Chronic kidney disease (CKD) is one of the leading major health problems worldwide(Reference Jha, Garcia-Garcia and Iseki1,Reference Kalantar-Zadeh, Jafar and Nitsch2) . Since 1990, the global incidence, prevalence, mortality, and disability-adjusted life-years of CKD have all increased dramatically, driven by population growth and ageing(Reference Xie, Bowe and Mokdad3). The estimated global prevalence of CKD is approximately 10–27 %(Reference Jha, Garcia-Garcia and Iseki1,Reference Petermann-Rocha, Balntzi and Gray4) , which causes 1·2 million deaths and 28 million years of life lost in 2017(Reference Xie, Bowe and Mokdad3,5) . The increased prevalence of CKD can further lead to an increased risk of kidney failure, CVD and mortality(Reference James, Hemmelgarn and Tonelli6–Reference Afkarian, Sachs and Kestenbaum8).
Now, sarcopenia is a public health concern with the physiological ageing process(Reference Cruz-Jentoft and Sayer9,Reference Bauer, Morley and Schols10) , and the reported estimated prevalence of sarcopenia among the Asia elderly ranges from 5·5 % to 25·7 %(Reference Kitamura, Seino and Abe11–Reference Chen, Liu and Woo13). Evidences from previous studies indicated that sarcopenia was associated with an increased risk of falls, frailty, mortality in general population and adverse clinical outcomes in patients with several diseases(Reference Zhang, Huang and Dou14–Reference Takenaka, Oya and Takemoto16). Accumulating evidence has assessed the relationships of sarcopenia with progression to end-stage renal disease, cardiovascular events and mortality in CKD patients(Reference Wilkinson, Miksza and Yates17–Reference Androga, Sharma and Amodu19). Several studies also reported that low handgrip strength and decreased skeletal muscle index were significantly correlated with CKD(Reference Lee, Jin and Lim20,Reference Yan, Zheng and Lin21) . To our knowledge, there have been no large longitudinal prospective studies evaluating the association between sarcopenia and kidney function decline or progression to CKD, especially in individuals with normal kidney function.
In clinical, the high-sensitive detection methods, such as dual-energy X-ray absorptiometry, computed tomography and MRI, were recommended to measure the skeletal muscle mass(Reference Ishii, Tanaka and Shibasaki22) but hard to achieve in daily clinical practice of large-scale population due to cost, time consumption and radiation exposure. Therefore, several consensus panels have developed simple screening algorithms to identify sarcopenia based on the easily obtainable variable(Reference Chen, Liu and Woo13,Reference Cruz-Jentoft, Baeyens and Bauer23) . In 2019, the Asian Working Group for Sarcopenia (AWGS) combined the muscle mass, muscle strength and physical performance to redefine the sarcopenia (including possible sarcopenia, sarcopenia and severe sarcopenia)(Reference Chen, Woo and Assantachai12). The AWGS first presented the concept of possible sarcopenia in order to early stratify and identify sarcopenia risk. Therefore, it is of great interest to assess the relationship between possible sarcopenia and kidney function.
In the present study, we aimed to conduct cross-sectional and longitudinal analyses to investigate the associations between sarcopenia and possible sarcopenia with CKD outcomes, using data from the China Health and Retirement Longitudinal Study (CHARLS).
Methods
Study population
The CHARLS is an ongoing nationally representative and population-based study that uses a multistage clustering sample method to select participants and conducted to collect a series of data regarding demographics, economic status, social networks, physical and psychological health in China(Reference Zhao, Hu and Smith24). The first visit was accomplished in 2011–2012 (Wave 1) of 17 708 patients, subsequently third follow-up visits carried out after that, each nearly 2 years apart among survivors (2013–2014: Wave 2, 2015–2016: Wave 3 and 2017–2018: Wave 4). The ethics application for collecting data on human subjects in CHARLS was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015), and all CHARLS participants provided written informed consent. The details of the CHARLS data are available at its website (http://charls.pku.edu.cn/en).
In the present study, we conducted a cross-sectional and a longitudinal analysis using data from the three waves of CHARLS for 2011, 2013 and 2015 (blood sample collection in 2011 and 2015). In the cross-sectional analysis, we included participants according to the following criteria: (1) individuals ≥ 45 years old and (2) individuals with complete information about sarcopenia and CKD in 2011. A total of 9357 participants were included (Fig. 1). In the longitudinal analysis, we further included participants according to the following criteria: (1) individuals with normal kidney function and without reported CKD in baseline, (2) individuals with complete information about CKD and eGFRcr-cys in 2015 and (3) individuals who were successfully followed up. Finally, a total of 5864 individuals with normal kidney function (eGFRcr-cys ≥ 60 ml/min per 1·73 m2) at baseline were eligible for subsequent analysis (Fig. 1).
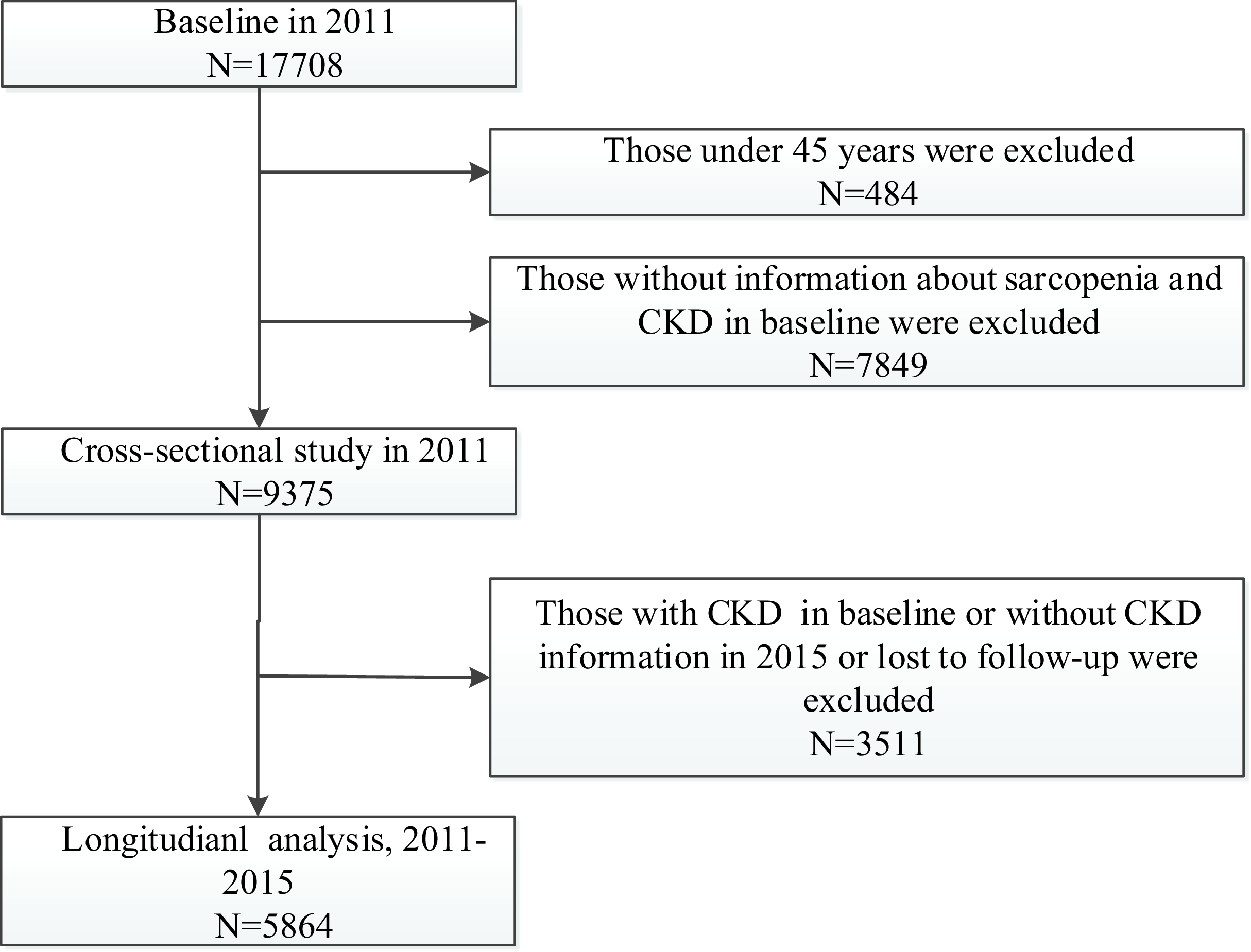
Fig. 1. Flow chart of sample selection and the exclusion criteria.
Assessment of sarcopenia status
The handgrip strength (kg) was measured in the dominant hand and non-dominant hand, with the participant squeezing a YuejianTM WL-1000 dynamometer (Nantong Yuejian Physical Measurement Instrument Co., Ltd.) as hard as possible. The cut-off points for low grip strength for men and women were < 28 and < 18 kg, respectively(Reference Wen, Wang and Jiang25). The muscle mass was estimated by the appendicular skeletal muscle mass (ASM) using a previously validated anthropometric equation in a Chinese population(Reference Wu, Li and Xu26), and the ASM/Ht2 values of < 5·63 kg/m2 in women and < 7·05 kg/m2 in men were considered as low muscle mass. For physical performance, the gait speed and the chair stand test were performed to evaluate individuals’ physical performance. The detail about sarcopenia evaluation and definition in CHARLS study has been described in the previous study(Reference Wu, Li and Xu26).
Sarcopenia status in current study was assessed according to the recommended diagnostic algorithm of AWGS 2019(Reference Chen, Woo and Assantachai12). Sarcopenia is diagnosed when low muscle mass and low muscle strength or low physical performance. When low muscle strength, low muscle mass and low physical performance are all detected, severe sarcopenia will be considered. Possible sarcopenia is defined by low muscle strength with or without reduced physical performance.
Assessment of study outcomes
The study outcome was the prevalence of CKD in the cross-sectional study. The CKD was assessed based on the self-reported physicians’ diagnosis ‘Have you ever been told by a doctor that you have kidney diseases?’ or personal eGFR level (eGFRcr-cys < 60 ml/min per 1·73 m2)). In the longitudinal analyses, the primary outcome was a combination of rapid decline in kidney function and progression to CKD. Secondary outcomes were separately those of rapid decline in kidney function and progression to CKD. Rapid kidney function decline defined as an annualised decline in eGFRcr-cys of 5 ml/min per 1·73 m2 or more(Reference Inker, Astor and Fox27). Annualised decline in eGFRcr-cys was estimated as (eGFRcr-cys at baseline (2011)- eGFRcr-cys at exit (2015))/follow-up time. Progression to CKD was defined as eGFRcr-cys < 60 ml/min per 1·73 m2 in 2015. The eGFRcr-cys was calculated using the latest CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equations by cystatin C and creatinine(Reference Inker, Schmid and Tighiouart28). Serum creatinine and cystatin C were measured using a rate-blanked and compensated Jaffe creatinine method and particle-enhanced turbidimetric assay, respectively.
Covariates assessments
The covariates were collected at baseline including age (continuous variable), sex, place of residence (rural v. urban), smoking status (ever smoking v. never smoking), educational level (illiteracy; primary school; middle school; high school or above), drinking status (ever drinking v. never drinking), the presence or absence of other chronic diseases (dyslipidaemia, diabetes mellitus, chronic lung disease and stroke) and medications (anti-hypertensive and anti-dyslipidaemic). ‘Ever smoking’ means that the respondent reported smoking at some point, and ‘never smoking’ means that the respondent reported never having smoked. ‘Ever drinking’ means that the respondent reports having had an alcoholic beverage in the past, and ‘never drinking’ means that the respondent reported not having any alcoholic beverage in the past. Blood pressure was measured with an electronic sphygmomanometer (Omron HEM-7200 Monitor) after 5 min of rest in the sitting position and was defined as the average of three separate measurements. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, current use of anti-hypertensive medications or self-reported history of hypertension. Moreover, dyslipidaemia was defined as TAG ≥ 150 mg/dl, or total cholesterol ≥ 240 mg/dl, or HDL-cholesterol < 40 mg/dl, or LDL-cholesterol ≥ 160 mg/dl, or current use of the lipid-lowering medications, or self-reported history of dyslipidaemia. And diabetes was defined as fasting glucose ≥ 126 mg/dl, or glycosylated HbA1c ≥ 6·5 %, or treatment for diabetes mellitus, or self-reported history of diabetes.
Statistical analysis
In the present study, only 323 (3·45 %) participants and 160 (2·73 %) participants had severe sarcopenia in cross-sectional and longitudinal analyses. Thus, we merged those with severe sarcopenia into sarcopenia group. Participants were divided into three subgroups according to sarcopenia status: no sarcopenia, possible sarcopenia and sarcopenia. Participants’ baseline characteristics are presented as percentages for categorical variables, as the means with standard deviation for normally distributed continuous variables and as medians with interquartile range for non-normally distributed variables. Demographic and clinical characteristics were compared by ANOVA or Kruskal–Wallis test for continuous variables and χ 2 test for categorical variables. Multivariable logistic regression models were applied to calculate the OR and 95 % CI between sarcopenia status and CKD outcomes. Potential covariates, such as eGFR, age, sex, place of residence, education level, smoking, drinking, systolic blood pressure, chronic diseases (dyslipidaemia, diabetes mellitus, chronic lung disease and stroke) and medications (anti-hypertensive, anti-dyslipidaemic and anti-diabetic) were included in the multivariable models.
In the longitudinal analysis, subgroup analyses were performed to evaluate the association between sarcopenia status and the risk of primary outcome according to sex, age, place of residence, hypertension, smoking, drinking, diabetes mellitus and BMI. Furthermore, sensitivity analyses were conducted to test the robustness of our findings. We first merged those with possible sarcopenia into sarcopenia group. Another sensitivity analysis was carried out by adjusting for age-squared and metabolic biomarkers in the participants who underwent metabolic examinations. In addition, we evaluated the associations of muscle mass, muscle strength and physical performance alone with the combination of rapid decline in kidney function and progression to CKD. Two tailed P < 0·05 was considered statistically significance. All statistical analyses were conducted using SAS statistical software (version 9.4).
Results
Characteristics of participants in the cross-sectional and longitudinal study
In the current study, a total of 9375 participants (4379 men and 4996 women) were included in the cross-sectional analysis, and the average age was 58·73 (sd 8·74) years. Among the 9375 participants, the prevalence of possible sarcopenia and sarcopenia was 23·04 % and 14·06 %, respectively. As shown in online Supplementary Table S1, baseline characteristics such as age, sex, living place, education level, history of dyslipidaemia and diabetes mellitus, smoking and drinking status, metabolic biomarkers levels, fasting blood glucose and diastolic blood pressure were significantly different among the three subgroups (online Supplementary Table S1).
In the longitudinal study of 5864 participants with eGFRcr-cys ≥ 60 ml/min per 1·73 m2, the average values of eGFRcr-cys were 105·89 (sd 13·63) ml/min per 1·73 m2 at baseline and 103·69 (sd 16·02) ml/min per 1·73 m2 at 2015, and the mean annualised decline in eGFRcr-cys was −0·8 (sd 12·58) ml/min per 1·73 m2. Table 1 shows the characteristics of the 5864 participants according to the sarcopenia status.
Table 1. Baseline characteristics of the study participants according to sarcopenia status in longitudinal analyses (n 5864) (Numbers and percentages; mean values and standard deviations)

TC, total cholesterol; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Continuous variables are expressed as mean ± standard deviation, or as median (interquartile range). Categorical variables are expressed as frequency (percent).
Cross-sectional associations of sarcopenia status with chronic kidney disease
In the cross-sectional study, the prevalence of CKD was 6·85 %, 8·38 % and 8·80 % in individuals with no sarcopenia, possible sarcopenia and sarcopenia, respectively. After adjusted for baseline eGFR at baseline, both possible sarcopenia and sarcopenia were significantly associated with an increased risk of CKD. When age, sex and other covariates were further adjusted in model 2, the associations between both possible sarcopenia, sarcopenia and CKD were still positive with the corresponding OR were 1·32 (95 % CI 1·08, 1·62) and 1·44 (95 % CI 1·11, 1·85), respectively (online Supplementary Table S2).
Longitudinal associations between sarcopenia status and chronic kidney disease outcomes
During the 4 years of follow-up, 359 (6·12 %) participants experienced rapid decline in kidney function and 126 (2·15 %) participants progressed to CKD. The associations between sarcopenia status and primary and secondary outcomes are shown in Table 2. After adjusted for baseline eGFR, age and other covariates in model 2, individuals with possible sarcopenia had an increased risk of the composite outcome of rapid decline in kidney function and progression to CKD, and rapid decline in kidney function alone, with the corresponding OR were 1·33 (95 % CI 1·01, 2·12) and 1·41 (95 % CI 1·06, 1·87), respectively. Consistently, individuals with diagnosed sarcopenia were associated with 49 % (OR 1·49, 95 % CI 1·05, 2·12), 64 % (OR 1·64, 95 % CI 1·13, 2·37) and 25 % (OR 1·25, 95 % CI 1·04, 2·25) increased risk of the composite outcome of kidney function and progression to CKD, rapid decline in kidney function alone and progression to CKD alone, respectively.
Table 2. Longitudinal association between sarcopenia status and CKD outcomes in longitudinal analyses (Numbers and percentages)

CKD, chronic kidney disease.
Model 1 was adjusted for eGFR at baseline.
Model 2 was further adjusted for age, sex, place of residence, education level, smoking, drinking, systolic blood pressure, chronic diseases (dyslipidaemia, diabetes mellitus, chronic lung disease and stroke) and medications (anti-hypertensive, anti-dyslipidaemic and anti-diabetic).
In addition, individuals with low muscle mass alone (OR 1·47, 95 % CI 1·08, 2·00), low muscle strength alone (OR 1·29, 95 % CI 1·05, 1·77) and low physical performance alone (OR 1·44, 95 % CI 1·08, 1·87) were associated with the composite outcome of rapid decline in kidney function. Those with low muscle mass alone (OR 1·57, 95 % CI 1·13, 2·17) and low muscle strength alone (OR 1·40, 95 % CI 1·01, 1·94) were associated with the rapid decline in kidney function. (Fig. 2).
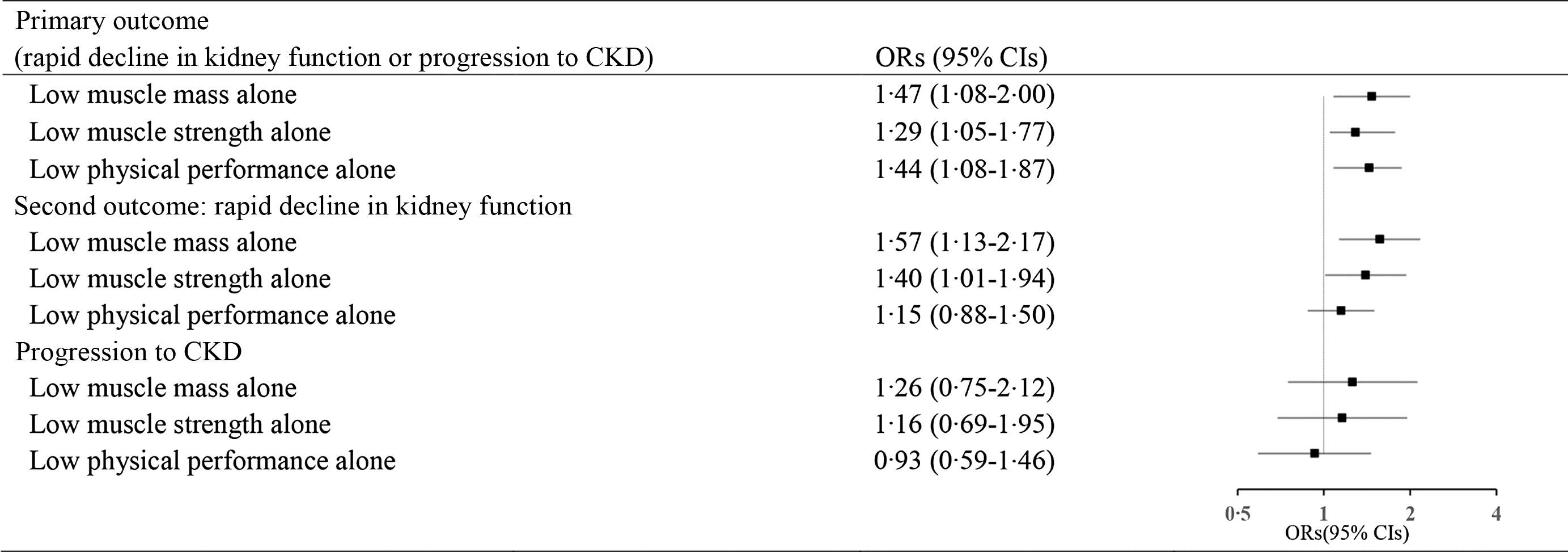
Fig. 2. Longitudinal association of low muscle mass alone, low muscle strength alone and low physical performance alone with CKD outcomes. (OR was adjusted for eGFR at baseline, age, sex, place of residence, education level, smoking, drinking, systolic blood pressure, chronic diseases (dyslipidaemia, diabetes mellitus, chronic lung disease and stroke) and medications (anti-hypertensive, anti-dyslipidaemic and anti-diabetic)).
In the subgroup analysis, the significant associations between sarcopenia with risk of primary outcome were observed in almost all subgroups (Table 3). There was a significant interaction between sarcopenia and hypertension, in relation to risk of primary outcome (p for interaction = 0·031). Sarcopenia was associated with an increased risk of primary outcome in participants with hypertension (OR 3·45, 95 % CI 1·68, 7·08) but not in those without hypertension (OR 1·13, 95 % CI 0·74, 1·71). In sensitivity analyses, we first merged individuals with possible sarcopenia into the sarcopenia group and found sarcopenia participants were more likely to experience the composite outcome of rapid decline in kidney function and progression to CKD, and rapid decline in kidney function than those without sarcopenia (online Supplementary Table S3). When we further adjusted for age-squared, possible sarcopenia and sarcopenia remained significantly associated with primary and secondary outcomes (online Supplementary Table S4). The longitudinal associations between possible sarcopenia, sarcopenia and study outcomes were not significantly changed after further adjusting for metabolic biomarkers (online Supplementary Table S5).
Table 3. Subgroup analysis of OR (95 % CI) of sarcopenia status and the primary outcome in longitudinal analyses (Odds ratios and 95 % confidence intervals)

In the multivariate models, confounding factors such as eGFR at baseline, age, sex, place of residence, education level, smoking, physical activity, drinking, systolic blood pressure, chronic diseases (dyslipidaemia, diabetes mellitus, chronic lung disease and stroke) and medications (anti-hypertensive, anti-dyslipidaemic and anti-diabetic) were included unless the variable was used as a subgroup variable.
Discussion
In this nationwide longitudinal prospective cohort study of Chinese adults aged 45 years and above, we first demonstrated that possible sarcopenia and sarcopenia, assessed using the AWGS 2019 algorithm, were independently associated with the prevalence of CKD in the cross-sectional analysis and were associated with rapid decline in kidney function and progression to CKD in individuals with normal kidney function. Subgroup and sensitivity analyses further confirmed these prospective findings. Furthermore, participants with low muscle mass or low muscle strength alone had an increased risk of rapid decline in kidney function and progression to CKD.
In 2017, sarcopenia was recognised by the International Classification of Diseases (ICD-10-MC) as a progressive muscle disease. Actually, the diagnostic criteria for sarcopenia have long been unclear and most clinicians are not very clear about the diagnosis and treatment of the sarcopenia, especially possible sarcopenia. The reported prevalence of sarcopenia ranges from 5·5 % to 25·7 % in Asian general population depending on the definition used(Reference Kitamura, Seino and Abe11–Reference Chen, Liu and Woo13). In an analysis of CHARLS, the prevalence of possible sarcopenia, sarcopenia and severe sarcopenia was 38·5 %, 18·6 % and 8·0 % among 6172 participants aged 60 years(Reference Wu, Li and Xu26). In our study of normal kidney function participants from CHARLS, the corresponding prevalence was still high (22·54 %, 9·57 % and 2·73 %, respectively). Considering the high prevalence of possible sarcopenia and sarcopenia, the insufficient diagnosis and treatment in clinical, as well as the poor prognosis of sarcopenia, our study provides evidences for future study in the prevention and control of sarcopenia.
Muscle loss is frequently occurred in CKD, and the prevalence of sarcopenia in CKD patients ranges from 4 to 49 %(Reference Inker, Astor and Fox27,Reference Sabatino, Cuppari and Stenvinkel29) . Accumulating evidence from cohort studies and meta-analysis reported that diagnosed sarcopenia was associated with an increased risk of hospitalisation, end-stage kidney disease progression and mortality in CKD patients(Reference Wilkinson, Miksza and Yates17,Reference Ribeiro, Neri and Oliveira30) . In an analysis of 428 320 participants from the UK Biobank, the prevalence of sarcopenia was twice in individuals with CKD than those without, and sarcopenia increases the risk of progression to end-stage renal disease in CKD patients(Reference Wilkinson, Miksza and Yates17). In a study of 360 Chinese diabetes patients, sarcopenia was independently associated with risk of albuminuria(Reference Yan, Zheng and Lin21). Consistent with the previous studies, our cross-sectional analysis found both possible sarcopenia and sarcopenia were associated with the prevalence of CKD. In addition, the present longitudinal prospective study indicated a significant association between sarcopenia and rapid decline in kidney function and progression to CKD in Chinese adults with normal kidney function.
To our knowledge, this is the first study to report that sarcopenia status, especially possible sarcopenia, assessed by the AWGS 2019 criteria, was associated with a rapid decline in kidney function and progression to CKD in Chinese adults with normal kidney function. Similarly, in another study from CHARLS, individuals with the diagnosis of possible sarcopenia were more likely to have new onset CVD than non-sarcopenia peers(Reference Gao, Cao and Ma31). Several previous studies also have separately evaluated the relationships between sarcopenia components (low muscle mass, low muscle strength or low physical performance) and CKD. Low et al. demonstrated that low baseline skeletal muscle mass and its reduction over time were associated with an increased risk of progression of CKD and albuminuria independently of conventional risk factors among type 2 diabetes participants(Reference Low, Pek and Moh32). Findings from the Korean National Health and Nutrition Examination Survey 2014–2017 study indicated that the prevalence of low handgrip strength was higher in patients with CKD, and handgrip strength was significantly positively correlated with eGFR in both men (R2 = 0·069) and women (R2 = 0·045)(Reference Lee, Jin and Lim20). Similarly, another study from the Korea National Health and Nutrition Examination Surveys 2008–2011 also found that there was a significant correlation between eGFR and ASM/Wt (appendicular skeletal muscle mass as a percentage of body weight) in general population(Reference Moon, Kim and Yoon33). As part of the current results, our findings also supported the evidence that three main components of sarcopenia (low muscle mass alone, low muscle strength alone and low physical performance alone) were associated with the composite outcome of rapid decline in kidney function and progression to CKD. Besides, those with low muscle mass alone and low muscle strength alone were related to higher risk of rapid decline in kidney function. The present study provides more valid appraisal of the relationships between possible sarcopenia and CKD outcomes, especially in those with normal kidney function.
In addition, there was significant interaction between sarcopenia and hypertension, and the stratified analyses showed stronger associations between sarcopenia and primary outcome in individuals with hypertension. Previous study had found sarcopenia-related parameters (muscle mass/function and physical performance) were independently and negatively related to hypertension(Reference Lee, Jung and Kim34,Reference Kara, Kara and Ceran35) . While hypertension was also with increased risk of sarcopenia. In patient with anti-hypertensive drugs use, anti-hypertensive drugs use seems to be related to higher muscle mass and gait speed values, and angiotensin converting enzyme inhibitors use is more likely to be related to higher muscle function only in female patients(Reference Kara, Kara and Ceran35). In light of these findings, it might be worth paying more attention to screen sarcopenia in hypertensive individuals.
The potential mechanisms of the relation between sarcopenia and CKD outcomes are multifactorial, but a number of hypotheses have been identified, including insulin resistance, endothelial dysfunction, inflammation, oxidative stress and activation of renin-angiotensin system(Reference Moon, Kim and Yoon33,Reference Kitada, Ogura and Monno36) . Skeletal muscle is the most important organ responsible for insulin-mediated human glucose processing. Low skeletal muscle mass is not only associated with insulin resistance diabetes and the metabolic syndrome but also causes glomerular endothelial cell dysfunction, glomerular hyperfiltration and renal tubular permeability(Reference Pryzbek, MacDonald and Stratford37,Reference Groop, Forsblom and Thomas38) . In clinical, several interventions like use of oral energy and protein supplementation combined with supervised physical resistance exercise may help to reverse sarcopenia, although findings have not always been consistent in available randomised controlled trials(Reference Sabatino, Cuppari and Stenvinkel29). Further studies are also warranted to explore more effective interventions.
The current study has several strengths. First, the study was based on the data from the CHARLS study, which is a large nationally representative cohort study with a high response rate. And potential confounders including metabolic biomarkers were collected and controlled in the multivariable models, which makes the result more credible. Second, unlike previous studies, this is the first to assess the potential relationships between possible sarcopenia, sarcopenia and rapid decline in kidney function and progression to CKD in individuals with normal kidney function. Third, the kidney function in the present study was based on eGFRcr-cys, which has higher reliability and validity. The AWGS 2019 definition of sarcopenia provides a simple and easy way to detect possible sarcopenia and sarcopenia. Our findings together with previous studies support the importance of screening for sarcopenia traits in general population and those with preexisting diseases. Thus, it is of clinical interest for clinicians to screen for possible sarcopenia and sarcopenia as an effective tool for early risk stratification.
Several potential limitations of present study need to be mentioned. First, the present study used observational data from CHARLS. Although, we had adjusted a series of confounders. This observational analysis could be influenced by potential biases and confounding factors. Second, the CHARLS study was exclusively a Chinese population aged 45 years and older. Thus, the findings from our study might not be generalisable to other populations or younger individuals. Third, some of the participants were excluded from analysis due to incomplete sarcopenia or outcome data. Finally, due to data about urine protein or urine albumin were not collected in the CHARLS, we could not evaluate the influence of sarcopenia on urine protein, which was closely associated with kidney function.
In conclusion, our findings demonstrated that both possible sarcopenia and sarcopenia, assessed by the AWGS 2019 criteria, were associated with an increased CKD risk and rapid decline in kidney function among Chinese adults. Our findings provide more valid evidence for screening and preventing possible sarcopenia and sarcopenia, which may be beneficial in reducing the incidence and disease burden of CKD.
Acknowledgements
This analysis uses data or information from the Harmonized CHARLS dataset and Codebook, Version C as of April 2018 developed by the Gateway to Global Aging Data. The development of the Harmonized CHARLS was funded by the National Institute on Ageing (R01 AG030153, RC2 AG036619, R03 AG043052). For more information, please refer to www.g2aging.org.
The sponsors had no role in the design, methods, data collection, analysis or preparation of the manuscript.
X. Z. and C. Z. conceived and designed the research; X. R., M. J., L. H. and X. Z. wrote the manuscript and L. H. and X. Z. performed the data analysis. All authors reviewed the manuscript.
The authors declare that they have no competing interests.
This analysis uses data or information from the Harmonized CHARLS dataset and Codebook, Version C as of April 2018 developed by the Gateway to Global Aging Data.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114523002313








