Ca is recognised as a key nutrient for human health. Dairy products, including milk, are a major food contributor of Ca due to their high bioavailability(Reference Beto1). Where recommended daily intakes of Ca cannot be obtained through food sources, or for populations at risk of deficiency, Ca supplements are often advised to prevent osteoporosis(Reference Cosman, de Beur and LeBoff2). However, in the last decade, several studies have linked dietary and supplemental Ca with a number of adverse health outcomes. In particular, the literature has indicated a potential link between Ca supplementation and adverse cardiovascular events(Reference Bolland, Avenell and Baron3). It has been suggested that excessive Ca supplementation accelerates vascular calcification(Reference Reid, Bolland and Grey4), which is an independent predictor of cardiovascular mortality(Reference Wilson, Kauppila and O’Donnell5,Reference Pletcher, Tice and Pignone6) . There have also been reports of increased risk of prostate cancer in men with use of Ca supplements(Reference Chan and Giovannucci7,Reference Rodriguez, McCullough and Mondul8) , proposed to be due to the down-regulation of calcitriol levels, which in turn may increase cell proliferation in the prostate(Reference Lokeshwar, Schwartz and Selzer9). However, meta-analyses of both interventional and observational studies have failed to demonstrate this association(Reference Bristow, Bolland and MacLennan10,Reference Schwingshackl, Boeing and stelmach-mardas11) . To our knowledge, the relationship between Ca supplements and respiratory mortality has not been investigated in adults, although parenteral Ca administration to critically ill patients has been associated with an increased risk of acute respiratory failure(Reference Dotson, Larabell and Patel12). In line with supplementation, evidence on the association between milk consumption with disease risk and mortality has produced conflicting results. Some studies have reported an increased risk between milk consumption and mortality(Reference Tognon, Rothenberg and Petrolo13,Reference Michaelsson, Wolk and Langenskiold14) , while others found an inverse relationship(Reference Ness, Smith and Hart15,Reference Wang, Chen and Ouyang16) or no evidence for an association(Reference Farvid, Malekshah and Pourshams17–Reference Umesawa, Iso and Date22). Overall, the relationship between supplemental and dietary Ca and mortality risk in the general population remains unclear and is likely to depend on the underlying pathology. In this prospective study, we used information from nearly 500 000 participants in the UK Biobank to examine the associations of supplemental Ca and dairy milk consumption with all-cause and cause-specific mortality.
Methods
Study design and participants
The study design has been previously described elsewhere(23). Briefly, between March 2006 and July 2010, the UK Biobank recruited 502 316 men and women from across England, Scotland and Wales. Inclusion criteria included being aged 40–69 years and living within a reasonable travelling distance (10 miles) from one of the twenty-two assessment centres. Participants attended their closest assessment centre and provided baseline information, physical measures and biological samples. In the present study, we included all participants in the UK Biobank who provided information on Ca supplementation or milk intake during the baseline assessment (n 497 828). This research was conducted under UK Biobank application 20175. The UK Biobank study was approved by the National Information Governance Board for Health and Social Care and North West Multicentre Research Ethics Committee (11/NW/0382).
Outcomes
Mortality data were obtained from the National Death Registries through linkage to records held by the Office for National Statistics for England and Wales and the Registrar General’s Office for Scotland(24). At the time of analysis, information on mortality outcomes was available up to July 2016, with the latest death date recorded in February 2016. Information on hospitalisations after the baseline data collection was identified through linkage to Hospital Episode Statistics for participants from England and Wales and through Scottish morbidity records for participants from Scotland(25). Primary causes of death were defined using the International Classification of Diseases edition 10(26) for cancer (code C00-D48), CVD (code I00-I89) and respiratory disease (J09-J22, J40-J47). Respiratory hospital admissions were further categorised as acute and infectious respiratory conditions (code J09-J22), chronic obstructive pulmonary diseases (code J40-J44) and asthma (code J45-J46).
Exposures and covariates
Information on supplemental Ca and dairy milk consumption was self-reported at baseline using computer-based touchscreen questionnaires(23). Use of Ca supplements referred to current use (‘Do you take any of the following [mineral supplements]?’). Dairy milk consumption was based on the question asking about the use and type of milk typically used (full cream, semi-skimmed, skimmed, soya, other type of milk, never/rarely have milk). Participants who answered ‘Do not know’ or ‘Prefer not to answer’ were coded as missing. Data on dietary Ca intake were available for a subsample that completed the 24-h recall questionnaire (web-based)(23). Participants were presented with 200 commonly consumed foods and drinks and reported the amount of each food consumed in the preceding 24-h period.
Covariate data were also self-reported via the touchscreen questionnaire(23). We considered demographic, socio-economic, lifestyle and health factors as potential confounders based on their known associations with mortality and Ca intake. Demographic variables included sex, age group (<65 or ≥65 years), ethnicity (White British, Asian/Asian British, Black/Black British or Mixed/Other) and BMI, which was calculated based on height and weight (categorised as underweight (<18·5 kg/m2), normal weight (18·5 to <25·0 kg/m2), overweight (25·0 to <30·0 kg/m2) or obese (≥30 kg/m2). BMI was based on measures obtained during the baseline visit. Key socio-economic determinants of health were considered, including highest education qualification (none, high school, National Vocational Qualification/Certificate of Secondary Education/Advanced levels or university degree or higher) and Townsend deprivation index (divided into five quintiles). Smoking status (never, former smoker or current smoker), alcohol consumption (daily or almost daily, 3–4 times/week, 1–2 times/week, 1–3 times/month or special occasions only) and physical activity intensity (none, light/moderate, vigorous) were regarded as confounding lifestyle factors. Health-related confounders included the number of treatments or medication taken (none, 1–2, 3–4 or ≥5) and self-reported assessment of general health (self-rated as excellent, good, fair or poor). Use of hormone replacement therapy was also considered in sensitivity analyses.
With regard to respiratory disease, we deemed inhaled corticosteroids, combination of inhaled corticosteroids and long-acting β-2 agonists, as potential confounders by indication, based on the known influence on bone metabolism(Reference Biskobing27). Medications were coded by a trained nurse via a standardised computer-assisted personal interview and validated by linking with the participants’ past medical records.
Statistical methods
Our primary analyses examined associations between Ca supplementation and dairy milk intake with mortality from all causes, cancer, CVD and respiratory disease using logistic regression. Effect modification by demographic variables was tested by adding an interaction term to the logistic regression model. Due to the observed effect modification by age, main analyses and results were stratified by age group (<65 and ≥65 years). OR and 95 % CI were estimated, with P values <0·05 considered statistically significant. Crude analyses adjusted for sex, age, ethnicity and assessment centre. In multivariate analyses, the models were further adjusted for potential confounders at baseline, including BMI, education level, deprivation quintile, smoking status, alcohol consumption, physical activity intensity, number of treatments or medications taken and self-reported health.
Several sensitivity analyses were conducted to determine the influence of our assumptions on resulting associations. First, we examined associations between supplementation with nutrients other than Ca on mortality outcomes, with reference to individuals who used no supplements at all. With respect to respiratory mortality, we adjusted models for use of inhaled corticosteroids. Cross-sectional regression was also performed to determine trends between Ca supplementation and use of inhaled corticosteroids. In post hoc analyses, we investigated the association between Ca supplementation and hospital admissions for cause-specific respiratory diseases. We performed these analyses twice, with participants with mild to severe airflow limitation (FEV1:FVC ratio <70 %) or other chronic lower respiratory conditions at baseline both included then excluded from the control groups. All analyses were performed using STATA, version 14.1(28).
Results
After an average follow-up time of 4·2 years, we documented 14 255 total deaths, of which 8297 were due to cancer, 2959 attributed to CVD and 572 related to respiratory diseases. Baseline characteristics of the study sample are shown in Table 1. A total of 34 906 participants reported use of Ca supplements, and 454 756 participants were consumers of dairy milk (7·56 % full cream; 70·48 % semi-skimmed; 21·96 % skimmed). Compared with participants who did not use Ca supplements, users tended to be female, aged ≥65 years, underweight, never smoke or consume alcohol, engage in light or moderate physical activity and have a degree or professional qualification. Ca supplement use was also associated with taking more total treatments or medications and poor self-reported health. Participants who consumed dairy milk were more likely to be male, of White or Asian descent and never consume alcohol. Rates of all-cause mortality were highest in males, participants aged ≥65 years and those of White ethnicity. In addition, participants that died from all causes were more likely to be underweight, current smokers at baseline, never consume alcohol, more materially deprived and less likely to engage in physical activity or have higher degree education. As expected, mortality was also associated with higher use of treatments or medications and worse self-reported health.
Table 1. Characteristics of the UK Biobank participants (Numbers and percentages)
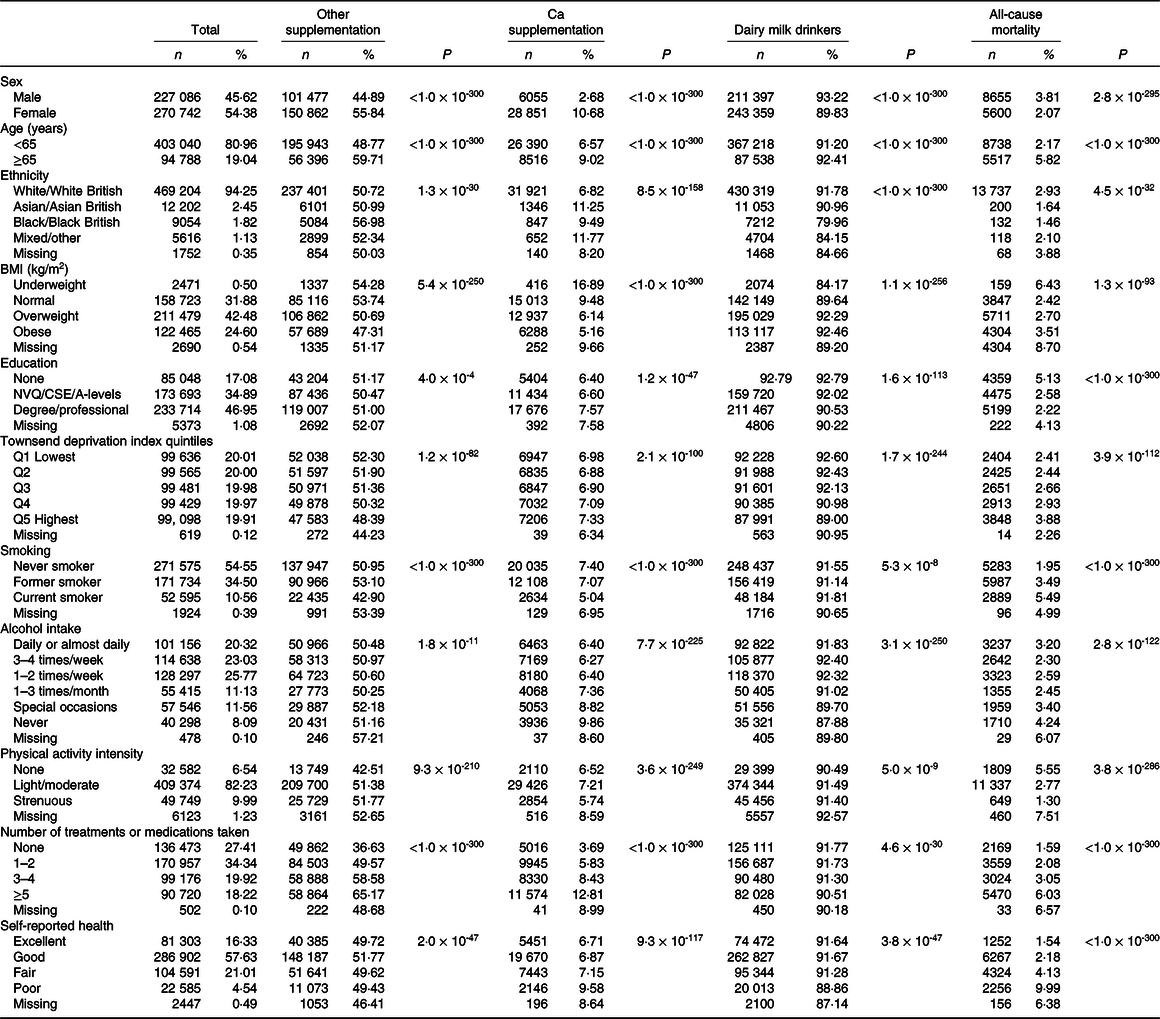
NVQ, National Vocational Qualification; CSE, Certificate of Secondary Education; A-levels, Advanced level.
The association between Ca supplementation and milk consumption with mortality varied by age group (P interaction ≤ 0·04) but not by sex or ethnicity (P interaction ≥ 0·06). In simple models adjusted for age, sex, ethnicity and assessment centre, supplementation was associated with an increased risk of all-cause mortality in the full sample and in younger participants (<65 years), but this association was abolished or reversed after full adjustment for social, behavioural and health-related characteristics (Table 2). There was no evidence for an association between Ca supplementation with mortality from cancer or CVD after full adjustment. In contrast, mortality from respiratory diseases was elevated in participants who were aged younger than 65 years (fully adjusted OR 1·69, 95 % CI 1·16, 2·47) (Table 2). Multivariate analyses for the association between Ca supplementation and respiratory mortality in younger participants remained elevated and significant when controlling further for the use of inhaled corticosteroids (P = 0·006, data not shown). There was some evidence for an association between dairy milk intake and lower mortality risk in the simple models (Table 2). A borderline association remained after full adjustment in participants <65 years old with respect to all-cause mortality and cancer mortality (Table 2). In participants aged ≥65 years, there were no associations between milk intake and all-cause or cause-specific mortality before or after multivariate adjustment (Table 2). Dietary Ca intake was not associated with all-cause or cause-specific mortality in the sub-sample, using information available from the 24-h dietary recall (n 68 795, Table 2).
Table 2. Calcium supplementation, dairy milk consumption, dietary calcium and mortality (Odds ratios and 95 % confidence intervals)

Q, quartile.
* Crude OR with the adjustment of age, sex, ethnicity and assessment centre.
† Adjusted OR with the adjustment of age, sex, ethnicity, assessment centre, education level, deprivation index quintile, smoking, alcohol use, physical activity intensity, BMI, number of treatments or medications taken and self-reported health.
‡ Using data gathered via touchscreen questionnaire.
§ Using data gathered via 24-h recall.
‖ Quartile 1 = 0–689 mg.
¶ Quartile 2 = 690–914 mg.
** Quartile 3 = 915–1187 mg.
†† Quartile 4 = 1188–8012 mg.
Sensitivity analysis
In further analyses, we compared the patterns observed with Ca supplementation with those seen with the use of any other supplements. We found statistically significant decreases by the intake of other supplements (excluding Ca) in the odds of mortality from all causes, cancer and CVD (P < 0·01 for all comparisons), while no associations were seen for respiratory mortality (online Supplementary Table S2).
Due to the suggested association between Ca supplementation and mortality from respiratory diseases, analyses were undertaken to establish any related evidence with respect to hospitalisations for cause-specific respiratory conditions. As shown in Table 3, Ca supplementation was not associated with the odds of hospital admission for acute and infectious respiratory conditions, chronic obstructive pulmonary diseases or asthma (P > 0·10) after full confounder adjustment, despite observed increases in mortality. Cross-sectional analysis demonstrated that participants who used Ca supplements were slightly more likely to use inhaled corticosteroids (8·86 v. 6·97 %).
Table 3. Calcium supplementation and incident respiratory diseases (Odds ratios and 95 % confidence intervals)
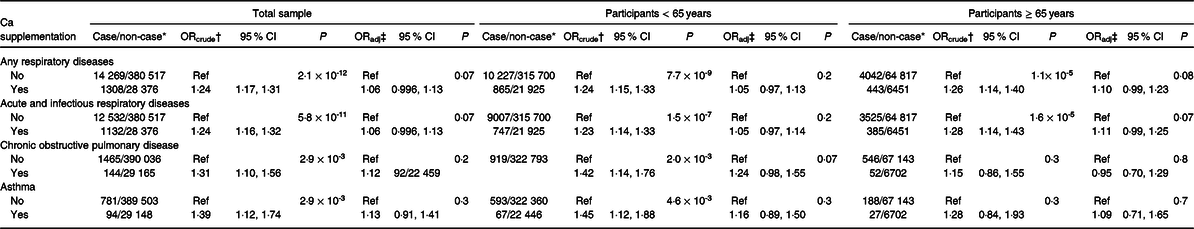
* Among non-cases, participants with the FEV1:FVC ratio greater than 70 % were removed from analyses.
† Crude OR with the adjustment of age, sex, ethnicity and assessment centre.
‡ Adjusted OR with the adjustment of age, sex, ethnicity, assessment centre, education level, deprivation index quintile, smoking, alcohol use, physical activity, intensity, BMI, number of treatments or medications taken and self-reported health.
In further interaction analyses, we found evidence for a differential effect of Ca supplementation on cancer mortality based on the previous use of hormone replacement therapy (P interaction = 0·0003). After full adjustment for covariates, Ca supplement use was associated with a decreased odds of cancer mortality in women using hormone replacement therapy but an increased risk in other women (fully adjusted OR 0·81, 95 % CI 0·69, 0·94 v. OR 1·17, 95 % CI 1·01, 1·35, respectively) (Table 4). This interaction was reflected in the relation between Ca supplementation and total mortality (P interaction = 0·015), while associations with CVD or respiratory disease mortality were not modified by hormone replacement therapy use.
Table 4. Calcium supplementation and mortality in women, with and without history of hormone replacement therapy use* (Odds ratios and 95 % confidence intervals)

* P value for interaction by the use of hormone replacement therapy is 0·015 and 0·0003 for total and cancer mortality, respectively.
† Using data gathered via touchscreen questionnaire.
‡ Adjusted OR with the adjustment of age, sex, ethnicity, assessment centre, education level, deprivation index quintile, smoking, alcohol use, physical activity intensity, BMI, number of treatments or medications taken and self-reported health.
Discussion
In this large-scale prospective study, we found little evidence to support the expressed concerns for possible adverse effects of Ca on mortality risk. In majority of these analyses, significant associations appeared to be due to confounding. However, we observed an association between Ca supplementation and elevated odds of mortality from respiratory diseases in participants aged younger than 65 years, with this association being robust to adjustment for social, lifestyle and health-related confounders, including the use of inhaled corticosteroid medications. However, the suggested increase in respiratory disease risk by Ca supplementation was not replicated in the analyses using data from hospital admissions, suggesting that independent data are required to confirm our findings.
Parenteral Ca administration to critically ill patients has been reported to more than double the mortality risk and the risk of acute respiratory failure(Reference Dotson, Larabell and Patel12). These observations are in line with animal studies and may be related to pro-inflammatory actions by Ca(Reference Collage, Howell and Zhang29). However, the evidence for an association between oral Ca supplementation and mortality from respiratory diseases was unexpected. To our knowledge, no study has specifically investigated the effect of Ca supplementation on respiratory diseases. Given the lack of reliable literature to support this finding, one possibility is that the observed association between Ca supplementation and respiratory mortality was due to confounding by indication. Theoretically, people with pre-existing respiratory conditions may have intentionally taken Ca supplements to prevent osteoporosis, which is a well-known complication of chronic inflammatory airway diseases(Reference Biskobing27,Reference Buehring, Viswanathan and Binkley30–Reference Weinstein33) . This is due to the systemic inflammatory response of the disease itself, as well as from chronic use of inhaled corticosteroids to control the disease(Reference Biskobing27). Although we are unable to fully discount possible influences by confounding or reverse causation, the association between Ca supplementation and mortality from respiratory diseases remained significant even when we controlled for use of inhaled corticosteroids in multivariate models, in addition to the broad range of social, behavioural and health-related covariates.
There are some biologically plausible mechanisms which might contribute to the suggestive association between Ca supplementation and increased respiratory mortality. Exacerbations of obstructive lung diseases such as chronic obstructive pulmonary diseases and asthma are characterised by airway obstruction, mucus hypersecretion and inflammation(Reference Cukic, Lovre and Dragisic34). The distinctive narrowing of the bronchi and bronchioles in chronic obstructive pulmonary diseases may result from contraction of the airway smooth muscle(Reference Cukic, Lovre and Dragisic34), which may be initiated by increases in intracellular Ca(Reference Kuo and Ehrlich35). An increase in cytosolic concentrations of free Ca must also develop to trigger mucous gland secretion, vagal nerve activity (which constricts the bronchi), mast cell mediator release (which induces inflammation) and the movement of inflammatory cells into the walls of the airways(Reference Middleton36). Thus, a possible action of Ca supplements on the respiratory system may be related to the acute increase in serum Ca, which has been observed after ingestion of Ca supplements, but not after consuming Ca-rich foods(Reference Machado, Bruce-Mensah and Whitmire37). Such a mechanism is plausible, as the ‘Calcium hypothesis of asthma’ suggests that Ca channel blocking agents may have a beneficial effect in asthma by inhibiting these actions(Reference Svedmyr38). However, bronchoprovocation studies to support this theory have been inconsistent(Reference Nair, Townley and Bewtra39–Reference Ann Twiss, Harman and Chesrown41). Overall, more mechanistic studies are needed to understand the true effects of Ca on the respiratory system.
Previous research is generally consistent with our results on Ca supplementation and mortality from all causes, cancer and CVD. Our results correspond to those of Avenell et al.(Reference Avenell, MacLennan and Jenkinson42), who conducted a randomised controlled trial examining supplementation with 1000 mg calcium carbonate v. placebo in 5292 people residing within the UK. They reported no significant increase in total, cancer or cardiovascular mortality with Ca supplementation. The participants are alike in terms of geographical location and that users were predominantly female (85 % women), but they were generally older (mean age 77 years old) than participants within the UK Biobank.
With regard to cancer-specific mortality, a meta-analysis of ten randomised controlled trials investigating Ca supplementation also reported null findings, although cancer was not a primary outcome of any included study(Reference Bristow, Bolland and MacLennan10).
Oestrogen therapy has been suggested to be a modifier of the effect of Ca (+vitamin D) supplementation on colorectal cancer(Reference Ding, Mehta and Fawzi43), and in line with this suggestion, we found some evidence for a differential association between Ca supplementation and cancer mortality in women based on the use of hormone replacement therapy. However, in the earlier study, women who did not receive oestrogen therapy but who were randomised to receive Ca and vitamin D were at suggestively decreased risk of developing colorectal cancer, while in our study, Ca supplementation was associated with an increased cancer risk in those who did not use hormone replacement therapy (and a decreased risk in those who did). While we cannot discount the role of methodological differences or chance, this discrepancy might have a biological explanation as variant in the CYP24A1 gene (involved in the regulation of Ca homeostasis) has been shown to interact with hormone replacement therapy effects on colorectal cancer(Reference Garcia-Albeniz, Rudolph and Hutter44).
Our findings are consistent with the European Prospective Investigation into Cancer and Nutrition study, where researchers found no evidence for an association between Ca supplementation and cardiovascular mortality(Reference Li, Kaaks and Linseisen45).
On the contrary, in the prospective NIH-AARP Diet and Health Study (n 388 229), Ca supplementation was associated with an increased risk of cardiovascular mortality in men (but not women)(Reference Xiao, Murphy and Houston46), while we found no evidence for interaction by sex. The proportion of men reporting the use of Ca supplements in the UK Biobank was notably lower compared with this earlier study (2·7 v. 23 %, respectively), which may explain the discrepancy.
Our findings are relatively comparable with previous observational studies on milk consumption and mortality. A meta-analysis of twelve prospective studies found no consistent association between milk intake and mortality from all causes, cancer or CVD(Reference Larsson, Crippa and Orsini19). However, similar to our analyses, when studies were stratified by age, results in younger cohorts (≤55 years) suggested a borderline inverse association with all-cause mortality. We were unable to locate published studies examining the effects of milk consumption on respiratory mortality, which highlights a gap in the literature. It could also reflect a lack of causal effect of Ca on respiratory diseases, as non-significant findings tend to be less likely to be published(Reference Easterbrook, Gopalan and Berlin47).
Overall, findings on milk consumption differed from those on Ca supplements, with weak inverse associations observed for all-cause and cancer mortality, and null associations for respiratory mortality. It seems pertinent to note that sensitivity analyses demonstrated null or protective effects of supplementation with nutrients besides Ca and mortality outcomes. Thus, differences between Ca supplements and dairy milk may be attributable to the presence of other micronutrients in milk.
Strengths and limitations
The large, prospective study design with rich data on potential confounding factors and standardised protocols for data collection were clear strengths of the present study. However, we recognise that the present study also had several limitations. Firstly, information on Ca supplementation and milk intake was based on self-report, which may have resulted in misclassification of participants with respect to the main exposure. However, any response bias is likely to be unselected, as there is no apparent reason for individuals to be more inclined to report consumption of dairy milk or use of Ca supplements. We only considered baseline exposure data, and therefore changes in diet over time may have confounded diet–disease associations. Only limited information on diet and supplement use was available for the whole cohort, while total Ca intakes could be estimated for a sub-sample.
It is also acknowledged that UK Biobank participants are not representative of the general population in terms of prevalence of obesity and co-morbidities(Reference Fry, Littlejohns and Sudlow48). There is evidence of a ‘healthy volunteer’ selection bias in this cohort, which is likely to have reduced the incidences of mortality. Efforts were made to control for this through multivariate adjustments(Reference Suresh, Gajendragad and Rahman49) but, at the same time, multivariate statistical models also had the potential to ‘over-adjust’ for morbidity(Reference Weinberg50).
Conclusion
In conclusion, the present study suggests that Ca intake is not associated with increases in all-cause mortality. Ca supplementation, however, may modestly increase mortality from respiratory diseases, but we cannot exclude the possibility that our analysis captured a reverse causation phenomenon. Future research should be directed towards determining causality using experimental study designs. Meanwhile, dairy milk may be a safer source of attaining recommended daily intakes of Ca in terms of longevity.
Acknowledgements
This work was supported by the National Health and Medical Research Council, Australia grant to EH (project GNT1123603). The UK Biobank was supported by the National Health Service and received funding by the UK Medical Research Council, Wellcome Trust and Department of Health. The funders had no role in the analysis or writing of this manuscript.
E. H. conceived and supervised the study. L. S. and A. Z. conducted data analysis, and all authors interpreted the results. L. S. drafted the manuscript with input from all authors. All authors approved the final version for submission.
There are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519003076







