The metabolic syndrome (MetS) consists of several metabolic risk factors such as abdominal obesity, hypertension, insulin resistance, hyperglycaemia and dyslipidaemia( Reference Grundy, Hansen and Smith 1 ). Substantial evidence has indicated that the MetS increased the risk of vascular diseases, diabetes, stroke( Reference Farias, Pereira and Rosa 2 , Reference Dekker, Girman and Rhodes 3 ), atherosclerotic CVD( Reference Agarwal, Jacobs and Vaidya 4 , Reference Wang, Ruotsalainen and Moilanen 5 ) and so on. In recent years, because of its increasing prevalence and socio-economic burden, the MetS has received widespread attention( Reference O’Neill and O’Driscoll 6 ). A meta-analysis incorporating studies from 2005 to 2015 showed that the pooled prevalence of MetS was 24·5 (95 % CI 22·0, 26·9)% in Mainland China( Reference Li, Li and Lun 7 ). According to the latest national study, the prevalence of the MetS has reached 33·9 %, which indicates that the MetS affects approximately 454 million adults in China( Reference Lu, Wang and Li 8 ). Experimental and epidemiological studies have indicated that dietary factors could be important for determining the MetS( Reference Liu, Song and Ford 9 , Reference Esposito, Marfella and Ciotola 10 ).
Na and K are important electrolytes that are necessary for normal cellular function. Because of increasing risks of hypertension( Reference Zhang, Cogswell and Gillespie 11 , Reference Park, Kwock and Yang 12 ), CVD( Reference Cook, Obarzanek and Cutler 13 , Reference Yang, Liu and Kuklina 14 ), obesity( Reference Ge, Zhang and Chen 15 , Reference Jain, Minhajuddin and Neeland 16 ) and the MetS( Reference Ge, Guo and Chen 17 ), high Na and low K intake has become a serious public health challenge worldwide. These two electrolytes have an inverse relation and intake imbalance in the human body. Dietary K intake can attenuate salt sensitivity and the pressor impacts of salt( Reference Morris, Sebastian and Forman 18 ). Regarding the biological interaction of Na and K, some studies have suggested that Na:K ratio is more strongly associated with blood pressure, and that it can lead to a higher risk of CVD compared with Na or K intake alone( Reference Park, Kwock and Yang 12 , Reference Cook, Obarzanek and Cutler 13 , Reference Okayama, Okuda and Miura 19 ). Several studies have examined the relationship between Na or K intake alone and the MetS risk, but the results were inconsistent( Reference Ge, Guo and Chen 17 , Reference Hoffmann and Cubeddu 20 – Reference Shin, Joh and Kim 22 ). Unfortunately, none of the population-based studies has examined the relationship between dietary Na:K ratio and MetS risk. Therefore, it is important to explore the ratio of these two electrolytes instead of exploring Na or K intake alone in the diets.
Therefore, this study aims to examine whether high dietary Na:K ratio is an independent risk factor for the MetS, as well as its components.
Methods
Study population and sampling strategy
The data used in this study were derived from a part of the 5th Chinese national nutrition and health survey, which was conducted from 2010 to 2013. In this cross-sectional study, a stratified, multi-stage probability cluster sampling design was used to recruit participants who had lived in Nanjing city for at least 6 months. In the first stage, two rural sampling sites (Pukou and Lishui) and four urban sampling sites (Qinhuai, primary Baixia, Yuhua and Gulou) were randomly selected. In the second stage, six villages/communities were randomly selected in each site, using a probability-proportional-to-size sampling scheme. In each village/community, seventy-five households were further selected randomly. All the members in the households were invited to take part in the study. In addition, thirty households were interviewed for 3-d, 24-h dietary recalls, twenty-five households were interviewed for FFQ and twenty households were interviewed for instant food questionnaires. All family members aged 6 years and older were invited to contribute a fasting blood sample for measuring blood glucose, blood lipids and so on.
The survey was conducted between August and October in each year. Subjects younger than 18 years of age were excluded from this analysis. Initially, 5832 subjects (2680 men and 3152 women; 2782 households) were recruited, and 2272 adults (1077 men and 1195 women; 1062 households) completed the 3-d, 24-h dietary recalls. A total of 279 individuals did not have information on laboratory examination; finally, 1993 adults were included. Written consents were obtained from all participants. This study was approved by the Medical Ethics Committee of the Chinese Centre of Disease Control and Prevention.
Demographic and socio-economic factors
The demographic factors and health behaviours of participants were collected by trained interviewers through face-to-face interviews, including age, sex and highest educational level achieved (primary school or below, middle or junior school and high school or more), and per-capita annual household income was categorised into low (<20 000 Yuan/person), moderate (20 000–34 999Yuan/person) and high (≥35 000 Yuan/person). The health behavioural variables included current smoking (never smoker or current smoker for the past 1 month), alcohol consumption (g alcohol/d) and physical activity. The types, frequency and amount of alcohol/wine consumption were also collected and then converted into the amount of pure alcohol (in g) consumed per day. Current drinking was defined as consuming more than 0 g of alcohol daily for the past 1 month. Physical activity was quantified as a metabolic equivalent of task minutes per week (MET-min/week), and then classified as low (<600 MET-min/week), moderate (600–2999 MET-min/week) or high (≥3000 MET-min/week).
Assessment of dietary intake
Dietary intake was assessed by 3 consecutive days of dietary recollection combined with condiments weighing method, including 2 weekdays and 1 weekend day. Participants were asked to recall all food content and quantities they had consumed during the past 24 h. The foods included staple foods, vegetables, fruits, snacks and so on. Measuring tools such as food models, food volumes and containers were used to enhance recall and conduct to the estimation of an average portion size. In addition, home condiment consumption (including cooking salt, monosodium glutamate, soya sauce, vinegar, sauces and other salt-containing condiments) and cooking oil in each household were recorded using a household food inventory weighing method on the same 3 d. According to the ratio of individual energy intake:the energy intake of all family members, the percentage of the condiments and oil of each family member was calculated. Further, daily condiments consumption was calculated for each family member. Finally, the daily food consumption data of the participants were obtained by a combination of 3 consecutive days of dietary recollection with condiments weighing method. On the basis of these food consumption data, the nutrient contents of the diet, including total energy, Na and K, were analysed and calculated using the Chinese Food Composition Table (2nd ed.)( Reference Yang, Wang and Pan 23 ).
Anthropometric measures
At each study site, body weight and height were obtained for each adult participant by trained staff using standard protocols to the nearest 0·1 kg and 0·1 cm, respectively. BMI was computed as weight (kg) divided by height square (m2). Waist circumference (WC) was measured at the narrowest point between the inferior margin of the last rib and the iliac crest in a horizontal plane. After 5 min of rest in the sitting posture, blood pressure was measured twice with a mercury sphygmomanometer (Shanghai ShangDa Medical Instrument Co. Ltd) on the right upper arm of the subject and then averaged.
Laboratory evaluation
Antecubital vein blood samples after fasting for 10–12 h were collected into a 2-ml vacuum, lithium heparin anticoagulant tube and a 4-ml vacuum tube, respectively, and immediately centrifuged at 1500 g for 10–15 min. These blood samples were used to evaluate glucose levels (mmol/l), total cholesterol (mmol/l), TAG (mmol/l) and HDL-cholesterol (mmol/l). After a fasting venous blood sample was collected, each participant received a 75-g oral glucose tolerance test, except for those with a known diagnosis of diabetes.
The blood samples were analysed in the Medical Center for Physical Examination, Nanjing Centre of Disease Control and Prevention for fasting blood glucose, serum cholesterol, TAG, HDL- and LDL-cholesterol, using the Hitachi 7180 auto-analyzer (Hitachi).
Definition of the metabolic syndrome
According to the American Heart Association and the National Heart, Lung and Blood Institute scientific statement( Reference Grundy, Cleeman and Daniels 24 ), the MetS was defined as the presence of three or more of the following criteria: (1) central obesity, WC ≥90 cm in men and WC ≥80 cm in women, and this criterion of WC was appropriate for Asians( Reference Gu, Reynolds and Wu 25 ); (2) hyperglycaemia, fasting glucose level ≥5·56 mmol/l (100 mg/dl) or on drug treatment with elevated blood glucose; (3) low HDL-cholesterol, HDL-cholesterol<1·03 mmol/l (40 mg/dl) in men and HDL-cholesterol <1·3 mmol/l (50 mg/dl) in women or on drug treatment for reduced HDL-cholesterol; (4) hypertriacylglycerolaemia, TAG level ≥1·7 mmol/l (150 mg/dl) or on drug treatment for this lipid abnormality; and (5) high blood pressure, systolic blood pressure/diastolic blood pressure ≥130/85 mmHg or on antihypertensive drug treatment for a history of hypertension.
Statistical analysis
The subjects of this study were divided into quartiles based on their Na:K ratio levels, and then the means of the MetS and its related components prevalence of the quartiles were compared. Dietary variables were adjusted for total energy intake by the residual method. Energy-adjusted Na:K ratio values were divided into quartiles, with the lowest (quartile 1) as the reference group. Quartiles of Na:K ratio 1 to 4 were ≤1·98, 1·99–2·95, 2·96–4·32 and ≥4·33. Linear regression analysis was used to test for trend across dietary Na:K ratio quartiles. Differences among groups were tested by one-way ANOVA, and post hoc comparisons were performed using Bonferroni correction. Comparisons between categorical variables were performed with the χ 2 test.
For further analysis, the association between dietary Na:K ratio and the risk of the MetS and its components was assessed using multivariate-adjusted logistic regression models. OR and 95 % CI were calculated. Three models were introduced. Model 1 was adjusted for age (continuous), sex, the ratio of TAG:HDL-cholesterol (not included in the MetS components analysis), BMI (continuous) and area of residence. Model 2 was further adjusted for education, physical activity, drinking and smoking status and the use of anti-diabetic, hypolipidaemic and antihypertension medications. Dietary factors such as total energy, carbohydrate and total fat were additionally adjusted for continuous variables in model 3. Multiplicative interactions were tested by adding the interaction terms (sex×quartiles of dietary Na:K ratio) using likelihood ratio test.
Statistical significance was considered when P<0·05 (two-sided). All analyses were performed using the Statistical Package for Social Sciences software version 17.0 (SPSS Inc.).
Results
General characteristics of the study population
Among the 1993 enrolled individuals, 907 were men and 1086 were women. There were 728 participants diagnosed with Mets and the prevalence rate was 36·5 %. Participants with the MetS had significantly higher dietary Na:K ratio than those without MetS (energy-adjusted means, 3·7 (sd 2·6) v. 3·3 (sd 2·1), respectively, P<0·01). The general characteristics of subjects by quartiles and the potential confounding variables are shown in Table 1. Compared with the lowest quartile, participants in the highest quartile of dietary Na:K ratio consumed more Na, more fat and they had higher BMI level (all P for trend <0·05); they were also more likely to be daily smokers and farmers.
Table 1 Summary statistics of the study population divided into quartiles of the sodium:potassium ratio (Mean values and standard deviations and percentages)
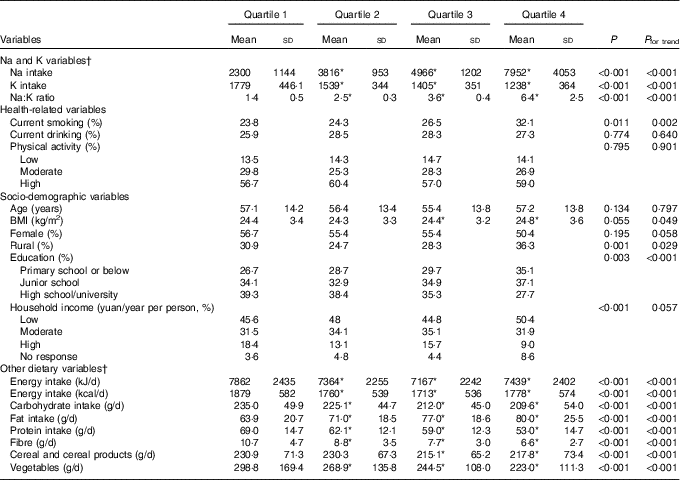
* P<0·05 compared with quartile 1.
† Dietary variables were total energy-adjusted values by residual method.
Association of the sodium:potassium ratio with the metabolic syndrome and its components
The prevalence rates of the MetS components were 48·4 % for central obesity, 56·2 % for high blood pressure, 32·6 % for hypertriacylglycerolaemia, 22·2 % for low HDL-cholesterol and 41·6 % for hyperglycaemia. The prevalence rates of the MetS and its related components in relation to quartiles are shown in Fig. 1. The prevalence rates of the MetS, hyperglycaemia and hypertriacylglycerolaemia tended to increase with the dietary Na:K ratio quartiles (all P for trend <0·05). However, there were no obvious changes in high blood pressure, central obesity and low HDL-cholesterol. In addition, a direct relationship was shown between the mean dietary Na:K ratio and the number of MetS components. In Fig. 2, the mean dietary Na:K ratio significantly increased with the number of MetS components after adjusting for age and sex (P for trend <0·001).
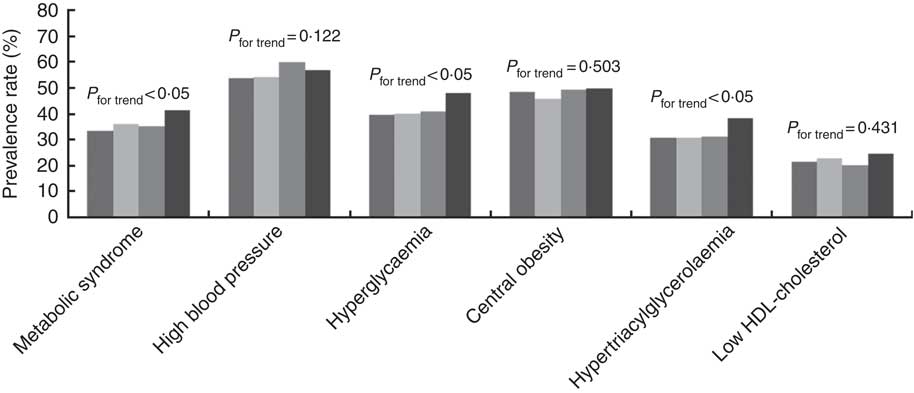
Fig. 1 Prevalence rates of the metabolic syndrome and its components in different dietary sodium:potassium ratio quartiles. ![]() , Quartile 1;
, Quartile 1; ![]() , quartile 2;
, quartile 2; ![]() , quartile 3;
, quartile 3; ![]() , quartile 4.
, quartile 4.
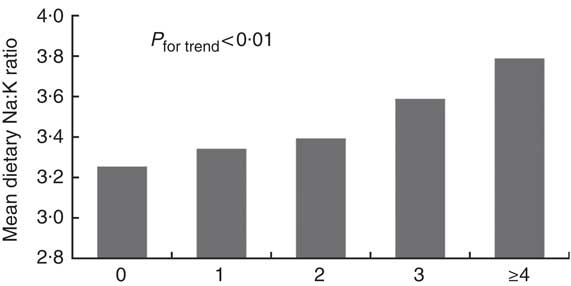
Fig. 2 Mean of dietary sodium:potassium ratio in the different number of the metabolic syndrome components (![]() ) after adjusting for age and sex (0, n 316; 1, n 449; 2, n 499; 3, n 437; ≥4, n 292).
) after adjusting for age and sex (0, n 316; 1, n 449; 2, n 499; 3, n 437; ≥4, n 292).
Dietary Na:K ratio was independently and positively associated with the risk of prevalent MetS (Table 2). Compared with subjects in the lowest quartile of dietary Na:K ratio, multivariate-adjusted OR for the MetS in quartile 2, quartile 3 and quartile 4 were 1·495 (95 % CI 1·038, 2·151), 1·347 (95 % CI 0·927, 1·957) and 1·602 (95 % CI 1·090, 2·353), respectively, and a statistically significant dose–response relationship between dietary Na:K ratio and the odds of the MetS was documented (P for trend=0·040). Each 1-sd increase in dietary Na:K ratio was still associated with a higher risk of prevalent MetS (OR=1·166; 95 % CI 1·018, 1·336). Furthermore, dietary Na:K ratio was positively associated with individual components of the MetS, especially high blood pressure, hyperglycaemia and hypertriacylglycerolaemia. Higher dietary Na:K ratio was associated with an increased risk of high blood pressure after adjusting for various factors in model 3 (quartile 3: OR=1·656; 95 % CI 1·228, 2·256), compared with the lowest quartile. The positive association between hyperglycaemia and dietary Na:K ratio was attenuated after adjusting for dietary factors. However, the positive association between hypertriacylglycerolaemia and dietary Na:K ratio was enhanced after adjusting for dietary factors. No significant association was found between dietary Na:K ratio and central obesity or low HDL-cholesterol.
Table 2 Multivariate OR for the metabolic syndrome and its components according to quartiles of dietary sodium:potassium ratio ratio (Odds ratios and 95 % confidence intervals)
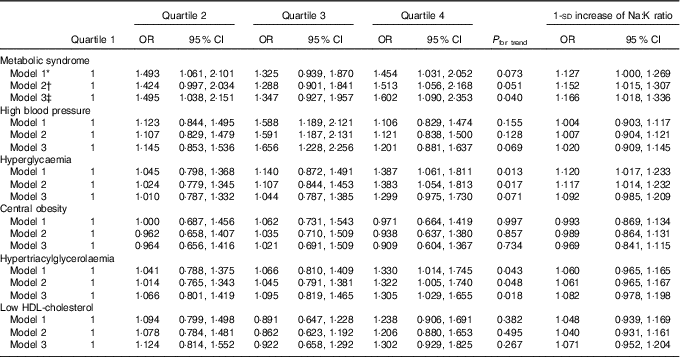
* Model 1 was adjusted for age (continuous), sex, the ratio of TAG:HDL-cholesterol, BMI (continuous) and area of residence.
† Model 2 was further adjusted for education, physical activity, drinking and smoking status and the use of anti-diabetic, hypolipidaemic and antihypertension medications.
‡ Model 3 was adjusted for total energy, carbohydrate and total fat intakes as continuous variables in addition to model 2.
The effects of the interaction of dietary Na:K ratio with sex on the association with the MetS and its components were not statistically significant (all P for interaction>0·1).
Discussion
To our knowledge, this is the first epidemiological study on the association between dietary Na:K ratio and the risk of the MetS among Chinese adults. We found a significant dose–response relationship between dietary Na:K ratio and the MetS, even after adjusting for possible confounding factors. Our study also identified that dietary Na:K ratio significantly increased with the number of MetS components after adjusting for age and sex. It indicated that dietary Na:K ratio was an independent risk factor of the MetS, and it offered a potentially modifiable strategy of the reduction of Na intake and increase of K intake to prevent the MetS.
Na and K are important electrolytes required by the body, and some studies have revealed the positive link between high Na or low K intake and metabolic disorders. Numerous mechanisms have been hypothesised to explain why high Na intake contributes to metabolic disorders, with special emphasis on inadequate aldosterone suppression and increased mineralocorticoid receptor activation by factors other than aldosterone( Reference Rossi, Belfiore and Bernini 26 , Reference Shibata 27 ). K has been shown to play a role as an endothelium-derived hyperpolarising factor( Reference Edwards, Dora and Gardener 28 ) and stipulate nitric oxide to maintain endothelial function( Reference Yang, Li and Weng 29 ). The impact of low K on metabolic disorders is mediated partly by the decrease of nitric oxide level and deterioration of endothelial function( Reference Reungjui, Pratipanawatr and Johnson 30 ), which may cause hyperglycaemia and insulin resistance( Reference Reungjui, Roncal and Mu 31 ).
Previous studies have examined the association of Na or K intake alone with the MetS, although the results had been controversial( Reference Ge, Guo and Chen 17 , Reference Hoffmann and Cubeddu 20 – Reference Shin, Joh and Kim 22 , Reference Cai, Li and Fan 32 ). Rodrigues et al. ( Reference Rodrigues, Baldo and de Sá Cunha 21 ) found that no differences were observed in urinary Na excretion in normotensive individuals, regardless of the presence of MetS. Ge et al. ( Reference Ge, Guo and Chen 17 ) showed that urinary K excretion was not significantly associated with the risk of the MetS; however, high Na intake might be an independent risk factor for the MetS. Cai et al. ( Reference Cai, Li and Fan 32 ) demonstrated a protective effect of adequate K intake on the MetS and obesity. This dispute was possibly explained by the fact that high K intake can attenuate salt sensitivity and the pressor impacts of salt( Reference Morris, Sebastian and Forman 18 ).
Some studies have suggested that the Na:K ratio was more closely related to some chronic diseases than one mineral( Reference Cook, Obarzanek and Cutler 13 , Reference Okayama, Okuda and Miura 19 ). We considered that the Na: K ratio may be a sensitive indicator for assessing the risk of MetS, and the result requires more investigation. Therefore, we designed the study to observe the association between dietary Na:K ratio and the risk of the MetS.
For the MetS components, our study showed a positive association between dietary Na:K ratio and the risk of high blood pressure, which was consistent with previous studies( Reference Zhang, Cogswell and Gillespie 11 , Reference Park, Kwock and Yang 12 , Reference Du, Batis and Wang 33 ). It has been reported that positive correlations between dietary Na:K ratio, the level of serum cholesterol and LDL-cholesterol and high Na:K ratio may have a negative impact on lipid metabolism( Reference Bu, Kang and Kim 34 ). According to our results, individuals with higher Na:K ratio were more likely to have hypertriacylglycerolaemia, even after adjusting for multiple risk factors. Interestingly, this positive association was enhanced after further adjusting for dietary factors (model 3). However, no significant association was observed between dietary Na:K ratio and the risk of low HDL-cholesterol. This controversy could be because of the fact that lipid levels are affected by other nutrients, such as fats, proteins and carbohydrates( Reference Bu, Kang and Kim 34 ). Moreover, both Na and K intakes were closely tied to total energy intake( Reference Cobb, Anderson and Elliott 35 ). Our dietary data were adjusted for total energy intake by the residual method. However, dietary data were not adjusted for total energy intake in this study( Reference Bu, Kang and Kim 34 ).
To date, no studies have been conducted to examine the association between dietary Na:K ratio and blood glucose. Our study found the positive association between dietary Na:K ratio and hyperglycaemia, but the positive association was attenuated after further adjusting for dietary factors. This may be related to the effects of diet macronutrient composition on fasting glucose( Reference Gower, Goree and Chandlerlaney 36 ).
In conclusion, our study found an independent and dose–response association between dietary Na:K ratio and MetS among Chinese adults in Nanjing, Jiangsu Province. These novel findings suggest the important role of reducing Na and increasing K intakes in diet and open new avenues for potential interventional targets to decrease the risk of MetS.
Strengths and limitations
To our knowledge, this may be the first epidemiology study that has focused on the association between dietary Na:K ratio and the MetS. As a cross-sectional study of a relatively large sample, this study used the complex sampling method to explore the relationship of dietary Na:K ratio and the MetS, adjusting for various potential confounders, such as age, sex, smoking, drinking, physical activity, BMI, the ratio of TAG:HDL-cholesterol, drug use and dietary factors in the multiple regression model; the overall results are relatively reliable.
The study has several limitations. First, the cross-sectional nature of our study makes it difficult to determine any causal relationship. In addition, 3 consecutive days of dietary recollection combined with condiments weighing method to assess the accuracy of the dietary Na and K intakes was not as good as the multiple 24-h urine collection method. Multiple 24-h urine collection method not only requires more funding but also adds a financial burden for the study participants. Furthermore, because of the high daily variability in Na and K excretion, when taking the effects of total energy into account, there were lower random errors in the method that we used to evaluate the Na and K intake, compared with spot or overnight urines, a single 24-h dietary recall or urine collection( Reference Cobb, Anderson and Elliott 35 ).
Acknowledgements
The authors acknowledge the contributions of the participants and investigators to the present study, from local Centers of Disease Control and Prevention, including Nanjing, Gulou, Qinhuai, primary Baixia, Yuhua, Pukou and Lishui, for their support in the survey implementation. The authors specially thank Prof. Fei Xu, Nanjing Municipal Center for Disease Control and Prevention, for his kind help in the article revision.
This study was financed by Nanjing Municipal Science and Technology Development Foundation (grant no. 201108006).
G. X. designed the study and was responsible for the review of the overall study. X. L. was in charge of the design of the study concept, data collection, interpretation, analysis and the first manuscript writing. B. G. was in charge of the study coordination, quality control and manuscript revision. D. J. was in charge of the study coordination, quality control and data analysis. Y. W., Y. J., B. Z., L. M., H. Z. and Y. C. conducted the research and analysed data.
The authors declare that there are no conflicts of interest.







