Ageing, a physiological state of natural development, is featured by physical and cognitive decline(Reference Pais, Ruano and Moreira1,2) . Once cognitive impairment occurs, dementia and mortality rates increase in the elderly(Reference Park, Kwon and Jung3), the typical symptoms are difficulties with memory, language, problem-solving and other thinking skills, which affect a person’s ability to perform daily activities(4). In the USA, more than 16 million family members and other unpaid caregivers provided approximately 18·6 billion hours of care for patients with low cognitive performance in 2019, and the total cost was estimated to be $305 billion in 2020(5). Before breakthroughs are made in prevention or treatment, low cognitive performance will pose an increasing challenge to the health care system around the world(Reference Nichols, Szoeke and Vollset6). Therefore, it is necessary and important to detect the preclinical manifestations of low cognition as early as possible and to conduct the intervention.
Trace elements are combined with biological macromolecules such as proteins and nucleic acids to participate in regulating the function of the nervous system(Reference Belaidi and Bush7–Reference Duce and Bush9). Homoeostasis imbalance of trace elements in the brain may cause cognitive impairment(Reference Duce and Bush9). Previous studies have suggested that Fe, Mg and Zn may help to maintain normal cognitive function and enhanced happiness by reducing mental and physical fatigue and positive emotions(Reference Belaidi and Bush7,Reference Horning, Caito and Tipps8,Reference Tardy, Pouteau and Marquez10) . Several studies showed that the level of Fe and Se in the elderly decreased with age, and Se deficiency may cause cognitive decline in the elderly(Reference Ferry and Roussel11–Reference Lonnerdal13). Moreover, Pb, Cd and Hg exposure were risks for cognitive dysfunction because they had long-term effects on the brain(Reference Karri, Schuhmacher and Kumar14,Reference Meramat, Rajab and Shahar15) .
This study was to assess the association between six trace elements (Fe, Pb, Cd, Hg, Se and Mn) in blood and low cognitive performance in the elderly over 60 years old, using the data from the National Health and Nutrition Examination Survey (NHANES) database. Subgroup analyses were conducted based on hypertension and diabetes to evaluate the association in different subpopulations.
Methods
Study population
Data were extracted from 2011–2012 and 2013–2014 NHANES, a representative cross-sectional survey of all non-institutionalised civilian population in the USA. NHANES is a major project of the National Center for Health Statistics (NCHS), a part of the Centers for Disease Control and Prevention (CDC) and is responsible for compiling life and health statistics. Participants completed surveys about demographics, health history, and diet, and they submitted blood and urine samples during physical examinations. Cognitive assessment was conducted in a household interview or at a Mobile Examination Center (MEC) using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Learning Test, the CERAD Word List Recall Test, the Animal Fluency test and the Digit Symbol Substitution Test (DSST)(17,18) . The NHANES protocols were approved by the NCHS Ethics Review Board of the US CDC. All individuals provided written informed consent during the survey. More information about the NHANES database can be obtained at: http://www.cdc.gov/nhanes.
Cognitive performance
The CERAD Word Learning subtest (CERAD W-L) assessed immediate and delayed learning of new language information (memory subdomain) (Reference Morris, Mohs and Rogers19). The CERAD test consisted of three consecutive learning trials and one delayed recall. In each learning tests, participants read ten unrelated words aloud at a time. Then the words from the learning trial were shown on a computer monitor in a random order, and the participants were asked to recall as many of them as possible. Each test was scored between 0 and 10 points, with one point for each correct word. The total score of the three tests and one delayed recall was the CERAD total score.
The Animal Fluency test assessed categorical verbal fluency. Participants were asked to name as many animals as possible in 1 min, and one point was scored for each animal correctly named.
DSST was a performance module in the Wechsler Adult Intelligence Scale (WAIS III), which was used to test sustained attention and working memory(Reference Wechsler20). Before the participants started the main test, they performed an exercise using a paper with a key at the top pairing numbers with nine symbols. Participants were asked to copy the corresponding symbols from the 133 boxes with numbers within 120 s. In NHANES, participants who were unable to correctly copy the symbols and numbers in the pretest practice did not continue. In the specified time, the score is the total number of correct matches, and the highest score is 133 points.
The low cognitive performance was assessed by the cut-off points of the 25th percentile of the CERAD, Animal Fluency and DSST tests, which was consistent with methods used in the published studies(Reference Dong, Li and Chen21,Reference Chen, Bhattacharya and Pershing22) . Participants scoring below the lowest quartile were defined as having low cognitive performance in each cognitive test. The threshold scores of the CERAD, Animal Fluency and DSST tests were 5, 14 and 41 points, respectively.
Serum iron, blood lead, cadmium, mercury, selenium and manganese exposure assessment
A phlebotomist at the MEC collected blood from participants, and the blood was processed and divided into vials. They were then refrigerated or frozen for storage and transported to laboratories across the USA, where most of the tests were completed by remote laboratories. The determination method of serum Fe concentration was a timed-endpoint method, which was determined by DcX800 method. The change in absorbance of the reaction system was monitored at 560 nm at a fixed-time interval, and the change in absorbance was directly proportional to the concentration of Fe in the sample. The concentrations of blood Pb, Cd, Hg, Se and Mn were determined by quadrupole inductively coupled plasma-MS (ICP-MS) technology. For details on the specific methods of blood metal detection and measurement, see NHANES laboratory manual (http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/PbCd_G_met_blood%20metals.pdf).
Potential covariates
Demographic information and history of diseases of participants were extracted from the database. Sex, age and race were self-reported demographic information. Education was assessed by the question ‘What is the highest grade or level of school you have completed or the highest degree you have received?’ (less than 9th grade/9–11th grade (includes 12th grade with no diploma)/high school graduate or general educational development (GED) or equivalent/some college or AA degree (associate of arts)/college graduate or above). Diabetes and hypertension history were examined by self-reported questionnaire. The BMI was calculated by dividing the weight of the participant by the square of the height (kg/m2). Smoking history was assessed by the question ‘Have you smoked at least 100 cigarettes in your entire life?’ (yes/no). The income-poverty ratio (IPR) was a measure of household income divided by the poverty guidelines to calculate the ratio of family income to poverty based on family size, year and state. Alcohol information (yes/no) and marital status (yes/no) were collected through personal interviews.
Statistical analysis
All statistical analyses were performed by SAS software (version 9.4, SAS Institute). Using the proc surveyfreq in SAS software, the final sample size was weighted with WTMEC2YR, SDMVPSU and SDMVSTRA. WTMEC2YR is the MEC exam weight (wtmec2yr) used for weighting. SDMVPSU means that the masked variance unit pseudo-substrate is sdmvstra, and the masked variance unit pseudo-primary sampling unit (PSU) is sdmvpsu. SDMVSTRA refers to the CI being applied to assess the reliability of an estimate. Quantitative data were tested for normality using Kolmogorov–Smirnov. Abnormal distribution data were presented by median and quartile (M (Q1, Q3)), and comparisons between the two groups were performed using Mann–Whitney U test. Qualitative data were expressed as the number and proportion (n (%)), and the χ 2 test was used for comparison between the two groups.
All participants were divided into the low cognitive performance and normal cognitive performance groups according to the cut-off points of the 25th percentile of the CERAD, Animal Fluency and DSST tests. Covariates were screened by the difference analysis between the two groups. Weighted univariable and multivariate logistic regression analyses were conducted to evaluate the associations between six trace elements and the risk of low cognitive performance. Model 1 was a crude model without adjusting covariates. Model 2 was first adjusted for demographic information (sex, age, race and marital status) and BMI. Model 3 was the fully adjusted model accounted for these variables as well as IPR, alcohol drinking, diabetes and hypertension history, and smoking status. Weighted random interpolation was performed for missing data. Sensitivity analysis was used to assess the results before and after interpolation. The associations were further explored in individuals with history of diabetes and hypertension. Two-sided P < 0·05 was considered as statistically significant.
Results
Description of the study population
A total of 19 931 participants were included from NHANES 2011–2012 and 2013–2014. Participants without cognitive function score (n 16 995) and laboratory values of trace elements (n 934) were excluded. The final inclusion of 2002 individuals was weighted to represent 37,242,171 individuals over the age of 60 years. The flow chart of population screening was shown in Fig. 1.

Fig. 1. The flow chart of population screening. NHANES, National Health and Nutritional Examination Survey.
Characteristics of old adults with low and normal cognitive performance
The characteristics of participants with low and normal cognitive performance according to the CERAD, Animal Fluency and DSST assessments were shown in Tables 1–3. For the CERAD test in Table 1, compared with old adults with normal cognitive performance, the median levels of blood Pb (1·45 ug/dl v. 1·38 ug/dl), Cd (0·38 ug/dl v. 0·35 ug/dl) and Se (193·03 ug/dl v. 192·50 ug/dl) were high, while the median levels of serum Fe (81·00 ug/dl v. 86·00 ug/dl), blood Hg (0·86 ug/dl v. 1·00 ug/dl) and Mn (8·64 ug/dl v. 8·80 ug/dl) were low in participants with low cognitive performance. Differences were found in age, sex, education level, BMI, IPR, diabetes history and hypertension history between the two groups (P < 0·05). For the Animal Fluency test in Table 2, there were statistical differences in age, race, education level, marital status, BMI, IPR, diabetes history and hypertension history (P < 0·05). The median levels of blood Pb (1·52 ug/dl v. 1·37 ug/dl) and Cd (0·42 ug/dl v. 0·34 ug/dl) were higher, while the median levels of serum Fe (76·00 ug/dl v. 86·00 ug/dl), blood Hg (0·73 ug/dl v. 1·03 ug/dl), Se (186·19 ug/dl v. 195·84 ug/dl) and Mn (8·73 ug/dl v. 8·79 ug/dl) were lower in the elderly with low cognitive performance than these in the elderly with normal cognitive performance. For the DSST test (Table 3), the results showed the differences in age, race, education level, BMI, IPR, alcohol drinking, diabetes history and hypertension history (P < 0·05). The median blood Cd (0·40 ug/dl v. 0·35 ug/dl) and Mn (8·80 ug/dl v. 8·78 ug/dl) levels were high, while the median blood Pb (1·40 ug/dl v. 1·41 ug/dl), serum Fe (78·00 ug/dl v. 87·00 ug/dl), blood Hg (0·76 ug/dl v. 0·99 ug/dl) and Se (190·33 ug/dl v. 193·94 ug/dl) levels were low in the elderly with low cognitive performance in comparison with these in the elderly with normal cognitive performance.
Table 1. Characteristics of elderly over 60 years of age by cognitive performance status according to CERAD test

CERAD, consortium to establish a registry for Alzheimer’s disease; GED, general educational development; IPR: income-poverty ratio.
Table 2. Characteristics of elderly over 60 years of age by cognitive performance status according to Animal Fluency test
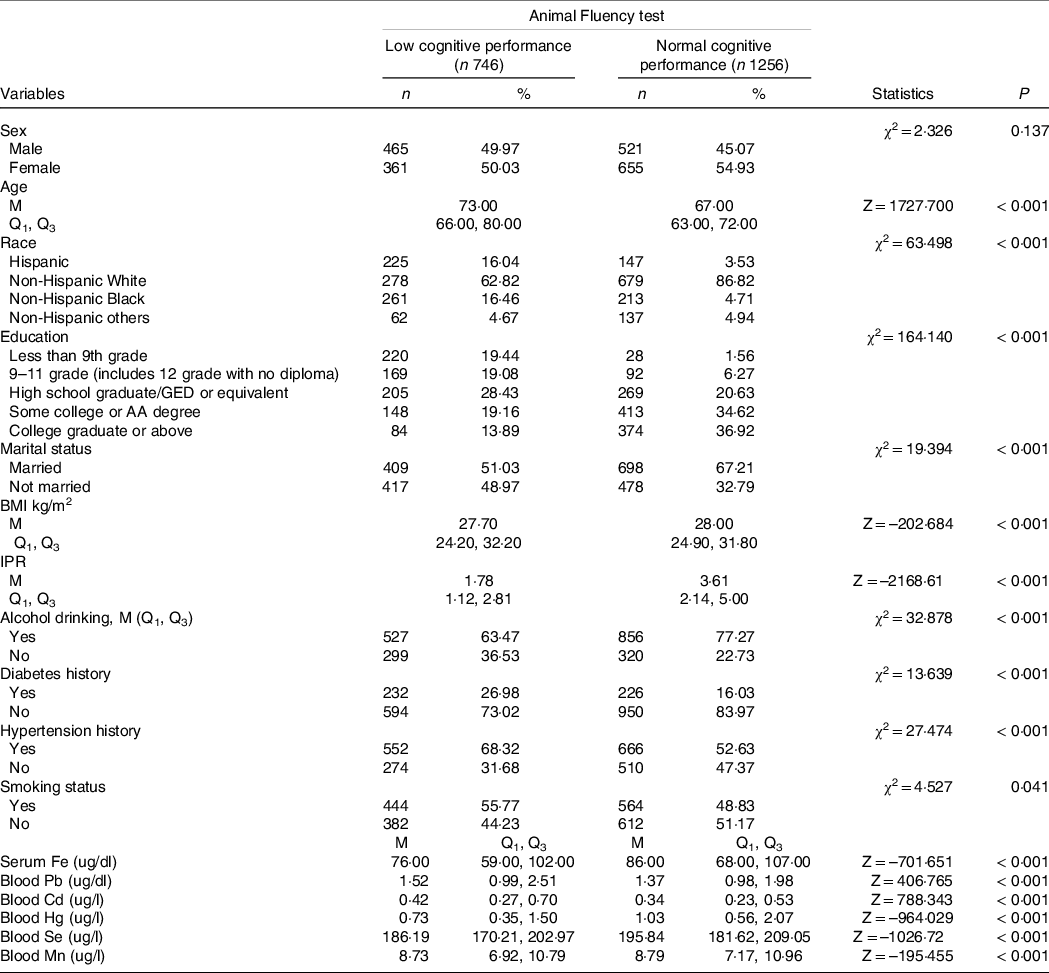
GED, general educational development; IPR: income-poverty ratio.
Table 3. Characteristics of elderly over 60 years of age by cognitive performance status according to DSST

DSST, Digit Symbol Substitution Test; GED, general educational development; IPR: income-poverty ratio.
Association between six trace elements and low cognitive performance
The associations between six trace elements and cognitive performance were shown in Fig. 2. After adjusting age, sex, race, BMI, IPR, alcohol drinking, diabetes and hypertension history, elevated serum Fe levels were associated with the decreased risk of low cognitive performance (OR = 0·995, 95 % CI 0·990, 0·999) in the Animal Fluency test. High blood Pb levels were related to the high odds of low cognitive performance (OR = 1·102, 95 % CI 1·019, 1·192), while high blood Se levels were linked to the decreased risk of low cognitive performance (OR = 0·987, 95 % CI 0·981, 0·993) in the DSST test among old adults. In addition, the results before interpolation showed that elevated blood Se levels were associated with the decreased risk of low cognitive performance in the DSST test (OR = 0·987, 95 % CI 0·981, 0·991), which was consistent with the results after filling (Supplementary Fig. 1).

Fig. 2. Association between six trace elements and low cognitive performance in the CERAD, Animal Fluency and DSST tests. CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; DSST, Digit Symbol Substitution Test.
Associations between six trace elements and low cognitive performance in old adults with or without diabetes and hypertension history
Figures 3 shows the associations between six trace elements (Fe, Pb, Cd, Hg, Se and Mn) and low cognitive performance in the elderly with or without diabetes and hypertension history. In the Animal Fluency test, after adjusting all covariates, high serum Fe levels were related to the decreased risk of low cognitive performance in old people without diabetes history (OR = 0·992, 95 % CI 0·988, 0·997) and with hypertension history (OR = 0·993, 95 % CI 0·988, 0·997). High blood Cd (OR = 2·900, 95 % CI 1·311, 6·417) and blood Mn (OR = 1·037, 95 % CI 1·009, 1·066) levels were associated with the high odds of low cognitive performance in old diabetic and hypertensive adults, respectively. In the DSST test, high blood Pb levels were associated with the increased risk of low cognitive performance in the elderly without diabetes (OR = 1·126, 95 % CI 1·026, 1·235) and hypertension (OR = 1·121, 95 % CI 1·002, 1·255) history. Elevated blood Cd levels were connected with low cognitive performance in old diabetic (OR = 3·177, 95 % CI 1·323, 7·27) and hypertensive (OR = 1·896, 95 % CI 1·056, 3·403) people. High blood Se levels were related to the decreased risk of low cognitive performance in old adults with (OR = 0·989, 95 % CI 0·981, 0·998) and without (OR = 0·986, 95 % CI 0·978, 0·993) diabetes history, with (OR = 0·992, 95 % CI 0·986, 0·997) and without (OR = 0·976, 95 % CI 0·962, 0·990) hypertension history.

Fig. 3. Association between six trace elements and low cognitive performance of the elderly in different subgroups. (a) With diabetes history; (b) without diabetes history; (c) with hypertension history; (D) without hypertension history. CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; DSST, Digit Symbol Substitution Test.
Discussion
In the present study, we investigated the associations between six trace elements and the risk of low cognitive performance among old adults. Our findings showed that elevated serum Fe levels were related to the decreased risk of low cognitive performance, especially in the elderly without diabetes history and with hypertension history in the Animal Fluency test. High blood Cd and blood Mn levels were associated with the high odds of low cognitive performance in old adults with diabetes and hypertension history, respectively. In the DSST test, high blood Pb levels were associated with the increased risk of low cognitive performance, especially in the elderly without diabetes and hypertension history. Elevated blood Cd levels were connected with low cognitive performance in old diabetic and hypertensive people. High blood Se levels were related to the decreased risk of low cognitive performance in all old adults. No relationships between trace elements and low cognitive performance were discovered in the CERAD test.
Fe plays an important role in brain development, myelination and cognitive function (Reference Lonnerdal13,Reference Butnariu23) . Elevated serum Fe levels were related to the decreased risk of low cognitive performance in the elderly, which was consistent with findings proposed by Kweon et al. (Reference Kweon, Youn and Lim24). Hare et al. reported that the transferrin-related Fe content in Alzheimer’s disease (AD) patients significantly decreased, which may be due to the decreased serum Fe content (Reference Hare, Doecke and Faux25). Fe is a cofactor of ribonucleotide reductase, which is responsible for the rate-limiting step of DNA synthesis, making Fe essential for cell division and neural tube formation(Reference Kim and Connor26,Reference Puig, Ramos-Alonso and Romero27) . Beyond cell division, neurons need timely and adequate Fe supply for neurotransmitter synthesis, synapse formation and dendritic arborisation (Reference Kim and Connor26). Excess redox-active Fe can also lead to oxidative damage and cell death (Reference Kim and Connor26). Appropriate Fe supplementation and Fe-rich foods intake may be beneficial for cognitive function in the elderly.
We also found high blood Se levels was associated with the decreased risk of low cognitive performance in all old adults. Se deficiency causes irreversible brain injury(Reference Ying and Zhang28). Lower plasma Se levels were associated with a high risk of cognitive impairment(Reference Ferry and Roussel11,Reference Vaz, Fermino and Haskel29) . A previous study reported the Se concentrations in the erythrocytes, plasma and nails were low in AD patients(Reference Cardoso, Ong and Jacob-Filho30). A similar association was found in people with diabetes. Serum and urine Se were indicators of body status in diabetes patients(Reference Navarro-Alarcón, Serrana and Pérez-Valero31). In addition, studies have confirmed that there were some common pathophysiological mechanisms between diabetes and cognitive impairment(Reference Spauwen, Eupen and Köhler32,Reference Klimova, Kuca and Maresova33) . Appropriate Se supplementation and Se-rich foods intake may be beneficial for cognitive health in the elderly, especially in diabetic and hypertensive individuals.
High Pb exposure was associated with cognitive impairment in older Americans. The existing reports suggested that the presence of Pb in the brain has a potential pro-inflammatory effect on the central nervous system, and neuronal death may be connected with the production of various cytokines and chemokine(Reference Chibowska, Baranowska-Bosiacka and Falkowska34,Reference Struzynska, Dabrowska-Bouta and Koza35) . Early or long-term accumulation of Pb exposure may be related to accelerated cognitive decline in old age(Reference Van Wijngaarden, Winters and Cory-Slechta36). Results of a previous meta-analysis showed that high blood Pb concentrations were associated with poor cognitive performance regarding in verbal and visuospatial abilities, memory, attention and psychomotor function(Reference Vlasak, Jordakieva and Gnambs37). These were consistent with our findings. Shalan et al. found that vitamin C might reduce intestinal absorption of Pb by reducing ferric iron in the duodenum to ferrous iron to make ferrous iron compete with Pb for intestinal absorption(Reference Shalan, Mostafa and Hassouna38). Appropriate vitamin C supplementation helps reduce Pb levels in the blood, liver and kidneys(Reference Vij, Satija and Flora39), which may be beneficial for cognitive health in older adults.
Furthermore, blood Cd exposure was in correlation with cognitive function in diabetic and hypertensive elder people. Previous studies showed that Cd homoeostasis is abnormal in individuals who had hypertension or diabetes history(Reference Alissa and Ferns40,Reference Tinkov, Filippini and Ajsuvakova41) . Cognitive dysfunction was one of the diabetes and hypertension complications(Reference Kodl and Seaquist42,Reference Iadecola and Gottesman43) . Blood Cd concentration was associated with slower gait speed, which is an early predictor of cognitive decline and dementia(Reference Kim, Garcia-Esquinas and Navas-Acien44–Reference Beauchet, Annweiler and Callisaya46). A cross-sectional study suggested that increased blood Cd was associated with worse cognitive function in adults aged 60 years or older in the USA(Reference Li, Wang and Fu47). Exposure to Cd could elevate the activity of acetylcholinesterase, leading to hydrolysis and concentration reduction of acetylcholine(Reference Karri, Schuhmacher and Kumar48). Reduced release of acetylcholine was associated with decreased cognitive abilities(Reference Karami, Darreh-Shori and Schultzberg49). Besides, evidence has indicated that the generation of reactive oxygen species was induced by Cd(Reference Genchi, Sinicropi and Lauria50). An excess of reactive oxygen species may result in inflammation, eventually causing neuron injury and death(Reference Genchi, Sinicropi and Lauria50,Reference Moyano, de Frias and Lobo51) .
Damage to Mn homoeostasis may alter the activities of Mn-dependent enzymes and Mn sensitivity pathways, leading to neurotoxicity and the pathophysiology of neurodegenerative diseases(Reference Horning, Caito and Tipps8). Mn is transported across the blood–brain barrier by the divalent metal transporter 1 and the transferrin receptor system and accumulates in Fe-rich regions of the basal ganglia(Reference Aschner, Erikson and Hernández52,Reference Balachandran, Mukhopadhyay and McBride53) . Mn efflux and inflow transporter genes (SLC30A10 and SLC30A8) mutations can change Mn levels in the Golgi apparatus and induce neurotoxicity through abnormal vesicular trafficking(Reference Balachandran, Mukhopadhyay and McBride53,Reference Carmona, Zogzas and Roudeau54) . We found that elevated blood Mn was associated with the odds of low cognitive performance among the elderly with hypertension. High blood pressure was associated with cognitive impairment caused by vascular factors (Reference Iadecola and Gottesman43). Although Hg exposure may impair nervous system function(Reference Chakraborty55,Reference Cabral Pinto, Marinho-Reis and Almeida56) , no association with low cognitive performance was discovered in our research. Future studies need to further explore these associations.
Relationships between several trace elements and low cognitive performance were discovered in the Animal Fluency and DSST tests, but not in the CERAD test. The CERAD test is used to assess episodic memory. The Animal Fluency test is utilised to assess verbal fluency and semantic-based memory function, and the sensitive measurement of frontal lobe executive function is evaluated using the DSST test. Thus, the differences in test results may be due to differences in the cognitive areas emphasised by each test.
Herein, we provided reference for the early prevention of cognitive health in the elderly on the relationship between six trace elements in blood and cognitive dysfunction. A representative and high-quality NHANES database was used, and the regression models were adjusted considering the covariates. Moreover, we further explored the association between blood micronutrients and cognitive impairment in old adults with diabetes and hypertension. Several limitations need caution in interpreting our findings. The levels of trace elements in serum or blood measured reflect daily and recent exposure and are considered an indication of current exposure, rather than long-term and lifetime exposure. And heavy metals in urine are considered to be indicators of long-term exposure before kidney damage (Reference Satarug, Garrett and Sens57). It is also necessary to study the effect of exposure to trace elements in urine on cognitive decline in the elderly. In this paper, we focus on trace elements in blood, and future studies in this area will be carried out. In addition, the cognitive tests selected for ease of management and usability did not cover all areas of cognitive function.
Conclusion
Elevated serum Fe and blood Se levels were associated with the decreased risk of low cognitive performance, while high blood Pb, Cd and Mn exposure were related to the high odds of low cognitive performance in old adults, especially in diabetic and hypertensive individuals. Appropriate Fe, Se supplementation and Fe-, Se-rich foods intake, while reducing exposure to Pb, Cd and Mn may be beneficial for cognitive function in the elderly.
Acknowledgements
None.
This research was supported by the Social Public Welfare Science and Technology Research Project of Zhongshan City (Grant No. 2019B1099).
K. L. and W. W. designed the study. K. L. wrote the manuscript. K. L.,T. L., X. W., J. Z. and Z. O. collected, analysed and interpreted the data. W. W. critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
There are no conflicts of interest.









