Sarcopenia is indicated by decreased skeletal muscle during the ageing process(Reference Rosenberg2). In addition to skeletal muscle loss, a decline in muscle strength and physical performance simultaneously develops, leading to health hazards, including falls, disability, frailty, quality of life deterioration, frequent hospitalisation and mortality(Reference Janssen, Heymsfield and Ross1,Reference Kamel3,Reference Newman, Kupelian and Visser4) . Recently, studies have started to focus on the process of changing body composition with ageing, including decreasing muscle mass and increasing fat mass(Reference Flegal, Shepherd and Looker5). Although sarcopenia has a negative impact on health, the impact of obesity on morbidity and mortality in older adults (≥ 65 years old) has been inconclusive(Reference Oreopoulos, Kalantar-Zadeh and Sharma6,Reference Winter, MacInnis and Wattanapenpaiboon7) . Reasonable weight gain could enhance the health capacity for the unpredicted stress of multiple illnesses(Reference Javed, Aljied and Allison8); in contrast, some researchers have suggested that obesity-induced insulin resistance, the metabolic syndrome (MS) and cardiovascular events result in poor health outcomes(Reference Adams, Schatzkin and Harris9,Reference Butler, Rodondi and Zhu10) . Therefore, it seems that sarcopenia and obesity together might have a greater or lesser impact in older adults. Many studies have focused on the health impact when sarcopenia and obesity are combined, so-called ‘sarcopenic obesity’ (SO)(Reference Prado, Wells and Smith11). SO seems to be associated with a substantial risk for low physical function, CVD and mortality(Reference Atkins, Whincup and Morris12,Reference Levine and Crimmins13) . As a consequence, understanding the impact and management of SO is especially important.
In sarcopenia, there are several consensuses on a clinical definition(Reference Chen, Liu and Woo14,Reference Cruz-Jentoft, Bahat and Bauer15) ; however, the definition of SO remains controversial(Reference Donini, Busetto and Bauer16). The prevalence and associated outcomes are significantly affected by the variable definitions and diagnostic criteria. One study in Singapore with a small number of participants showed a high level of variations in the prevalence rates across different SO definitions(Reference Khor, Lim and Tay17). Different indicators of clinical outcomes were also assessed in different studies examining SO(Reference Donini, Busetto and Bauer16). Almost all previous studies of SO defined sarcopenia as synonymous with low muscle mass(Reference Donini, Busetto and Bauer16). However, current consensuses suggest that the sarcopenia definition should include both low muscle mass and low muscle strength(Reference Cruz-Jentoft, Bahat and Bauer15,Reference Bhasin, Travison and Manini18) . In addition, most studies use only BMI to define obesity. However, BMI may be a less than accurate tool for measuring body fat in the older population, which partly explains the obesity paradox(Reference Seidell and Visscher19). To date, studies with comprehensive combinations of different obesity parameters and different sarcopenia indices to evaluate the impact of SO remain lacking. It is crucial to identify patients at risk for the research and targeting of treatment approaches. A harmonising definition of SO is needed to make the clinical diagnosis and management of SO more practical(Reference Batsis and Cook20). Sarcopenia and its components have been noted to be associated with the risk of fall, but whether obesity enhances the risk is not conclusive. Additionally, obesity has been noted to be associated with the risk of MS, but whether sarcopenia and its components aggravate the risk is unclear.
The aim of the present study was to determine the best measurement of SO by estimating the impact on fall risk and MS. In addition, we also independently estimated muscle mass, muscle strength and muscle function with obesity parameters to provide further insight into the influence of individual sarcopenia parameters.
Methods
Study participants
In Taipei city, Taiwan, yearly regular health check-ups for individuals who are 65 years of age or older are provided free by the government. We enrolled participants who were 65 years of age or older, did not experience chest pain or bone pain while exercising, did not have problems of cognitive impairment or congestive heart failure, were not currently receiving regular haemodialysis or medical therapy for malignancy and had received health check-up examinations at the health examination centre of the Tri-Service General Hospital (TSGH) between 2015 and 2017. All participants provided demographic details, health condition information and data related to physical activities by structured questionnaires. Written informed consent was obtained from all participants prior to participation in the health screening programme. The study protocol was approved by the institutional review board of TSGH, Taiwan.
Measurement of body composition
Body weight and body height were measured with a digital scale and a stadiometer and were recorded to the nearest 0·01 kg and 0·1 cm, respectively. BMI was calculated as body weight in kilograms divided by the square of body height in metres. The horizontal plane at the level of the middle point between the uppermost border of the bilateral iliac crests and the lower border of the 12th rib was measured as waist circumference. We used a Bioelectronics Impedance Analyzer (BIA) to measure appendicular skeletal muscle (ASM) (InBody 720, Biospace) and used body height in metres to adjust ASM to ASM/ht2 to define the skeletal muscle mass index. We also measured body fat mass and body fat percentage. All participants avoided eating or drinking anything at least 8 h before this procedure. We used the sex-specific cut-offs proposed by the consensus from the Asia Working Group of Sarcopenia (AWGS) in 2014 for low skeletal muscle mass index (7.0 kg/m2 in men and 5·7 kg/m2 in women)(Reference Chen, Liu and Woo14).
Functional performance measurement
Measuring and calculating the mean value of three measurements of the dominant hand’s grip strength was conducted using a dynamometer (Exacta™ Hydraulic Hand Dynamometer; North Coast Medical Inc.). Walking time was measured over a straight line 6 metres long at their usual gait speed. Gait speed was calculated as the distance divided by walking time. Based on the sarcopenia definition by AWGS in 2014, the cut-off value for low handgrip strength was less than 26 kg for men and less than 18 kg for women, and a gait speed less than 0·8 m/s was used as the cut-off for low gait speed(Reference Chen, Liu and Woo14).
Definition of sarcopenia
Sarcopenia was defined as low skeletal muscle mass index with either low handgrip strength or low gait speed or both, according to the consensus of the European Working Group on Sarcopenia in Older People (EWGSOP) in 2010(Reference Cruz-Jentoft, Baeyens and Bauer21) The cut-off values of low skeletal muscle mass index, low handgrip strength and low gait speed were in accordance with the consensus of AWGS in 2014(Reference Chen, Liu and Woo14) Our study was conducted between 2015 and 2017. The EWGSOP2 was published in 2019(Reference Cruz-Jentoft, Bahat and Bauer15) Therefore, we used EWGSOP 2010. In addition, one of the strengths was using EWGSOP 2010 because it enabled us to compare our results to those of studies in the same period. Most studies on SO were conducted before EWGSOP2 was published.
Definitions of sarcopenic obesity
SO was defined by the above sarcopenia definition plus obesity. However, the definition of obesity among older adults has been inconsistent, and we attempted to examine the impact on clinical outcomes in community-dwelling older adults based on different definitions of obesity. We defined obesity by three different parameters based on the definition of obesity from the Health Promotion Administration in Taiwan: BMI ≥ 27 kg/m2; waist circumference of ≥ 90 cm for men and ≥ 80 cm for women; and body fat percentage of > 25 % for men and > 30 % for women(Reference Chang, Wu and Chang22). In addition, we also used each component of sarcopenia (low muscle mass, low handgrip and slow gait speed) combined with the obesity parameters to define different phenotypes of SO.
Definition of the metabolic syndrome
We used the National Cholesterol Education Program (NCEP) expert panel on the detection, evaluation and treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) guidelines(Reference Ervin23) to define the cut-off points for hypertension, hyperglycemia, hypertriacylglycerolaemia and low levels of HDL. Modified criteria of abdominal obesity for Asian people were based on guidelines from the International Diabetes Federation(Reference Alberti, Eckel and Grundy24). MS was defined as the fulfilment of three or more of the following conditions:(1) hypertension with systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; (2) hyperglycemia with fasting glucose ≥ 100 mg/dl;(3) hypertriacylglycerolaemia with serum TAG ≥ 150 mg/dl;(4) low level of HDL cholesterol as < 40 mg/dl in males and < 50 mg/dl in females, and (5) abdominal obesity as waist circumference ≥ 90 cm in males or ≥ 80 cm in females.
Covariates
Age, cigarette smoking and alcohol consumption were ascertained from personal identification cards and by self-report. Alcohol consumption was defined as drinking at least once every week. Positive smoking status was defined as ever smoking in life. The presence of hypertension was defined as average blood pressure ≥ 140/90 mmHg, based on a doctor’s diagnosis by self-report, or the regular use of medications for blood pressure control. Diabetes mellitus was defined as fasting plasma glucose ≥ 126 mg/dl, by self-report of a physician’s diagnosis, or the current use of diabetic medications (including oral hypoglycemic agents or insulin injection). Medical histories of stroke, coronary artery disease, chronic obstructive pulmonary disease, arthritis, osteoporosis and use of antipsychotic agents or sedative agents were obtained by self-report. Physical activities were assessed by the International Physical Activity Questionnaire Short Form (IPAQ)(Reference Qu and Li25). The 5-times sit-to-stand test was administered by asking the participant to stand up from a chair with their arms folded across their chest and then sit down for five repetitions as quickly as possible. The total time was recorded with a stop watch. A subset of the participants were asked one additional question regarding whether they had experienced fall events in the past year.
Statistical analysis
Continuous variables are presented as the mean and standard deviation. Categorical variables are expressed as the number (percentage). ANOVA was conducted to examine the differences in continuous variables among participants categorised as robust and with obesity, sarcopenia and SO. χ 2 tests were used to compare categorical variables. We used multiple logistic regression analyses to examine the fall risk and MS among robust, obesity, sarcopenia and SO groups. In fall risk analysis, binary logistic regression analysis was performed according to sarcopenia and obesity status after adjustment for confounding variables. Models were initially adjusted for age and sex and then further adjusted for health behaviours (smoking and alcohol consumption), MS, physical activities, osteoporosis, arthritis, use of antipsychotic agents and sedative agents. In the MS analysis, adjustments for different confounding variables were also applied. Models were initially adjusted for age and sex and then further adjusted for health behaviours (smoking and alcohol consumption), physical activities, uric acid, stroke and coronary artery disease. We also analysed data after combining individual components of sarcopenia with different obesity parameters. All analyses were performed with IBM SPSS Statistics version 22. Significance was set as a P-value < 0·05 in a two-sided test.
Results
There were 765 older adults who completed an interview as well as laboratory and clinical examinations enrolled in our study. Some of the participants (n 468) comprised the subgroup for the analysis of fall risk. In the analysis of waist circumference-defined SO, twenty-nine older adults had SO. These participants with SO tended to have an older age, higher serum levels of TAG, lower fasting sugar, longer completion time in the 5-times sit-to-stand test, lower handgrip strength, slower gait speed, higher body fat percentage, higher fat-to-muscle ratio, higher likelihood of being female, more likely to have the chronic diseases of hypertension and arthritis, more likely to have a drug history of antipsychotic use and higher prevalence of falls in the past year than participants with a robust status (Table 1).
Table 1. Characteristics of the participants by waist-defined sarcopenic obesity
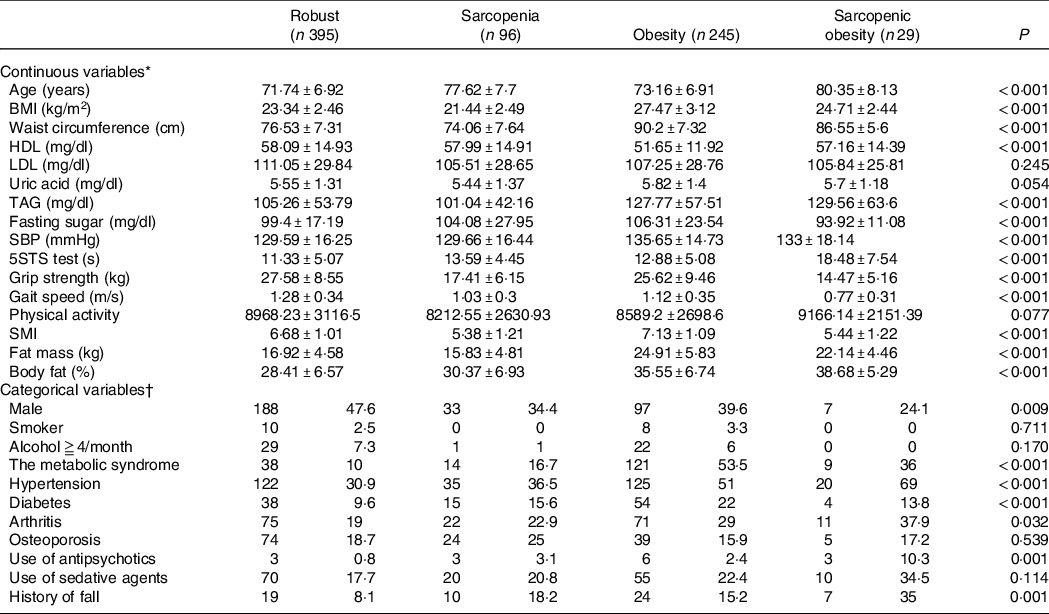
SBP, systolic blood pressure; 5STS, 5-times sit-to-stand up; SMI, skeletal muscle mass index.
ANOVA was conducted to examine the differences in continuous variables among participants categorised as robust and with obesity, sarcopenia and sarcopenic obesity. χ 2 tests were used to compare the categorical variables among the participants categorised as robust and those with obesity, sarcopenia and sarcopenic obesity; P < 0·05 was considered statistically significant.
* Values in the continuous variables are expressed as the mean and standard deviation unless otherwise specified.
† Values in the categorical variables are expressed as the number (percentage).
In the analysis of participants with SO defined by BMI, only seven older adults fit this definition. This group with SO showed relatively older age, lower serum HDL-cholesterol levels, higher serum uric acid levels, higher serum TAG levels, lower fasting sugar, longer completion time for the 5-times sit-to-stand test, lower handgrip strength, lower gait speed, higher fat mass, higher body fat percentage and higher fat-to-muscle ratio than participants with a robust status. The participants with SO tended to be female, have a higher percentage of taking antipsychotics and have a higher percentage of individuals with falls in the past 12 months than participants with a robust status (online Supplementary Table 1a). There were ninety older adults with higher body fat percentages and sarcopenia. They had longer completion times for the 5-times sit-to-stand test, higher body fat percentage and higher fat-to-muscle ratios and tended to be female, with a higher prevalence of a history of hypertension compared with robust participants (online Supplementary Table 1b).
With the definition of obesity based on higher BMI, we examined the fall risk among the older adults with non-sarcopenia/non-obesity, sarcopenia/non-obesity, non-sarcopenia/obesity and SO and treated the non-sarcopenia/non-obesity participants as the reference group. The SO group had a higher OR to fall (OR: 4·66; 95 % CI 1·42, 15·29; P = 0·011) compared with the robust group after adjusting for confounding covariates. In the analysis of the fall risk among older adults with SO defined by waist circumference, the participants with non-sarcopenia/non-obesity were treated as the reference group. The OR to fall in the participants with SO was 10·16 (95 % CI 2·71, 38·13; P = 0·001) after fully adjusting for confounding covariates. When body fat percentage was used to define obesity, the participants with SO had a higher OR to fall (OR: 3·33; 95 % CI 1·07, 10·36; P = 0·038) compared with the robust group after controlling for possible confounding factors (Table 2).
Table 2. Associations of the fall risk between participants with sarcopenia and obesity defined by different obesity parameters

Logistic regression analyses to examine the fall risk among the robust, obesity, sarcopenia and sarcopenic obesity groups. The robust group is the reference group for the other groups; P < 0·05 was considered statistically significant.
* Adjusted covariates: Model 1 = age, sex; Model 2 = Model 1 + health behaviours (smoking and alcohol consumption), the metabolic syndrome, physical activity, osteoporosis, arthritis, and the use of antipsychotic agents and sedative agents.
In the analysis of MS risk between participants with SO, we first defined obesity by higher BMI, and the participants with SO had an OR of 3·65 (95 % CI 0·49, 27·25; P = 0·207) after adjusting for confounders compared with the robust group. When defining SO by waist circumference, the older adults with SO had a higher OR of having MS (OR: 5·27; 95 % CI 2·06, 13·45; P = 0·001) after fully adjusting for possible confounding factors compared with the robust group. In the analysis of SO defined by body fat percentage, the participants with SO tended to have a higher OR of MS (OR: 2·66; 95 % CI 1·28, 5·50; P = 0·009) after adjusting for possible confounding factors compared with the robust group (Table 3).
Table 3. Associations of the metabolic syndrome risk between participants with sarcopenia and obesity defined by different obesity parameters

Logistic regression analyses to examine the metabolic syndrome risk among robust, obesity, sarcopenia and sarcopenic obesity groups. The robust group is the reference group for other groups; P < 0·05 was considered statistically significant.
* Adjusted covariates: Model 1 = age, sex; Model 2 = Model 1 + health behaviours (smoking and alcohol consumption), physical activity, uric acid, stroke and coronary artery disease.
In the analysis of fall risk between the participants with individual components of sarcopenia coupled with obesity, the risks were statistically significant when considering low gait speed and low grip strength but not low muscle mass (Table 4). In the analysis of MS risk between the participants with individual components of sarcopenia coupled with obesity defined by waist circumference, the risks were also statistically significant when considering low gait speed (OR: 7·19; 95 % CI 3·61, 14·30) and low grip strength (OR: 9·19; 95 % CI 5·00, 16·91) but not low muscle mass (Table 5).
Table 4. Associations of the fall risk between participants with individual components of sarcopenia and obesity defined by different obesity parameters
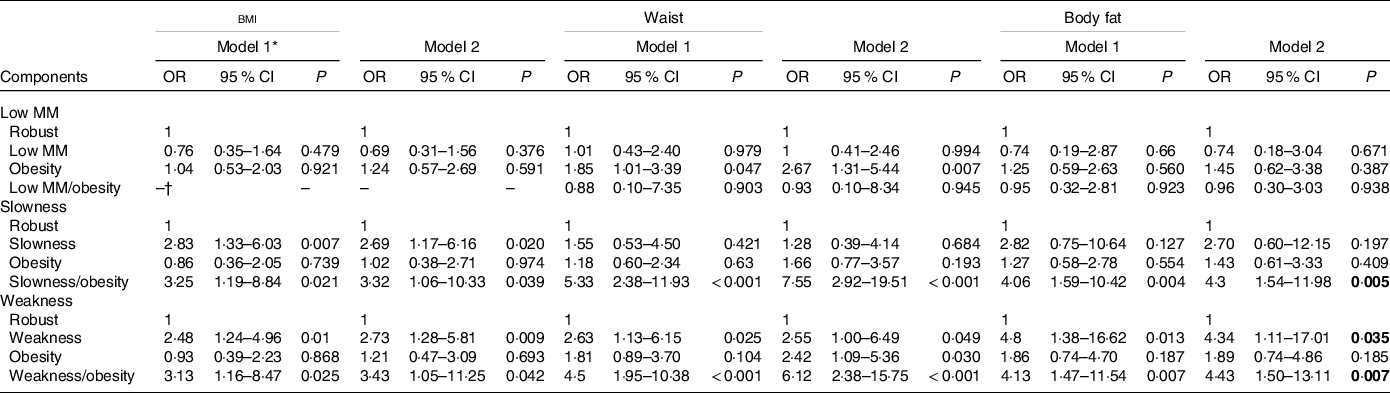
Low MM, low muscle mass
Logistic regression analyses to examine the fall risk among low MM, slowness and weakness components. The robust group in each components is the reference group for other groups; P < 0·05 was considered statistically significant.
* Adjusted covariates: Model 1 = age, sex; Model 2 = Model 1 + health behaviours (smoking and alcohol consumption), the metabolic syndrome, physical activity, osteoporosis, arthritis, use of antipsychotic agents and sedative agents.
† The case number is too small to analysis.
Table 5. Associations of the metabolic syndrome risk between participants with individual components of sarcopenia and obesity defined by different obesity parameters
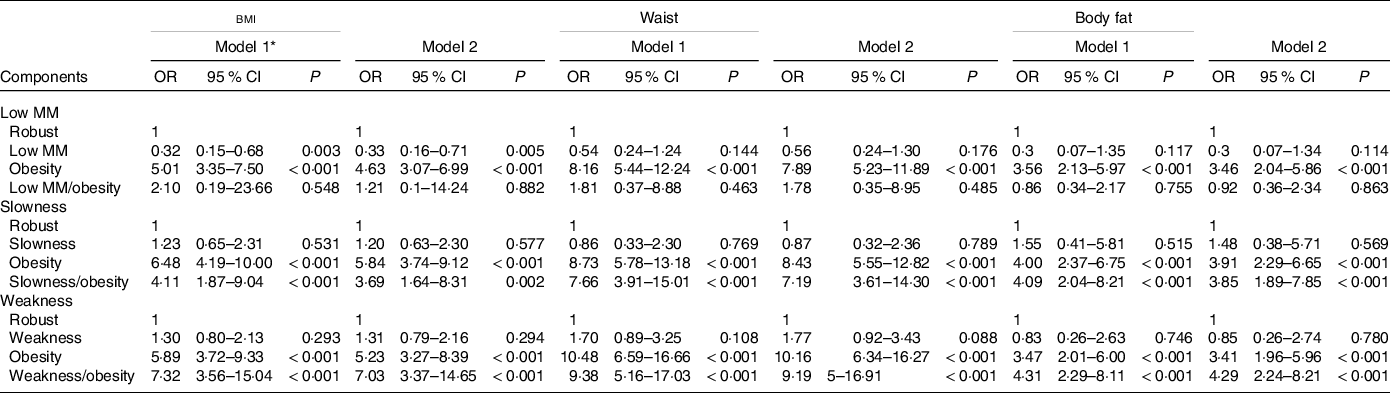
Low MM, low muscle mass
Logistic regression analyses to examine the metabolic syndrome risk among low MM, slowness and weakness components. The robust group in each components is the reference group for other groups; P < 0·05 was considered statistically significant.
* Adjusted covariates: Model 1 = age, sex; Model 2 = Model 1 + health behaviours (smoking and alcohol consumption), physical activities, uric acid, stroke and coronary artery disease.
Discussion
To our knowledge, this is the first study to assess the risk in falls and MS across a wide range of SO measurement. Our study found that different measurement of obesity resulted in a wide range of SO prevalence rates. BMI-defined obesity yielded lower prevalence results and underestimated clinical outcomes of the MS, while waist circumference-defined and body fat percentage-defined obesity resulted in relatively reasonable related to clinical outcomes of SO. These discrepancies indicated that the different measurement of obesity may overestimate or underestimate the prevalence of SO. In terms of clinical outcomes, a synergistic effect existed in the fall risk estimation among the three different definitions of obesity parameters, especially waist-defined obesity. In the MS analyses, the risk existed only in SO defined by waist circumference and body fat, and sarcopenia alone was not the risk factor for MS. Obesity may have additional detrimental effects rather than protective effects when combined with sarcopenia in this population.
Sarcopenia is a major issue in the ageing population globally(Reference Cao and Morley26). In addition, the prevalence of obesity is also rising(Reference Jaacks, Vandevijvere and Pan27). The coexistence of these two epidemics has increased the SO population(Reference Zamboni, Rubele and Rossi28). Sarcopenia aggravated the adverse effects of obesity in older adults and vice versa (Reference Zamboni, Rubele and Rossi28). Therefore, this special population needs more awareness. In addition, when we encouraged the older adults with obesity to lose weight without accounting for muscle mass, muscle loss usually happened in conjunction with a decrease in fat(Reference Villareal, Aguirre and Gurney29). This will worsen the condition of SO. As a result, less than 5 % of all participants were found to have this condition. An accurate diagnosis for SO is needed to find the target population and develop different interventions for the management obesity and sarcopenia in this subset of participants.
The fall risk examination between these three different SO definitions revealed quite different results. Our results revealed that obesity defined by BMI and body fat did not increase the fall risk, while SO defined by BMI and body fat was associated with an increase in the fall risk. This means that coexisting sarcopenia and obesity indeed increased the risk of fall compared with sarcopenia or obesity only. This result is similar to the study in post-menopausal women that defined SO by body fat, which revealed that fall risk increased in those with SO but not in those with sarcopenia alone(Reference Follis, Cook and Bea30). Compared with Western populations, the Asian population with the same BMI value has a higher cardiovascular risk(31). However, some specific obese populations have lower mortality, which has also been called the obesity paradox(Reference Hainer and Aldhoon-Hainerova32). Therefore, using only BMI to define obesity is usually not precise. Waist circumference is a good indicator for central obesity. In our study, obesity defined by waist circumference was associated with an increased fall risk. SO defined by waist circumference also showed a synergistic effect on fall risk estimation. Therefore, it seems that SO defined by waist circumference is more closely associated with the risk of fall than SO defined by BMI and body fat.
In terms of MS analyses, obesity itself is a strong risk factor for this condition. Treating non-sarcopenia/non-obesity participants as the reference, the risk of MS was obvious in analyses by the three different obesity definitions. However, a risk of MS was not found in participants with sarcopenia plus obesity defined by BMI but was found in SO defined by waist circumference and body fat. This phenomenon reflected that sarcopenia alone was not a risk factor for MS, but SO carries the risk of MS; if older adults progress to sarcopenia concomitantly with obesity defined by waist or body fat, the risk of MS was greater than obesity alone. This conclusion differs from previous studies, which showed that sarcopenia is a risk factor for MS(Reference Lu, Yang and Chang33,Reference Park, Park and Park34) . Lu et al. and Park et al. defined sarcopenia by weight-adjusted muscle mass and found that sarcopenia was related to MS; however, in our study, we defined sarcopenia by height-adjusted muscle mass. This phenomenon is concordant with our previous article on the association between sarcopenia and fatty liver, which revealed that the definition of sarcopenia is important(Reference Peng, Wu and Chen35). When we studied the outcomes of metabolic risk factors, such as MS, in those with sarcopenia, we need a cautious interpretation of the results based on the definition of sarcopenia.
We combined individual components of sarcopenia with obesity parameters as synonymous with SO, as previously mentioned(Reference Donini, Busetto and Bauer16). Neither low muscle mass alone nor coexisting low muscle mass and obesity increases the risk of falls. However, slow gait speed alone increased the fall risk, and a synergistic effect also existed when the participants had both slow gait speed and obesity. In addition, low grip strength alone increased the fall risk, and a synergistic effect also existed when participants had both low grip strength and obesity. The results are most consistent across different obesity parameters when low grip strength alone is considered sarcopenia. An Australian study also found that coexisting low muscle strength and obesity but not low muscle mass and obesity were related to an increased fall risk score(Reference Scott, Sanders and Aitken36). As far as MS is concerned, low muscle mass alone did not increase the risk of MS and was without a synergistic effect when participants had both low muscle mass and obesity. However, there was an increased risk when the participants had both low grip strength and obesity or both low gait speed and obesity. Although previous studies have examined associations between SO and outcomes(Reference Donini, Busetto and Bauer16), our study compared the outcomes of SO in the same population for the first time. We examined a broader view of the different fall and MS risks among these groups. Therefore, we can conclude that regardless of whether fall or MS risk is of concern, the roles of low grip strength and slow gait speed are more important than that of low muscle mass. This is in line with recent EWGSOP2 guidelines(Reference Cruz-Jentoft, Bahat and Bauer15) highlighting that muscle strength but not muscle mass is the key characteristic of sarcopenia. In addition, the risk of clinical events with low muscle strength with obesity is increased compared with low muscle strength only or obesity only.
There were still some limitations that should be acknowledged in the present study. First, we used a BIA to evaluate muscle mass and body fat. More advanced equipment, such as computed tomography and MRI, is needed for accurate measurement of muscle mass and body fat. However, these techniques are expensive and have acquisition limitations. Most previous studies have also used bioelectrical impedance analysis to assess body composition. Moreover, the BIA has been validated against gold standard methods. Second, less than 5 % of participants were categorised as SO when obesity was defined by BMI and waist circumference, which led to relatively wide CI for the risk estimates. Third, we included only community-dwelling adults and an Asian population. Therefore, adults with significant functional limitations and frailty were not included. Other races were also excluded. Therefore, caution should be taken when interpret our results in these populations. Fourth, only a subset of participants were asked about falls. The question about falls was not standardly included in the regular health check-ups. Therefore, the patients who volunteered to answer the additional question might be healthier and proactive. The overall risk might be lower in this fall subset group. Therefore, it is likely that cases of falls were underreported. Misclassification of outcomes would likely attenuate the results towards the null. Fifth, participants in the SO group tended to have an older age compared with those in the robust group. This will increase fall events and the incidence of the MS. Therefore, we adjusted for age in the logistic regression models to minimise the effect of age. Finally, the present study was a cross-sectional design, so causality could not be determined. Further longitudinal studies are required to validate the results.
In conclusion, the coexistence of sarcopenia and obesity defined by waist circumference might be the most appropriate measurement for SO regarding correlations with clinical outcomes in our population. Among the three components of sarcopenia, low grip strength coexisting with abdominal obesity was the most representative of all other measurement in SO in our population. Further validation in other populations and databases need to be carried out.
Acknowledgements
The study was supported by the Ministry of Science and Technology (MOST) in Taiwan (MOST 104–2314-B-016–011-MY3). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Study design: T-C. P. and T-W. K. Data collection: T-C.P., W-L. C. and Y-Y. C. Data analysis: T-C. P., W-L.C. and L.-W.W. Drafting of the manuscript: T-C.P., W-L.C., Y-P.C. and T-W.K.
All authors read and approved the final version of the manuscript
There are no potential conflicts of interest to disclose.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521001288








