours before infant feeding on the 3rd day after delivery. Mature milk, including both foremilk and hindmilk, was delivered and collected from one breast before infant feeding using an electric pump (Medela) between 14.00 and 16.00 hours on days 42 and 90 in outpatient clinics. Milk samples were frozen immediately in sterilised plastic tubes at –80°C. Before quantifying the hormones in BM, the samples were thawed at 4°C, sonicated and centrifuged. Sonication was performed at 50 W for three bursts with 10-s intervals, and centrifuged at 100 000 g for 1 h at 4°C (Braun-sonic Sonicator; B. Braun). The supernatant fat was discarded and the skim milk was used for quantifying adiponectin, leptin, insulin and ghrelin by ELISA at the Key Laboratory of Endocrinology, Peking Union Medical College Hospital. The intra- and inter-assay CV were <5·4 and <8·5 % for adiponectin, and <7·4 and <9·3 % for leptin, respectively. The cross-reactivity to proinsulin of the insulin assay was not significant (<0·05 %). The sensitivity of insulin assay was 0·5 mU/l, and the intra- and inter-assay CV were <4·1 and <9·0 %. Total ghrelin was tested using the total human ghrelin ELISA kit (Millipore). The intra- and inter-assay CV for the ghrelin assay were <1·9 and <7·7 %.
Statistical analysis
According to the study on colostrum ghrelin concentration in women with and without GDM( Reference Aydin, Geckil and Karatas 33 ), six mother–infant dyads at each group were needed to achieve the statistical power of 84 % and the two-sided significance level of 0·05( Reference Machin, Campbell and Fayers 34 ). According to the baseline adiponectin level and infant weight-for-height in another two cohorts examining the association between BM adiponectin and infant growth( Reference Woo, Guerrero and Altaye 24 ), a sample size of seventy-two (using data from Cincinnati group) or seventy-five (using data from Maxico group) mother–infant dyads was able to achieve a statistical power of 80 %( Reference Neter, Wasserman and Kutner 35 ). We had ninety-six mother–infant dyads at the baseline and seventy-eight on day 42, which was an acceptable sample size for detecting the association between BM adiponectin concentration and infant weight-for-height. The differences in the demographic characteristics and hormone concentrations between GDM and healthy groups were evaluated using independent sample t test and Mann–Whitney U test for continuous variables, and using χ 2 test for discrete variables. The association of maternal and obstetrical factors (i.e. pre-pregnancy BMI, maternal BMI during lactation, GDM, plasma glucose during pregnancy, gestational weight gain, gestational age and delivery mode) with the hormone concentrations was tested using generalised estimating equation (GEE), a semi-parametric analysis using all the longitudinal data points to estimate the overall average effects of maternal or obstetrical factors (e.g. BMI) on the hormone levels in BM. The association of the overall hormone concentrations in BM with infant weight-for-height gain and head circumference was also analysed using GEE. The statistical analysis was conducted using SPSS version 20.0 (SPSS Inc.). α was set to 0·05 for two-sided tests if otherwise mentioned. To be more conservative with our exploratory analysis, we used Bonferroni correction to control for potentially inflated type I error (α) in the regression analyses.
Results
In all, ninety-six of 121 eligible women agreed to participate and successfully delivered colostrum. Among them, forty-eight women were with GDM and forty-eight were healthy (Fig. 1). In the GDM group, twenty-two dyads dropped out due to the introduction of formula feeding (n 16), IGT (n 4) and type 2 diabetes (n 2). A total of thirteen healthy mothers dropped out due to the introduction of formula feeding (Fig. 1).
The plasma glucose of women with GDM at fasting, 1, 2 and 3 h in OGTT were 5·75 (sd 0·98), 10·96 (sd 1·51), 9·78 (sd 1·47) and 7·14 (sd 1·95) mmol/l, respectively (Table 1). As expected, women with GDM had higher plasma glucose at 1 h in the 50 g GLT during pregnancy compared with healthy women (11·49 (sd 2·42) v. 6·82 (sd 1·29), P<0·001; Table 1). We tested glycosylated Hb for women with a diagnosis of GDM during the 24th to 28th gestational weeks. The median glycosylated Hb level was 6·20 % (44 mmol/mol; ranged from 5·0 % (31 mmol/mol) to 10·0 % (86 mmol/mol); data not shown). In all, seventeen mothers with GDM received insulin injections. All mothers with GDM had favourable blood glucose control during pregnancy.
Table 1 Characteristics of mothers and infants (Mean values and standard deviations; numbers and percentages)
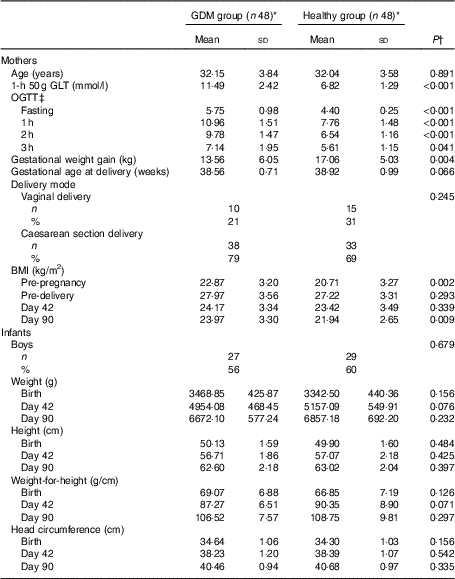
GDM, gestational diabetes mellitus; GLT, glucose loading test; OGTT, oral glucose tolerance test.
* The number of subjects at recruitment and first follow-up. The numbers of subjects at the second and third follow-ups are shown in Fig. 1.
† t Tests for continuous variables and χ 2 tests for categorical variables were used to evaluate the difference between GDM and healthy groups.
‡ All mothers with GDM and eight healthy mothers underwent OGTT during pregnancy.
The two maternal groups were comparable in age, gestational age and delivery mode (P>0·05; Table 1). Women with GDM, particularly those who received insulin injections, had higher BMI (P≤0·012) but less gestational weight gain (P=0·009) than healthy women (online Supplementary Table S1). We did not find significant differences between infants born to GDM and healthy women, regarding their weight, height, weight-for-height and head circumference at birth, on days 42 and 90 (P>0·05; Table 1).
Breast milk hormone concentrations and maternal factors
We collected colostrum samples on day 3, and mature milk samples on days 42 and 90 (Fig. 1). When compared with healthy women, women with GDM had lower concentrations of adiponectin and ghrelin in colostrum on day 3 (P adiponectin <0·001 and P ghrelin=0·011) and mature milk on day 90 (P adiponectin=0·009 and P ghrelin<0·001; Table 2). Women with GDM had higher concentration of insulin in colostrum (P=0·047) and mature milk (P=0·021; Table 2), especially in women who received insulin injections (P colostrum=0·049 and P mature milk <0·001; online Supplementary Table S2). The leptin concentration was not statistically different between women with GDM and healthy women (P>0·05; Table 2). All the four hormones were not significantly different between the two groups on day 42 (P>0·05; Table 2). Lactation time also played an important role in adiponectin, leptin and ghrelin levels in BM. Adiponectin and leptin concentration decreased across time and ghrelin concentration was highest at day 42 (Table 2).
Table 2 Breast milk hormone concentrations over lactation (Medians and interquartile ranges (IQR))
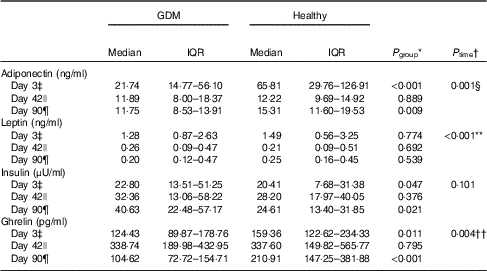
GDM, gestational diabetes mellitus.
* Mann–Whitney U tests were used to compare the difference between GDM and healthy groups at each lactation time point.
† General linear model for repeated measurements with GDM/without GDM as between-subject factor was used to compare the hormone concentrations over time. Hormone concentrations were natural log transformed before entering the models.
‡ n 48 for GDM group and n 48 for healthy group at day 3.
§ In subsequent within-subject contrasts, adiponectin concentration was significantly different between day 3 and day 42 (P=0·001).
|| n 35 for GDM group and n 43 for healthy group at day 42.
¶ n 26 for GDM group and n 35 for healthy group at day 90.
** In subsequent within-subject contrasts, leptin concentration was significantly different between days 3 and 42 (P<0·001) and between days 3 and 90 (P=0·002).
†† In subsequent within-subject contrasts, ghrelin concentration was significantly different between days 3 and 42 (P<0·001) and between days 42 and 90 (P=0·044).
We tested the association of maternal factors with overall hormone concentrations in BM using GEE. We found that adiponectin concentration was inversely associated with GDM (P=0·014), plasma glucose concentration at 1 h in the 50 g GLT during pregnancy (P<0·001), and caesarean section delivery (P=0·029), while it was positively associated with maternal BMI during lactation (P=0·001) and gestational age (P=0·017; Table 3). Leptin concentration was only positively associated with maternal body size, including pre-pregnancy BMI (P=0·017) and maternal BMI during lactation (P<0·001; Table 3). Furthermore, we noticed that pre-pregnancy BMI, maternal BMI during lactation, GDM and plasma glucose concentration at 1 h in 50 g GLT during pregnancy were positively associated with insulin concentration (P<0·001 for pre-pregnancy BMI, maternal BMI during lactation and GDM; P=0·035 for plasma glucose concentration), while inversely associated with ghrelin concentration in BM (P=0·031 for pre-pregnancy BMI, P<0·001 for maternal BMI during lactation and GDM, and P=0·007 for plasma glucose concentration; Table 3). However, some of the associations, such as GDM and gestational age with adiponectin, were insignificant with Bonferroni correction rendering further confirmation (Table 3).
Table 3 Associations between maternal factors and breast milk hormone concentrationsFootnote † (β-Coefficients and 95 % confidence intervals)
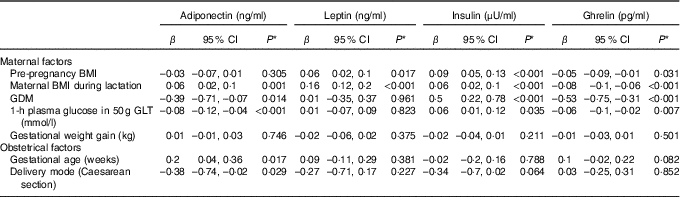
GDM, gestational diabetes mellitus; GLT, glucose load test.
* A P value ≤0·007 was considered significant with Bonferroni correction.
† Generalised estimating equation was used to test the association between maternal factors and milk hormones with adjustment for maternal age.
Breast milk hormone concentrations and infant growth
The overall adiponectin concentration in BM during the first 3 months was inversely associated with the infant weight-for-height in both the GDM (β=–2·49; 95 % CI –3·83, –1·15; P<0·001) and healthy groups (β=–1·42; 95 % CI –2·38, –0·46; P=0·003; Table 4). Adiponectin and insulin were associated with head circumference during the follow-up period in both the GDM (β=–0·39; 95 % CI –0·65, –0·13; P adiponectin=0·003; β=–0·39; 95 % CI –0·65, –0·13; P insulin=0·004) and healthy groups (β=–0·15; 95 % CI –2·38, –0·46; P adiponectin=0·007; β=–0·55; 95 % CI –1·11, 0·01; P insulin=0·049; Table 4). However, the association of insulin with head circumference in healthy subjects was insignificant after Bonferroni correction. We further plotted the BM hormone concentrations and weight-for-height at each time point to show the trend of the associations (online Supplementary Figure). The correlations shifted over time, which could be due to the differences in the concentrations between colostrum and mature milk.
Table 4 Associations of breast milk hormone concentrations with infant weight-for-height and head circumferenceFootnote † (β-Coefficients and 95 % confidence intervals)
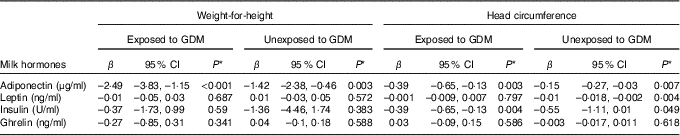
GDM, gestational diabetes mellitus.
* A P value ≤0·0125 was considered significant with Bonferroni correction.
† In each generalised estimation equation model, we included weight-for-height as the dependent variable, and the hormone concentrations (i.e. ghrelin, adiponectin, leptin or insulin), gestational age and infant’s sex as the independent variables.
Discussion
This study provides evidence supporting milk-borne adiponectin as an important nutritional mediator that links maternal metabolic status and early infant growth. Although women with GDM received dietary intervention or insulin therapy, adiponectin and ghrelin concentrations were decreased and insulin concentration was increased in their BM. Women with higher BMI had more adiponectin, leptin, insulin, but less ghrelin in BM. Among all the four hormones, adiponectin was inversely associated with early infant growth (i.e. weight-for-height and head circumference) in both women with GDM and healthy women, suggesting an independent modulatory role of milk-borne adiponectin in infant growth.
The concentrations of adiponectin( Reference Weyermann, Brenner and Rothenbacher 26 , Reference Newburg, Woo and Morrow 36 – Reference Savino, Liguori and Sorrenti 38 ), leptin( Reference Schuster, Hechler and Gebauer 18 , Reference Bronsky, Mitrova and Karpisek 37 – Reference Savino, Liguori and Petrucci 39 ) and insulin( Reference Ley, Hanley and Sermer 23 ) in BM were in the same range comparing to prior studies. The total ghrelin concentration was comparable to that reported by Aydin who also used ELISA to quantify the hormones( Reference Aydin 22 ). However, our read was ten times lower than the concentration measured using RIA, which could be due to the non-specific competition of proteins in BM( Reference Prudom, Liu and Patrie 40 ). To minimise the confounding effect of variation in the duration between milk delivery and collection( Reference Ley, Hanley and Sermer 23 ), we stored the BM samples immediately after collection. Other potential differences between GDM and healthy groups, including nulliparous mothers, maternal age, gestational age and delivery mode, were balanced between the GDM and healthy groups.
Women with controlled diabetes mellitus had normal milk lactose, glucose, protein, cholesterol, TAG and total fatty acid composition( Reference van Beusekom, Zeegers and Martini 31 ). However, their hormone concentrations varied and were associated with maternal BMI, glucose metabolism and the stage of lactation. Our results were consistent with prior studies that women with GDM or gravid hyperglycaemia had higher insulin( Reference Ley, Hanley and Sermer 23 ) and lower ghrelin concentration in their BM( Reference Aydin, Geckil and Karatas 33 ). Higher BMI was associated with more leptin( Reference Schuster, Hechler and Gebauer 18 , Reference Schueler, Alexander and Hart 41 , Reference Brunner, Schmid and Zang 42 ), insulin( Reference Ley, Hanley and Sermer 23 , Reference Ahuja, Boylan and Hart 43 ) and less ghrelin in BM, which was due to the corresponding maternal hormone levels in serum( Reference Andreas, Hyde and Gale 25 ). Maternal BMI seemed to be a more important factor modulating hormone levels in BM. When maternal BMI were comparable in GDM and healthy groups on day 42, all the four hormones were similar between groups. For adiponectin, prior studies and our study did not find a significant correlation between pre-pregnancy BMI and milk adiponectin( Reference Martin, Woo and Geraghty 19 , Reference Andreas, Hyde and Gale 25 ). Only two previous studies examined the association between post-pregnant BMI and adiponectin in BM( Reference Martin, Woo and Geraghty 19 , Reference Woo, Guerrero and Altaye 24 ). Both prior and our study found positive association between maternal BMI and milk adiponectin. Maternal BMI fluctuated during lactation and lactation month was associated with both maternal BMI and milk components( Reference Woo, Guerrero and Altaye 24 ). In our study, BMI during lactation was associated with BM adiponectin after accounting for time effect. It is noticeable that higher BMI was associated with a lower serum adiponectin concentration( Reference Cnop, Havel and Utzschneider 44 ) and a higher BM adiponectin concentration. This inconsistency between serum and BM can be due to the modulatory function of prolactin, a major determinant of mammary gland secretion and a negative modulator for adiponectin secretion. Excess maternal adipose tissue can down-regulate the secretion of prolactin, and consequently increase the adiponectin concentration in BM( Reference Martin, Woo and Geraghty 19 ). Although GDM was associated with higher BMI, GDM seemed to decrease the adiponectin concentration in BM independently and more robustly than the effect of adipose tissue possibly by decreasing the circulating adiponectin.
Hormones in BM were suggested to protect infants from the short-term acceleration of adipose deposit and the long-term obesity and diabetes( Reference Savino, Fissore and Liguori 45 ). Prior studies in healthy infants and toddlers have shown that breastfed infants exposed to higher concentration of adiponectin in BM had a lower weight gain during the first year and a greater weight gain during the second year( Reference Woo, Guerrero and Altaye 24 , Reference Woo, Guerrero and Guo 30 , Reference Brunner, Schmid and Zang 42 ). In Brunner’s study, milk adiponectin concentration at week 6 tended to be inversely associated with infant anthropometry in the first 4 months, but was positively associated with infant weight gain and fat mass till 2 years of age. In the study by Woo et al., a higher median level of adiponectin across baseline (week 1), months 1, 3, 5 and 6 was associated with accelerated weight trajectory during the second year. We also found such an inverse association during the early infancy in children born to mothers with GDM. Our results partially support the hypothesis that BM adiponectin could mitigate early weight gain in infancy when fat mass gain is dominant, and it may promote weight gain in the second year of life when lean body mass is dominant( Reference Woo, Guerrero and Guo 30 ). BM adiponectin, thus, protects children against obesity in later life. With favourable controlled blood glucose, breast-feeding could help the infants of women with GDM gain growth trajectory comparable to that of infants born to healthy women( Reference Whitmore, Trengove and Graham 46 ).
The strengths of this study include recruiting exclusively breastfed infants until 90 d before introduction of solid food, recruiting women with GDM under proper control to minimise the bias due to the effects of macronutrients in BM, and drawing both foremilk and hindmilk. However, limitations should be noted when interpreting the results. First, based on prior studies demonstrating the association of hormones in maternal serum, BM and infant serum( Reference Savino, Lupica and Benetti 47 , Reference Weyermann, Beermann and Brenner 48 ), maternal and infant serum hormone concentration was not analysed in this study. Second, twenty-two dyads with GDM dropped out due to IGT, type 2 diabetes and introduction of formula feeding during the follow-ups, which may make the samples on 42 and 90 d less representative of the population with GDM. Finally, infant growth was evaluated using anthropometric measurements. More accurate measurements of body composition, such as MRI, will help accurately evaluate the mediation effect of hormones in BM between maternal metabolic status and infant growth and their protective effects on infants’ metabolic health.
In conclusion, breastfed infants of women with controlled GDM gained normal growth trajectory. Milk-borne adiponectin could be an important nutritional mediator that links maternal metabolic status and early infant growth. It was decreased in BM of women with GDM and was associated with lower infant early weight gain. Further studies in infants fed by BM of women with diabetes, donor BM of healthy women, and formula are warranted to elucidate the role of milk-borne hormones as bioactive nutrients to attenuate the risk of childhood obesity associated with maternal diabetes.
Acknowledgements
The authors thank the parents who participated in the study. The authors also wish to thank Dr Simon Hugh Lam, Dr Albert Martin Li, Ms Yi Peng and Mr John Simmons Borchert for their review and advice on the manuscript.
This work was supported by grants from Beijing Municipal Science and Technology Commission (D111100000611001) and Beijing Science and Technology Star Program (2004A027). The sponsors did not influence the study design, analysis, interpretation of the results or writing of the manuscript.
X. Y. contributed to conception and design of the study, follow-up of study subjects, data acquisition, hormone assays, data analysis and interpretation, drafting and critical revision of the manuscript and final approval of the manuscript for submission. S. S. R. was involved in data acquisition, skim milk preparation, data analysis and interpretation, drafting and critical revision of the manuscript and final approval of the manuscript for submission. X. S., G. D. and W. W. contributed to the design of the study, follow-up of study subjects and revision of the manuscript. L. Z. and S. W. participated in recruitment of subjects and sample collection. M. L. contributed to the design of the study, hormone assays, critical review of the manuscript and final approval of the manuscript for submission. D. W. was involved in conception and design of the study, critical review of the manuscript and final approval of the manuscript for submission.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002933








