Chronic obstructive pulmonary disease (COPD) is currently the third cause of death worldwide( Reference Lozano, Naghavi and Foreman 1 ). Interestingly, poor lung function is an important predictor of mortality in patients with COPD and in the general population( Reference Sharma and Goodwin 2 – Reference Friedman, Klatsky and Siegelaub Reference Schunemann, Dorn and Grant 4 ).
Several studies have focused on evaluating the impact of nutritional therapy in COPD patients( Reference Itoh, Tsuji and Nemoto 5 ); however, it is unclear how specific dietary components may influence lung function( Reference Hanson, Rutten and Wouters 6 ).
Lutein is a carotenoid without vitamin A capacities, but with powerful antioxidant capacities( Reference Kijlstra, Tian and Kelly 7 ). The main sources of lutein are kale, spinach and collards( 8 ). Furthermore, lutein is well known for its antioxidant effect in the eye, where it protects the retina from inflammation and oxidative stress( Reference Kijlstra, Tian and Kelly 7 ). With this in mind, it may be the case that lutein has the same protective function in the lungs; counteracting a retardation in pulmonary function. It is hypothesised that carotenoids, including lutein, can protect the airways from inflammation-induced damage. Schunemann et al. reported in both their studies (cross-sectionally and prospectively) a strong antioxidant effect of lutein (serum and dietary intake) on lung function( Reference Schunemann, Grant and Freudenheim 9 , Reference Schunemann, McCann and Grant 10 ). It has been suggested that inadequate dietary intake of antioxidants is associated with the development of respiratory diseases( Reference Keranis, Makris and Rodopoulou 11 , Reference Tsiligianni and van der Molen 12 ).
The association between lutein intake and respiratory health has recently been reviewed( Reference Melo van Lent, Leermakers and Darweesh 13 ), and very few studies in adult populations have been carried out( Reference Schunemann, Grant and Freudenheim 9 , Reference Schunemann, McCann and Grant 10 , Reference Ochs-Balcom, Grant and Muti 14 – Reference Thyagarajan, AM and Smith 16 ). However, these studies combined lutein with zeaxanthin and did not extensively adjust for confounders or comprised selected populations (e.g. only in women, young adults or patients with COPD). To date, there are no recommended levels of intake for lutein; therefore, from a public health perspective, it is important to determine whether recommendations are required. We aimed to cross-sectionally evaluate the effect of lutein intake on lung function as measured by forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and the FEV1/FVC%, in adults from a general population (aged 45–79 years), taking into account socio-demographic, lifestyle and nutritional factors.
Methods
Study design
The present study was conducted with data from the Rotterdam Study, an ongoing, prospective, population-based cohort study since 1990, including adults aged 45 years or older living in the well-defined district Ommoord, a neighbourhood of Rotterdam, The Netherlands( Reference Ikram, van der Lugt and Niessen 17 ). The Rotterdam Study was approved by the Medical Ethics Committee of the Erasmus University, and written informed consents were collected from all participants( Reference Ikram, van der Lugt and Niessen 17 ). The original aim of the Rotterdam Study was to investigate factors related to frequently occurring diseases in the elderly, as this group is particularly vulnerable to diseases( Reference Ikram, van der Lugt and Niessen 17 , Reference Oeppen and Vaupel 18 ). Further information about the objectives and study design of the Rotterdam Study itself is described elsewhere( Reference Ikram, van der Lugt and Niessen 17 ).
The baseline visit of the first Rotterdam Study cohort, including 7983 participants aged 55 years or older, was completed between 1990 and 1993 (Rotterdam Study cohort I (RS-I)-visit 1). From 2000 to 2001, the cohort was extended with a second cohort of 3011 individuals, who are now 55 years old or have moved to Ommoord district (RS-II). From 2006 to 2008, a third cohort of 3932 individuals aged 45–54 years living in Ommoord (RS-III) was added( Reference Ikram, van der Lugt and Niessen 17 ). Participants were invited for follow-up measurements every 3–4 years.
Study participants
Data from the present study were collected from participants attending the fifth visit of the original cohort (RS-I-visit 5; 2009–2011; n 2140), the third visit of the second cohort (RS-II-visit 3; 2011–2012; n 1887) and the second visit of the third cohort (RS-III-visit 2; 2012–2013; n 3000). We excluded participants for whom dietary intake data were not available (n 1337), participants who had an abnormal total energy intake below 2092 kJ (500 kcal) or above 20 920 kJ (5000 kcal) (n 158) and participants who did not have an interpretable spirometry (n 1130). Thus, we included data from 4402 individuals in these analyses. A flow chart is given in Fig. 1.

Fig. 1 Flow chart of the participants included in the study (n 4402). RS-I–III-1–5, Rotterdam Study cohorts I–III-visits 1–5; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Dietary intake of lutein
For RS-I-visit 5 (2009–2011), RS-II-visit 3 (2011–2012) and RS-III-visit 1 (2006–2008), self-reported dietary intake was assessed using a FFQ. Participants were asked to fill in a FFQ at home to report their nutritional intake in the past year. The FFQ was a comprehensive, 389-item, semi-quantitative questionnaire based on an existing validated FFQ developed for Dutch adults( Reference Feunekes, Van Staveren and De Vries 19 , Reference Goldbohm, van den Brandt and Brants 20 ). The FFQ included questions such as the frequency of consumption of food items over the last month, the amount and type of food item and preparation methods. Portion sizes in g/d were estimated using standardised household measures( Reference Donders-Engelen and van der Heijden 21 ). Dietary data were converted into nutrient intakes (including daily lutein intake and total energy intake) using the Dutch Food Composition Tables of 2006 and 2011( Reference Westenbrink, Jansen-van der Vliet and Brants 22 , 23 ).
Lung function
The main outcomes assessed in the present study were the FEV1, FVC and FEV1/FVC%. Trained personnel measured lung function using Master Screen® PFT Pro (CareFusion) according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines( Reference Celli, MacNee and Force 24 ). To ensure reproducibility, multiple efforts were required. The values of the best acceptable effort were used for analyses. Spirometry tests that did not meet ATS/ERS acceptability and reproducibility criteria were classified as ‘not interpretable’.
Confounders
Before the baseline research centre visit, home interviews were conducted by trained interviewers. Thereafter, participants were invited for clinical examination and laboratory tests at the research centre. At the baseline visit of the original cohort (RS-I-visit 1 1990–1993), data of multiple factors were collected. For the present analyses, level of education and ethnicity of the baseline visit were used as proxies for education and ethnicity of the fifth visit (RS-I-visit 5; 2009–2011). Level of education was divided into two groups – low (primary education or less) and high (levels above primary education). Ethnicity of participants was based on the ethnic background of the participants’ grandparents, and was divided into Caucasian and non-Caucasian. Physical activity was measured in metabolic equivalent task-h per week, which was a combination of questions on sport, walking, cycling and gardening. Smoking status was divided into three categories – never smoker, former smoker and current smoker. Data on the following confounders used for the analyses were collected from the fifth visit itself (RS-I-visit 5; 2009–2011): age, sex, height, weight, total daily energy intake, physical activity and smoking status. In addition, other potential dietary confounders were collected by the FFQ: total fat intake, ratio of n-3:n-6 fatty acids (N3:N6), and intakes of fibre, alcohol, β-carotene, β-cryptoxanthin, lycopene and zeaxanthin.
At the baseline visit of the second cohort (RS-II-visit 1; 2000–2001), data on education and ethnicity were collected in the same way as for the baseline visit of the original cohort and were used as proxies for education and ethnicity of the third visit (RS-II-visit 3; 2011–2012). Ethnicity was based on the ethnic background of the participants’ parents, and was divided into the same categories as the RS-I-visit 1. The other factors were collected from the third visit itself.
Data on education, ethnicity, physical activity and dietary intake of the first visit of the third cohort (RS-III-visit 1; 2006–2008) were also collected according to the above-mentioned methods and used as proxies for education, ethnicity and dietary intake data of the second visit (RS-III-visit 2; 2012–2013). The other factors were collected from the second visit itself.
Statistical analysis
We used linear regression analysis to estimate cross-sectionally the effect of 1 sd increase in dietary lutein intake (continuous) on the change in FEV1 (ml), FVC (ml) and FEV1/FVC%. We also tested the relationship for non-linearity using natural splines (online Supplementary Fig. S1)( 25 ). There was no evidence for a non-linear relationship, but as no recommended daily intake of lutein exists, we decided to categorise dietary lutein intake into four quartiles. As there is no European reference data for lutein intake available, the second quartile (1·9–3·2 mg) was chosen as a reference group, as this quartile was in agreement with the average daily intake of lutein for males (2·0–2·3 mg) and females (1·7–2·0 mg) in the USA( Reference Johnson 26 ).
Confounders were selected on the basis of published literature and a 10 % change in the effect estimate of the association between dietary lutein intake and indices of lung function, as described by Mickey & Greenland( Reference Mickey and Greenland 27 ). Lutein and other dietary covariates were adjusted for total energy intake, using the residual method( Reference Willett, Howe and Kushi 28 ). Thereafter, analyses were performed with adjustment for social-demographic factors (age, sex, height, cohort, ethnicity and education) in the analyses labelled as model 1. Model 2 was additionally adjusted for lifestyle factors: weight, total daily energy intake, total fat intake, N3:N6, fibre intake, alcohol intake, smoking status and physical activity. To adjust for other carotenoids, model 3 included additional adjustment for intakes of β-carotene, β-cryptoxanthin, lycopene and zeaxanthin. α-Carotene could not be included in this model because of multicollinearity (the correlation between α-carotene and β-carotene was 0·96).
We tested for significant interactions (P<0·10) between lutein and sex, age, BMI (BMI=weight/height2), smoking status, previous diagnosis of lung cancer, asthma and COPD. Subgroup analyses were conducted for smoking and BMI, as a significant effect of BMI on lung volume has been demonstrated( Reference Jones and Nzekwu 29 ) in addition to an effect modification of smoking on lung function( Reference Larose, Brumpton and Langhammer 30 , Reference Lange, Sparrow and Vokonas 31 ). Stratified analysis was conducted by smoking status and BMI strata according to the World Health Organization( 32 ). In addition, the main analyses were repeated with the exclusion of participants with diabetes and/or CVD. Seven confounders contained missing values. In general, missing values were low. The percentages of missing values ranged from 0·02 (height and weight) to 3·4 % (ethnicity). To account for potential attrition bias, multiple imputation was used to create ten different possible copies of the original data set, in which the missing values were substituted by imputed values (online Supplementary Table S1). These imputed values were calculated from their predictive distribution based on the observed data( Reference Sterne, White and Carlin 33 ). Combined results of the created data sets (n 10) were then pooled in a separate pooled data set to account for the uncertainty about the missing values. An outline of the procedure is described in the online Supplementary Table S2.
In addition, the main results are presented as effect estimates and 95 % CI for the indices of lung function, and a P value <0·05 was considered statistically significant. IBM SPSS Statistics for Windows (release 21.0.0.1) was used to perform the analyses.
Results
Table 1 details the study population characteristics, subdivided by cohort. The average age per cohort ranged from 56 to 79 years; women were slightly more represented than men, and the population was almost exclusively Caucasian. The median daily lutein intake was 2·09 mg/d in RS-I-visit 5, 2·50 mg/d in RS-II-visit 3 and 3·12 mg/d in RS-III-visit 1. The median energy intake was 8101 kJ/d (1935 kcal/d) in RS-I-visit 5, 8240 kJ/d (1968 kcal/d) in RS-II-visit 3 and 9307 kJ/d (2223 kcal/d) in RS-III-visit 1. The prevalence of chronic diseases differed among the three cohorts, where 44, 32 and 27 % of the participants had diabetes, CVD, COPD, asthma or lung cancer in cohort RS-I-visit 5, RS-II-visit 3 and RS-III-visit 2, respectively. Table 2 shows the Spearman’s correlations between the carotenoids. Correlation of lutein with other carotenoids ranged from 0·19 (β-cryptoxanthin) to 0·76 (β-carotene).
Table 1 Characteristics of participants in the Rotterdam Study (n 4402) (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))Footnote *

FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; N3:N6, n-3:n-6 fatty acids ratio; MET h/week, metabolic equivalent of task-h per week; COPD, chronic obstructive pulmonary disease.
* Based on imputed data.
† Treatment for narrowed blood vessels, myocardial infarction, stroke, cerebral haemorrhage and cerebrovascular accident.
Table 2 Spearman’s correlations of dietary carotenoids (n 4402)
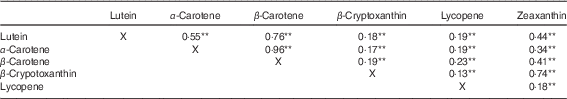
** P≤0·001, a P value <0·05 is considered to be statistical significant.
Lutein intake and lung function
In comparison with the second quartile, the first and lowest quartile of lutein intake was significantly associated with both a lower FEV1 and a lower FVC in the first model adjusted for socio-demographic factors (−53·07 (95 % CI −94·14, −12·01) ml, −49·34 (95 % CI −95·42,−3·25) ml, respectively) (Table 3). After additional adjustment for lifestyle factors (model 2), the associations were attenuated, and thus they were no longer significant. No significant associations were observed between the third and the fourth quartiles of lutein intake and FEV1, FVC and FEV1/FVC%, or for the linear associations between lutein and FEV1, FVC and FEV1/FVC%. Additional adjustment for carotenoids (model 3) did not have an effect on these results.
Table 3 Association between dietary lutein intake and forced expiratory volume in 1 s (FEV)1, forced vital capacity (FVC) and FEV1/FVC% (n 4402) – pooled analysis (β-Coefficients and 95 % confidence intervals)

Ref., reference values.
* P<0·05.
† Model 1 is adjusted for age, sex, height, ethnicity, cohort and education.
‡ Model 2 is adjusted as for model 1, plus weight, total daily energy intake, total fat intake, n-3:n-6 fatty acids ratio, fibre intake, alcohol intake, smoking status and physical activity measured in metabolic equivalent of task-h per week.
§ Model 3 was additionally adjusted for daily intakes of β-carotene, β-cryptoxanthin, lycopene and zeaxanthin.
Sensitivity analysis
We did not find a significant interaction between lutein and sex, age, lung cancer, asthma or COPD (P value all ≥0·133). Although we did not observe a significant interaction for smoking with FEV1, FVC or FEV1/FVC% (P interaction ≥0·133), stratified analyses revealed that lutein was significantly associated with lower FEV1 and FEV1/FVC% in smokers after adjusting for socio-demographic factors and lifestyle factors (−60·08 (95 % CI −115·84, −4·31) ml, −1·64 (95 % CI −2·63, −0·65) % per sd increase in lutein, respectively) (Tables 4 and 6). After adjustment for other carotenoids, some of the results were attenuated; however, the association remained significant for lutein and FEV1/FVC (−1·69 (95 % CI −2·93, −0·45) % per sd increase of lutein). As compared with the second quartile, the fourth and highest quartile of lutein intake was significantly associated with a lower FEV1 and a lower FVC in smokers (Tables 4 and 5). Moreover, the association remained significant after full adjustment (model 3: −157·55 ml fourth quartile v. second quartile (95 % CI −311·32, −3·79)) for FEV1 and attenuated for FVC. Before adjustment for lifestyle factors, lutein was not significantly associated with FEV1/FVC%, but the association became significant after adjustment (model 2: −2·79 % fourth quartile v. second quartile (95 % CI −5·15, −0·41)) (Table 6). After full adjustment, this association became borderline significant.
Table 4 Association between dietary lutein intake and forced expiratory volume in 1 s (FEV1), stratified by smoking status – pooled analysis (β-Coefficients and 95 % confidence intervals)

Ref., reference values.
* P<0·05 (P interaction=0·13).
† Model 1 is adjusted for age, sex, height, ethnicity, cohort and education.
‡ Model 2 is adjusted as for model 1, plus weight, total daily energy intake, total fat intake, n-3:n-6 fatty acids ratio, fibre intake, alcohol intake and physical activity measured in metabolic equivalent of task-h per week.
§ Model 3 was additionally adjusted for daily intakes of β-carotene, β-cryptoxanthin, lycopene and zeaxanthin.
Table 5 Association between dietary lutein intake and forced vital capacity (FVC), stratified by smoking status – pooled analysis (β-Coefficients and 95 % confidence intervals)

Ref., reference values.
P interaction=0·549, * P<0·05, ¥ P=0·071.
† Model 1 is adjusted for age, sex, height, ethnicity, cohort and education.
‡ Model 2 is adjusted as for model 1, plus weight, total daily energy intake, total fat intake, n-3:n-6 fatty acids ratio, fibre intake, alcohol intake and physical activity measured in metabolic equivalent of task-h per week.
§ Model 3 was additionally adjusted for daily intakes of β-carotene, β-cryptoxanthin, lycopene and zeaxanthin.
Table 6 Association between dietary lutein intake and forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC%), stratified by smoking status – pooled analysis (β-Coefficients and 95 % confidence intervals)

Ref., reference values.
P interaction=0·033, * P<0·05, ¥ P=0·056.
† Model 1 is adjusted for age, sex, height, ethnicity, cohort and education.
‡ Model 2 is adjusted as for model 1, plus weight, total daily energy intake, total fat intake, n-3:n-6 fatty acids ratio, fibre intake, alcohol intake and physical activity measured in metabolic equivalent of task-h per week.
§ Model 3 was additionally adjusted for daily intake of β-carotene, β-cryptoxanthin, lycopene and zeaxanthin.
In addition, we observed similar results after stratification by BMI and exclusion of participants with diabetes and/or CVD (data not shown).
Discussion
In a population-based prospective cohort study, we investigated the cross-sectional association between dietary lutein intake and lung function as measured by FEV1. We observed a weak association between lutein and FEV1 and FVC in adults aged 45 years and older, after adjustment for socio-demographic factors. However, this was mainly explained by other nutrients and lifestyle factors. Strikingly, we found that lutein intake was associated with lower lung function as measured by FEV1/FVC% in smokers. To date, there are no dietary recommendations regarding lutein intake; therefore, it is important to clarify to what extent lutein may be related to health outcomes.
Findings in other studies
Our finding that lutein is not associated with FEV1 and FVC is in line with a few other observational studies on FEV1 ( Reference Schunemann, Grant and Freudenheim 9 , Reference Schunemann, McCann and Grant 10 , Reference Semba, Chang and Sun 15 ) and one study on FVC( Reference Semba, Chang and Sun 15 ). A population-based prospective study found a significant association between lutein/zeaxanthin and FEV1, FVC and FEV1/FVC%; however, they investigated the association in adults with chronic airflow limitation( Reference Ochs-Balcom, Grant and Muti 14 ). A previous longitudinal study found a borderline significant association between lutein/zeaxanthin and a slower decline in FEV1, and a significant association between lutein/zeaxanthin and FVC. However, this study was conducted in young adults with a mean age of 25 years( Reference Thyagarajan, AM and Smith 16 ). A borderline significant association between lutein/zeaxanthin blood levels and FVC has been described( Reference Schunemann, Grant and Freudenheim 9 ), and these authors have also demonstrated a significant association between lutein/zeaxanthin intake and FVC( Reference Schunemann, McCann and Grant 10 ). In addition, all studies that investigated lutein/zeaxanthin also investigated the association between other carotenoids and/or antioxidant vitamins and lung function. Schunemann et al.( Reference Schunemann, Grant and Freudenheim 9 , Reference Schunemann, McCann and Grant 10 ) reported that lutein/zeaxanthin dietary intake and blood levels were strongly related to lung function, although not significant for FEV1, as compared with other nutrients with antioxidant capacities such as beta-carotene. This was also reported by Ochs-Balcom et al.( Reference Ochs-Balcom, Grant and Muti 14 ), who demonstrated that lutein/zeaxanthin, measured by dietary intake and blood levels, was strongly related to FEV1, FVC and FEV1/FVC%. Dietary lutein intake has been examined by two out of five studies that investigated lung function in adults( Reference Schunemann, Grant and Freudenheim 9 , Reference Schunemann, McCann and Grant 10 , Reference Ochs-Balcom, Grant and Muti 14 – Reference Thyagarajan, AM and Smith 16 ). A study found a significant positive association between lutein/zeaxanthin and all indices of lung function( Reference Ochs-Balcom, Grant and Muti 14 ). Moreover, a second study found a significantly positive association between lutein/zeaxanthin and FVC only( Reference Schunemann, McCann and Grant 10 ).
Interestingly, subgroup analysis by smoking status in our study revealed that lutein intake was associated with a lower lung function as measured by FEV1/FVC%. Although this has not been reported before, other studies have documented the potential harmful effects of other carotenoids on lung outcomes. For example, intervention studies suggest that carotenoid supplementation increased lung cancer and mortality in heavy smokers( Reference Slatore, Littman and Au 34 – Reference Omenn, Goodman and Thornquist 38 ). Indeed, the association between lutein intake and FEV1 in smokers was explained by adjustment for other carotenoids, suggesting that the inverse association between lutein intake and indices of lung function may be explained to a certain extent by other carotenoids.
Mechanisms
The belief that relates lutein intake with improved respiratory health is based mainly on counteracting oxidative stress. Antioxidants are known to act against reactive oxygen species (ROS), which can be divided into exogeneous oxidants (e.g. cigarette smoke) and endogeneous oxidants (i.e. produced by inflammatory cells)( Reference Halliwell 39 , Reference Genestra 40 ). Oxidative stress, an imbalance between antioxidant capacity and ROS( Reference MacNee 41 , Reference Schunemann, Freudenheim and Grant 42 ), is suggested to be associated with worsening of lung function. For instance, an association between increased markers of oxidative stress (i.e. malondialdehyde or oxidised LDL and decreased lung function in COPD patients has been found( Reference Shen, Yang and Guo 43 , Reference Ahmad, Shameem and Husain 44 ). However, our results are not in line with this hypothesis. On the basis of this mechanism, it can be suggested that the function of lutein (as an antioxidant) depends on the level of exposure to smoking (as an oxidant). For that reason, we performed stratified analyses by smoking status, as it has been proposed that a high intake of carotenoids may reduce their protective function against ROS, particularly in smokers and may even enhance smoke-induced oxidative stress( Reference Palozza, Serini and Trombino 45 ). A possible explanation for this lost function is that cigarette smoke modifies the chemical composition of these nutrients, turning antioxidants into pro-oxidants( Reference Hurst, Contreras and Siems 46 ). Hence, further studies on the potentially harmful effects of dietary lutein intake in smokers are required.
Methodological considerations
Several important strengths of our study can be acknowledged. In comparison with previous studies, we have a large population-based sample size, and were able to adjust for a wide range of confounders including lifestyle and dietary factors as well as intakes of other carotenoids, which were found to be very important.
However, some limitations also need to be taken into account. First, our study had an observational cross-sectional design, which prevents final conclusions about the causality of the observed associations. We found that other dietary and lifestyle factors largely explained the observed association. With this in mind, it may be important to study overall dietary patterns in relation to lung function as this takes the intercorrelation between dietary factors into account( Reference Hu 47 ). Indeed, several dietary patterns have been found to be associated with lung function( Reference Butland, Fehily and Elwood 48 – Reference Varraso, Fung and Hu 54 ). Second, we only had information on dietary intake of lutein and not on blood levels. It may be argued that the blood levels of lutein give a better reflection of lutein status in the human body. For example, when studying blood levels, unmeasured factors such as genetic factors can be better taken into account( Reference Gruber, Chappell and Millen 55 ). In contrast, when high oxidative stress is present, such as in COPD, blood levels of antioxidants may decrease because of an increased demand, which leads to lower blood levels of antioxidants as a consequence, such as lutein( Reference Ford, Mokdad and Giles 56 ). Hence, reverse causality might be present, as it remains unknown whether individuals with, for example, COPD have low blood levels of antioxidants as a consequence of the disease (e.g. physiologically or due to altered dietary intake), or whether low blood levels contribute to the development of the disease. Although our results were not different after excluding participants with chronic diseases, reverse causation might still partly explain the findings. Third, we used a FFQ for dietary assessment of lutein, which is subject to measurement error. To account for potential systematic measurement error, we adjusted lutein intake for total energy intake( Reference Willett, Howe and Kushi 28 ). However, non-differential misclassification may still be present, which may have led to bias towards the null( Reference Rothman 57 ). Fourth, selection bias or survival bias may be present, as we selected participants for this study on the basis of available information on their dietary intakes and lung function. Individuals with lower lung function or at a severe stage of chronic disease might not have visited the research centre, and therefore may have been excluded from the present study. This exclusion could have underestimated our association. However, the socio-demographic characteristics (e.g. age, sex, height, body weight, ethnicity and smoking status) of the included participants in our study did not differ from the total population of the Rotterdam Study( Reference Terzikhan, Verhamme and Hofman 58 ).
In conclusion, we investigated the cross-sectional association between dietary lutein intake and lung function in a large, population-based, prospective cohort study. Although we observed some associations between lutein intake and lung function, the majority of these disappeared after adjustment for other nutrients and lifestyle factors. This suggests that a combination of healthy dietary and lifestyle factors might contribute to an improved lung function instead of lutein intake alone. Future studies in the general population should focus on whole diet to identify patterns in foods linked to specific nutrients that are associated with lung function. Interestingly, we observed that higher lutein intake was associated with a lower lung function in smokers. This finding supports previous evidence on the adverse effect of high intakes of antioxidants in smokers. However, to date, there is no dietary recommendation for lutein, which is urgently needed in the clinical setting, as harmful effects might manifest when a high dose is taken by particular risk groups. Future studies should also be aware of the potential inverse association between high lutein intake and specific risk groups such as smokers. To date, the effect of high doses of nutrients that are included in nutritional supplements for the general population or risk groups is poorly studied. A trial by Omenn et al.( Reference Omenn, Goodman and Thornquist 38 ) investigated the protective effect of high doses of vitamin A and β-carotene on lung cancer, which were discontinued when the incident risk of lung cancer was shown to be higher in the treatment group as compared with the control group. Some trials supported their findings, and some other research groups did not( Reference Goralczyk 59 ). In addition, some risk groups might benefit from a nutrition supplement and others may not( Reference Goralczyk 59 ). For example, (pre) pregnant women are advised to take folate supplementation to avoid spina bifida; however, a high dose of folate increased the risk of adenomas in a trial investigating colorectal adenomas( Reference Cole, Baron and Sandler 60 ). These examples demonstrate that more research is needed to give a public health recommendation on nutritional supplements.
Acknowledgements
The authors gratefully thank Roisin McManus for revising the grammar of the manuscript.
The Rotterdam Study is funded by Erasmus MC and Erasmus University, Rotterdam, The Netherlands; the Netherlands Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
D. M. v. L., E. T. M. L., J. C. K.-d. J., L. L. and O. H. F. designed the study. D. M. v. L., conducted the study. A. H., O. H. F., G. G. B. and B. H. S. provided essential materials. D. M. v. L. and E. T. M. L. analysed the data. D. M. v. L, E. T. M. L., L. L. and J. C. K.-d. J. wrote the manuscript. L. L. and J. C. K.-d. J. had primary responsibility for the final content. A. H., B. H. S., G. G. B. and O. H. F. contributed to the intellectual content while drafting the manuscript. All the authors read and approved the final version of the manuscript.
D. M. v. L., E. T. M. L., J. C. K.-d. J. and O. H. F. work in ErasmusAGE, a centre for ageing research across the life course funded by Nestlé Nutrition (Nestec Ltd), Metagenics Inc. and AXA. Nestlé Nutrition (Nestec Ltd), Metagenics Inc. and AXA had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data as well as preparation, review or approval of the manuscript. L. L. is a Postdoctoral Fellow of the Research Foundation – Flanders (FWO).
Supplementary material
For supplementary material/s referred to in this article, please visit https:/doi.org/10.1017/S0007114517000319










